The primary purpose of the urinary system is to regulate the body's fluid balance, acid-base, electrolytes and regulate blood pressure. The kidneys also have a role in the excretion of metabolic waste and toxins, as well as in the secretion and metabolism of hormones.
Acute kidney injury is characterised by renal parenchymal injury. This represents an abrupt decline in glomerular filtration rate characterised by increased serum and plasma creatinine concentrations, uraemia (when severe enough) and, often, changes to urine output (Haskey, 2019). It is associated with decreased renal function, fluid, electrolyte and acid-base imbalances and retention of uraemic waste products (Bar-Nathan et al, 2022).
There are four phases of acute kidney injury based on pathophysiology (Monaghan et al, 2012):
- Initiation phase: the process begins with an insult or injury to the kidney, which leads to a decrease in glomerular filtration rate. However, there may not be any noticeable clinical signs at this stage. If the underlying cause is identified and addressed during this phase, it can help prevent further progression of the disease.
- Extension phase: during this phase, the kidney insult continues because of hypoxia, ischaemia and an ongoing inflammatory response. The glomerular filtration rate continues to decrease as tubular epithelial cells undergo apoptosis and necrosis.
- Maintenance phase: the glomerular filtration rate stabilises as kidney blood flow improves and cellular repair begins. However, it is important to note that uraemic complications such as vomiting, diarrhoea and changes in drinking and urinating may become more noticeable. Acute kidney injury is commonly diagnosed during this phase.
- Recovery phase: during the final stage – which can last for months to years – glomerular filtration rate increases as cellular repair takes place. However, it is also possible for fibrosis to develop, replacing normal renal architecture and leading to chronic kidney disease. To prevent relapses, it is crucial to avoid any further injury or insult during this stage.
Common causes
The causes of acute kidney injury can be divided into three categories:
Pre-renal
This occurs when renal blood flow is compromised due to certain conditions or situations such as dehydration, shock or congestive heart failure. It is important to remember the kidneys receive 20–25% of cardiac output (Waxman, 2020). In response, the kidneys conserve water and sodium, resulting in a reduction of urine output known as ‘physiological oliguria’. Local production of prostaglandins helps maintain renal blood flow, but this can be affected by drugs, including non-steroidal anti-inflammatories, and potentially lead to renal ischaemia. If left untreated, this can cause acute tubular necrosis and intrinsic renal failure. Therefore, it is crucial to recognise pre-renal failure promptly, identify and address the underlying cause and carefully choose drugs to avoid initiation.
Primary intrinsic
Primary intrinsic acute kidney injury may result from damage to the glomeruli, tubules, interstitial tissues or renal vessels and can have many causes. Most commonly, the condition results from exposure to nephrotoxins, renal ischaemia or infections, but the aetiology can be less evident initially in some cases (Segev et al, 2008). The insult causes injury and structural damage to the kidneys, leading to an abrupt reduction of function. Nephrotoxic agents include:
- Ethylene glycol
- Antibiotics such as aminoglycosides
- Cyclosporine
- Chemotherapeutic agents
- Heavy metals (lead, thallium, zinc, arsenic, mercury)
- Hypercalcaemia
- Miscellaneous agents such as iodinated contrast media and carbon tetrachloride (found in cleaning agents)
- Grapes and raisins in dogs
- Lilies in cats.
Post-renal acute kidney injury
Post-renal acute kidney injury occurs when an obstruction in the urinary tract after the kidneys causes waste to build up within the kidneys. This can be because of:
- Obstructive nephrolithiasis
- Ureteral obstruction
- Ureteral or renal avulsion injury
- Ureteral neoplasia
- Bladder obstructive neoplasia, especially transitional cell carcinoma of the trigone area
- Obstructive prostatic disease
- Urethral obstruction.
It is important to be aware that pre-renal and post-renal causes of acute kidney injury can lead to intrinsic acute kidney injury. Monitoring trends in creatinine, presence of cylindruria, or glucosuria and proteinuria is required after their correction. Acute kidney injury might also occur when an acute insult affects kidneys with pre-existing chronic disease – this is often referred to as acute-on-chronic renal disease. This is one of the more common aetiologies: in the face of chronic kidney disease, the kidneys are more vulnerable to injury.
Diagnosis
The diagnosis of an acute kidney injury is made using a combination of clinical history, physical examination findings, diagnostic test results and diagnostic imaging.
History and clinical examination
When an acute kidney injury is suspected, it is crucial to gather a detailed history. This helps distinguish acute kidney injury from chronic kidney disease, assess the patient's vaccination status (this is important in case of leptospirosis) and identify potential toxin exposure. Owners may report symptoms such as lethargy, loss of appetite, vomiting, diarrhoea and changes in urine production. Additionally, any other health issues that could increase the risk of acute kidney injury or complicate treatment, such as cardiac disease, should be noted. Evaluation of the patient's hydration status should be carried out to formulate an appropriate fluid plan. Uraemia is another important condition to be vigilant for, with signs including hypothermia, oral ulcers and halitosis.
Patients with an acute kidney injury may present with normalsized kidneys or renomegaly. Renal asymmetry could suggest unilateral ureteral obstruction or pyelonephritis (Wu et al, 2021). Small, irregular kidneys can be found with acute-on-chronic kidney disease. Palpation of the bladder to exclude urethral obstruction or to obtain a baseline size of the bladder when monitoring fluid balance is important. Retinal examination to detect ocular damage caused by secondary hypertension is also helpful to gauge whether the apparent renal disease is acute or more longstanding.
Complete blood count
Red blood cells are usually within the normal range in acute kidney injury, but an increase in packed cell volume may occur due to dehydration and haemoconcentration. In cases of chronic kidney disease, anaemia may be present because of reduced erythropoietin production. However, in patients with acute kidney injury, anaemia may also be present due to blood loss from gastrointestinal bleeding or haemolysis (Silverstein and Hopper, 2023).
Biochemistry
Byers and Giunti (2021) assert that creatinine is the primary diagnostic tool for acute kidney injury, while suggesting that other markers and clinicopathological data can also provide valuable insights for identifying this condition. Blood urea nitrogen can also indicate decreased kidney function, but it may be affected by other factors such as gastrointestinal bleeding or increased production, making it a less specific marker for kidney injury than creatinine. Symmetric dimethylarginine offers a more accurate reflection of kidney function compared to creatinine and urea, as it is not influenced by factors that might overstate glomerular filtration rate. Symmetric dimethylarginine is less susceptible to external factors such as body condition and the presence of other diseases (Hall et al, 2014). However, neither creatinine nor symmetric dimethylarginine alone can distinguish between acute kidney injury or chronic kidney disease. Hepatobiliary enzyme activities, such as alanine transferase and alkaline phosphatase, as well as bilirubin levels, may increase in cases of leptospirosis, optimising cardiac function and improving acid-base abnormalities.
Additional tests can be submitted to external laboratories and may prove helpful in the diagnosis of specific causes, such as leptospirosis tests, microagglutination tests, enzyme-linked immunosorbent assay, polymerase chain reaction or toxin screen tests (ethylene glycol).
Urine analysis
Urine should be examined shortly after collection to avoid artefactual changes in composition (Silverstein and Hopper, 2023). In healthy, hydrated dogs and cats, typical urine specific gravity ranges from 1.015–1.045 and 1.035–1.060, respectively (Watson et al, 2015). If the urine is highly concentrated (>1.045 in dogs and >1.060 in cats), it suggests pre-renal azotaemia and retention of concentrating ability at the level of the kidney. On the other hand, isosthenuria (urine specific gravity of 1.008–1.015) with azotaemia indicates intrinsic renal disease (Herold, 2017). Glycosuria with normoglycaemia and proteinuria suggest tubular damage or dysfunction. It is essential to examine a fresh urine sample under a microscope to check for the presence of white blood cells, red blood cells and bacteria, which may signify an infection. If these are detected in a free catch sample, a sterile sample should be obtained through cystocentesis and sent for bacterial culture and sensitivity testing. If there is active renal tubular damage or necrosis, it is common to find granular and hyaline casts. About 30% of dogs with acute kidney injury are reported to have casts during urine sediment examination (Herold, 2017). Struvite and calcium oxalate dihydrate crystals are frequently seen in canine and feline urine samples, while calcium oxalate monohydrate crystals are rare and associated with ethylene glycol toxicity.
Imaging
Abdominal radiographs can be used to assess kidney size and investigate for the possibility of urolithiasis (nephroliths, ureteroliths or urethroliths). A nephropylogram can be used to identify ureteric obstructions (Haskey, 2019).
Abdominal ultrasound allows an assessment of the renal architecture, and urolithiasis may be visualised directly, or there may be evidence of a ureteric obstruction. If ureteric and renal pelvic dilatation or hydronephrosis is present, this supports possible obstruction.
Staging systems
The International Renal Interest Society Acute Kidney Injury Grading scheme has been developed in veterinary medicine to determine appropriate grading in animals with acute kidney injury shown in Table 1 (Cowgill and Langston, 2011). There are others, but this is considered the most reliable and repeatable.
Table 1. International Renal Interest Society acute kidney injury grading criteria.
| Acute kidney injury grade | Blood creatinine | Clinical description |
|---|---|---|
| Grade I | < 1.6 mg/dl (140 µmol/l) | Nonazotaemic acute kidney injury:
|
| Grade II | 1.7–2.5 mg/dl (141–220 µmol/l) | Mild acute kidney injury:
|
| Grade III | 2.6–5.0 mg/dl (221–439 µmol/l) | Moderate to severe acute kidney injury:
|
| Grade IV | 5.1–10.0 mg/dl (440–880 µmol/l) | |
| Grade V | >10.0 mg/dl (880 µmol/l) |
‡Volume responsive is an increase in urine production to > 1ml/kg/h over 6 hours, or decrease in serum creatinine to baseline over 48 hours.
Treatment and nursing considerations
Treat the underlying cause if an underlying cause can be identified – the appropriate therapy should be incorporated into the treatment plan. This might include decontamination in cases of a toxic insult, antidote or reversal agent if appropriate and unblocking if obstructed. Antibiotics are imperative in infectious cases such as pyelonephritis and leptospirosis, and nurses should be barriered in cases of zoonotic disease. Administration of intravenous 20% ethanol can be prescribed to treat ethylene glycol toxicity (Thrall et al, 2013).
Hospitalisation of these patients can be for days to weeks, with nurses playing an integral role in their care, having a clear understanding of the treatment plan and aims, ensuring individualised care and maximising the potential for success of the patient.
Cardiovascular
Acute kidney injury can lead to various complications such as cardiac arrhythmias, congestive heart failure due to fluid overload and abnormal blood pressure levels (hyper- and hypotension) (Silverstein and Hopper, 2023). It is important to monitor a patient's heart rate and rhythm for any abnormalities. Electrocardiographic monitoring is particularly recommended for critical patients and those with hyperkalaemia so that trends can be observed. Regular blood pressure measurements should be taken to address any hyper- or hypotension.
Respiratory
It is important to monitor the respiratory rate, effort and pattern to detect any signs of respiratory distress which may be caused by fluid overload or pulmonary haemorrhage due to leptospirosis (Haskey, 2019). In order to provide appropriate treatment, it is crucial to identify and address the underlying cause while also administering oxygen therapy and closely monitoring the patient's breathing. Arterial blood gas sampling is the gold standard and is recommended to assess patients' oxygenation and ventilation. If this is not available, a pulse oximeter can be used.
Neurological
In some cases of severe uraemia, encephalopathy can develop, leading to impaired mentation, stupor, tremors and seizure-like activity. Treatment requires support therapies to reduce uraemia and manage seizures, and haemodialysis if appropriate. The nurse should evaluate the patient's mental state during each interaction to detect any changes.
Intravenous fluid therapy
Administering fluids for acute kidney injury and chronic kidney disease is aimed at improving circulating volume, boosting cardiac output and perfusion pressure and ultimately enhancing renal blood flow, oxygen supply to the kidneys and glomerular filtration rate.
Monitoring the patient's trends and fluid rate changes can be required quickly to counteract complications. It is imperative to be cautious with fluid therapy as it may cause fluid overload, leading to complications shown in Table 2. It is crucial to notify the veterinary surgeon promptly should any of these symptoms be noticed, particularly if accompanied by weight gain (Hughston, 2016).
Table 2 Clinical signs of fluid overload (adapted from Hensen, 2021).
| Tachypnoea |
| Hypertension |
| Heart mumur |
| Nasal discharge |
| Third spacing – ascites, pleural effusion, peripheral oedema |
| Change in mentation |
| Hypothermia |
| Tachycardia |
Urine output and urine specific gravity can be used as part of the fluid balance assessment of the patients. Vasopressor therapy might be required to support blood pressure in cases of hypotension refractory to fluid therapy (Haskey, 2019). Fluid therapy is the main cornerstone of treatment. Malbrain et al (2020) proposed four distinct phases (ROSE, Figure 1) of intravenous fluid therapy for humans with critical illness: resuscitation, optimisation, stabilisation and evacuation. In overview, the resuscitation phase anticipates an escalation of fluid therapy in patients with life-threatening shock, low blood pressure, signs of impaired perfusion or a combination of these, and is characterised by the use of fluid bolus therapy (rapid infusion to correct hypotensive shock; approximately 10 ml/kg balanced isotonic crystalloid over 15 minutes, typically not exceeding a total of three boluses). When transitioning to the optimisation phase, the patient should no longer be in immediate life-threatening danger, but in a stage of compensatory shock and at high risk for decompensation. At this point, additional fluid therapy is given more cautiously with the intent of optimising cardiac function and improving tissue perfusion. The goal is to administer the volume necessary to alleviate organ dysfunction while avoiding fluid overload (Malbrain et al, 2020).
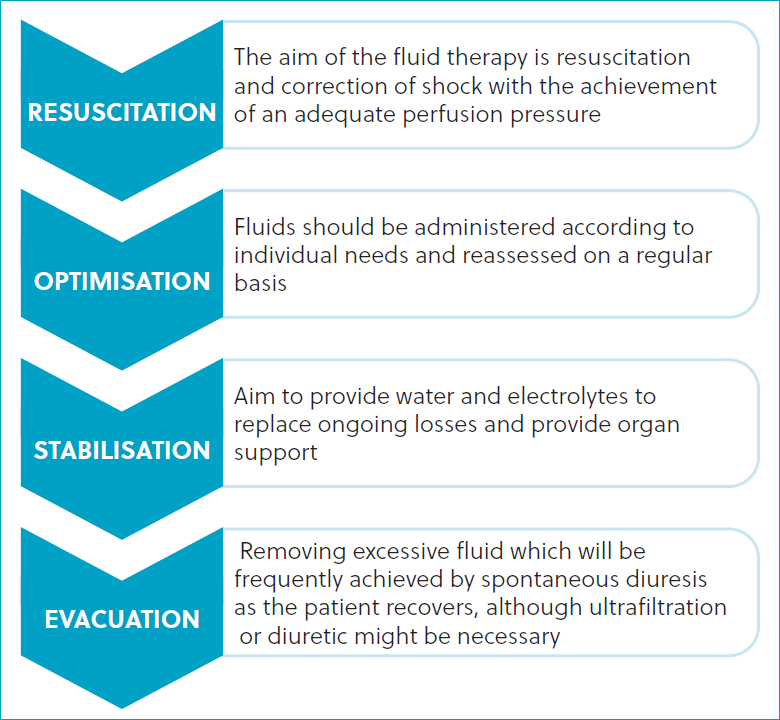
The stabilisation phase evolves over days, replacing insensible loss at 22 ml/kg/day plus replacement of urine volume and ongoing losses (such as vomiting). The volume of medications and nutrition account for part of the fluid administered; the remaining volume is typically administered as intravenous crystalloids.
During the evacuation phase, the fluid dose should be decreased by 10–20% every 12–24 hours and the patient monitored for dehydration (Malbrain et al, 2020). This enables the true recovery of the acute kidney injury to be assessed and a return of the azotaemia can be seen in cases as fluid therapy is withdrawn.
Acute kidney injury patients may benefit from the placement of a central venous catheter to facilitate effective fluid therapy, drug administration and frequent blood sampling. Nurses can be utilised in the placement of these jugular or saphenous (medial in cats, lateral in dogs) vein and kept in situ for an indefinite period so long as they are appropriately managed and there are no signs of complication (Kirby and Rudloff, 2017).
Management of electrolytes and acid-base
Hyperkalaemia can be a life-threatening complication in acute kidney injury (Silverstein and Hopper, 2023). It happens when the kidney is unable to excrete excess potassium. This can lead to bradycardia, ventricular tachycardia and atrial standstill. As a precaution, pa tients with hyperkalaemia should undergo electrocardiographic monitoring.
There are a number of methods to manage hyperkalaemia. One option is t use intravenous regular insulin and glucose which helps by driving potassium into cells, while the administration of calcium gluconate can reduce the risk of cardiac arrhythmias. Enabling diuresis via the establishment of urine flow is the primary goal in cases of hyperkalaemia. If the urinary obstruction is removed or the patient recovers from the oligoanuric acute kidney injury event, normal potassium concentration can be more easily achieved. In some patients, however, post-obstructive diuresis (osmotic) occurs – this can result in a paradoxical hypokalaemia because of marked polyuria. For this reason, it is crucial to closely monitor patients' blood electrolytes during their acute kidney injury event and recovery phase.
In patients, the most common acid-base issue is metabolic acidosis caused by a failure to excrete excess acid at the level of the kidney, leading to low serum bicarbonate. Usually, fluid therapy is enough to correct the acidosis. In rare cases, administration of sodium bicarbonate might be required. However, it must be administered with caution and careful monitoring of pH, bicarbonate, base excess, carbon dioxide and sodium. Side effects of sodium bicarbonate therapy include paradoxical central nervous system acidosis, secondary respiratory acidosis, hypokalaemia and hypernatraemia (Waddell, 2013). The amount of bicarbonate needed should be accurately calculated with formulae present in standard medicine textbooks.
Urine production
Quantification of urine output can be achieved (see Table 3) by placing a urinary catheter with a closed collection system. If this is not possible, collection of all urine that is voided during a walk or use of a litter tray can be performed. This also challenging to do – however, for quantification, 1 ml of urine is equivalent to 1 g in weight. Another method of approximating urine output is to regularly weigh the patient – whilst less accurate than collecting the urine, this provides a reasonable surrogate marker of fluid balance. Bladder size should be assessed regularly using quick ultrasound scans of the bladder, verifying the filling (or not) of the bladder, as a large bladder may indicate a blocked urinary catheter, while a small bladder with reduced urine output would raise concerns for anuria or oliguria (Haskey, 2019).
Table 3 Urine output calculation and definitions.
| Urine output | Volume of urine |
|---|---|
| Normal urine output | 1–2 ml/kg/hour |
| Oliguria | <0.5 ml/kg/hour |
| Anuria | None or negligible amount |
Urine output (ml/kg/hour) = Volume of urine (ml)/time(hours)/patient weight (kg)
One of the hallmarks of acute kidney injury is a drastic reduction in urine output, which can manifest as anuria or oliguria. The difference between an acute kidney injury and a dehydrated patient is profound, and understanding and addressing this is crucial for optimal clinical outcomes. Normally hydrated dogs and cats should produce approximately 25–50 ml/kg/day of urine; the difference is stark, and it is important to remember that acute kidney injury patients, especially in the oliguric or anuric phase, are in a precarious position of fluid overload. Nurses should:
- Calculate fluid needs
- Administer cautiously, especially during the initial hours
- Monitor frequently, check the patient response as often as every 20 minutes looking for signs of fluid overload
- Regularly re-assess. Acute kidney injuries are dynamic, and what worked in the first hour might not be appropriate in the third or fourth hour.
The placement of a urinary catheter can establish urine flow in cases where obstruction is suspected. This can also enable the ‘ins and outs’ approach to be used which is the most accurate method for assessing fluid balance. The nursing team should have an understanding of which urinary catheters are appropriate for the patient and how to place them. The clinic should have a standard operating procedure for any device left in situ so the nursing team are aware of how to manage the device and to minimise the risk of complications or hospital-acquired infections.
Antiemetics and gastroprotection
Nausea or vomiting and gastrointestinal ulceration secondary to uraemia is quite common in these patients. In such cases, the veterinary surgeon should be alerted of signs such as anorexia, inappetence, lip-smacking and hypersalivation. It is also important to be vigilant for signs of melaena. Administration of antiemetics, for example, maropitant, ondansetron and metoclopramide can be administered to alleviate nausea. In addition, sucralfate is an important medication to use when signs of uraemic ulceration are present.
Analgesia
Opioids are often the first-choice analgesic for critical patients as they are well tolerated and can be given intravenously. Carrying out regular pain scores will help to identify patients in need of analgesia but also tailor their individual plan. Non-steroidal antiinflammatory drugs should be avoided as they are nephrotoxic and may cause further damage (Haskey, 2019).
Nutrition outcome
Nutrition is always a challenge in the critical patient, and provision of nutrition should be established at the earliest opportunity. Nurses play a vital role in ensuring that patients meet their recommended energy requirement. Many patients with acute kidney injuries will not voluntarily eat, and so assisted feeding methods will be required to provide adequate nutrition. Naso-oesophageal feeding tubes work well in these patients as they can be placed without sedation or anaesthesia, can be kept in situ for several days and are generally well tolerated as long as vomiting and regurgitation is managed, in the author's opinion. Nurses should be able to calculate the patient's recommended energy requirement and devise a suitable nutritional plan – for example, day 1: 33% of recommended energy requirement; day 2: 66% of recommended energy requirement; and day 3: 100% of recommended energy requirement. An ideal diet for acute kidney injury patients has yet to be identified, however, diets similar to those for chronic kidney disease patients (moderated protein, potassium and phosphate) or highly digestible bland diets appear logical.
Prognosis
The survival rates for acute kidney injury in cats and dogs vary and are dependent on the underlying cause. Studies suggest that acute kidney injury has a combined mortality rate of 47% (Vaden et al, 1997; Segev et al, 2008; Legatti et al, 2018). It has been observed that non-infectious causes of acute kidney injury have a worse prognosis than infectious causes, with cats having a slightly higher mortality rate than dogs. However, there is no single parameter that has been proven to predict prognosis, as neither urea nor creatinine have been statistically significant prognostic factors. During the recovery phase, patients may require ongoing medical management and support, as this phase can take several months.
Conclusions
It is crucial for a veterinary nurse to recognise the difference between an acute kidney injury and chronic kidney disease because it enables them to educate clients, support patients, assist in their treatment and provide both appropriate and effective nursing care. While an acute kidney injury can be a serious condition for some patients, it is possible for others to recover and maintain a good quality of life. Therefore, it is important that acute kidney injuries are diagnosed quickly to commence aggressive medical management and prevent any further damage to the kidneys. Patients with acute kidney injuries require dedicated and specific nursing care to support them during the recovery phase.
KEY POINTS
- Acute kidney injury indicates a sudden and often reversible reduction in kidney function.
- Acute kidney injury can be categorised as pre-renal, primary intrinsic or post-renal.
- The diagnosis of an acute kidney injury is made using a combination of clinical history, physical examination findings, diagnostic test results and diagnostic imaging.
- Survival rates of acute kidney injuries vary depending on the underlying cause.
- The difference between an acute kidney injury and a dehydrated patient is profound. Understanding and addressing this is crucial for optimal clinical outcomes.


