Infective endocarditis is a disease that is associated with high morbidity and mortality in both cats and dogs (Miller et al, 2004; Palerme et al, 2016; Reagan et al, 2022). Endocarditis is usually seen in middle-aged to older, large-breed dogs (Sykes et al, 2006). Infective endocarditis in cats is uncommon; two case series exist (Malik et al, 1999; Palerme et al, 2016) but otherwise, the literature is limited to single case reports (Perez et al, 2010; Dixon-Jimenez and Margiocco, 2011; Doyle et al, 2011; Varanat et al, 2012; Vercelli et al, 2019; Brennan et al, 2020; van Loon et al, 2020). The prevalence of canine infective endocarditis reported in referral centres is low (0.04–0.13%), possibly as a result of non-specific clinical signs and difficulty of diagnosis (MacDonald et al, 2004; Sykes et al, 2006). In small animals, the mitral and aortic valves are most commonly affected, and proliferative or erosive lesions often result in valvular insufficiency (MacDonald et al, 2004).
Pathogenesis
The normal endocardium is resistant to adherence of pathogens, but it can become susceptible when damaged. Mechanical or inflammatory lesions can cause bacterial seeding within the endothelium. In one study by Kienle et al (1994), dogs with subaortic stenosis were found to be predisposed to developing infective endocarditis. However, this association was not identified in a more recent study by Reagan et al (2022). Dental procedures have been thought to increase the risk, but the pre-existing risk is considered relatively low (Glickman et al, 2009; Peddle et al, 2009). Typical locations of vegetations are on the valve edge, and on the lower-pressure side of a regurgitant valve; commonly the ventricular side of the aortic valve (Figure 1) and the atrial side of the mitral valve. These lesions may extend across the valve leaflet, to adjacent endocardium or chordae tendineae (Miller et al, 2004).
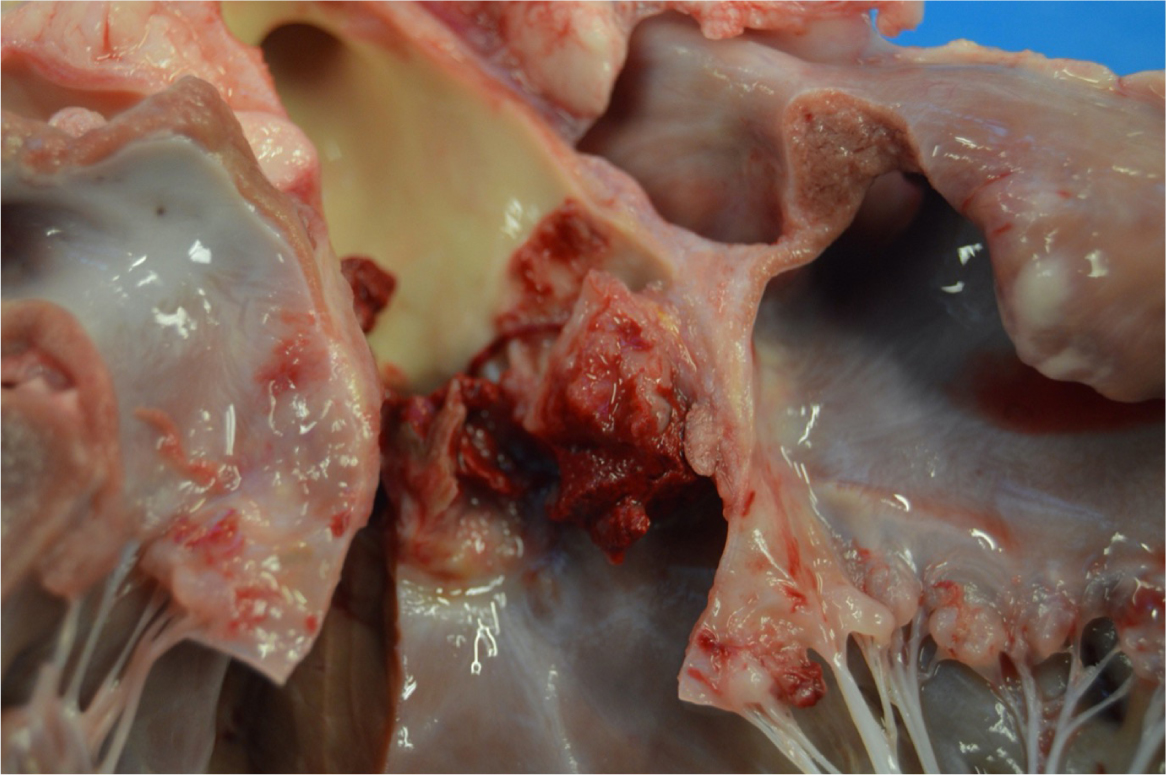
During damage to the endothelium, extracellular matrix proteins, thromboplastin and tissue factor trigger coagulation and form a coagulum. This coagulum contains fibrin, fibrinogen and platelet proteins and can bind bacteria. Inflammatory process stimulates endothelial cell expression of integrins that bind bacteria and fibronectin to the extracellular matrix. Bacteria must be present and able to adhere to the coagulum for colonisation to occur (Miller et al, 2004). This adherence may occur via a variety of mechanisms, one of which includes the adherence of microbial surface components recognising adhesive matrix molecules (MSCRAMMS) that are present on the surface of some bacteria. This adherence was shown to occur in Staphylococcus aureus, as an example (Holland et al, 2014). S. aureus has receptors for MSCRAMMS and the ability to adhere to damaged valves. This bacteria can then trigger tissue factor production and induce platelet aggregation, consequently increasing the size of the lesion (Foster et al, 2014). However, MSCRAMMS may not be the sole factor allowing bacteria adherence. Bacteria also can excrete enzymes that cause further damage to valve tissue and can even rupture chordae tendineae. Very high concentrations of bacteria (109–1011 bacteria/gram of tissue) may accumulate within the vegetative lesion (Sexton and Bashore, 2002).
The most common bacteria causing infective endocarditis include Staphylococcus spp., Streptococcus spp., Escherichia coli, Bartonella spp., and Pseudomonas spp. (Sykes et al, 2006; Reagan et al, 2022). Less commonly isolated bacteria include Pasteurella spp., Erysipelothrix rhusiopathiae and Enterobacter spp., Corynebacterium spp. and Proteus spp. (Sykes et al, 2006; Nakamura et al, 2012; Kern et al, 2019). Bacteria such as S. aureus and Bartonella spp. may become internalised within the endothelial cells and may not be detected by the immune system. Bartonella spp. also evades the immune system by colonising red blood cells without causing haemolysis. In one study, Bartonella spp. was found to be the most common cause of culture-negative endocarditis, responsible for 45% of dogs with negative blood cultures (MacDonald et al, 2004).
Candida spp. are the most commonly isolated organisms causing fungal endocarditis in humans (Pasha et al, 2016). In small animals, fungal infective endocarditis is very rare, but there have been documented cases secondary to foreign body migration (Costa et al, 2014).
A large number of cases have no detectable primary source of infection (Sisson and Thomas, 1984). However, infective endocarditis has been reported in dogs secondary to prostatitis, pneumonia, polyarthritis, disco-spondylitis, urinary tract infections, pyoderma and periodontal disease (Zanotti et al, 1989; Miller et al, 2004; Sykes et al, 2006; Tou et al, 2008; Woodruff et al, 2019).
Clinical presentation
Infective endocarditis is possibly often misdiagnosed owing to its non-specific clinical signs and false negative results of diagnostic tests. The most common clinical signs in dogs and cats include lethargy, lameness, anorexia, increased respiratory rate and effort, weakness, collapse episodes, neurological signs and haematuria (MacDonald et al, 2004). On clinical examination, a heart murmur is commonly present (76–96% of dogs) (Sisson and Thomas, 1984; MacDonald et al, 2004; Reagan et al, 2022).
Depending on the valve affected the presence of left apical, systolic or left basilar, systolic or diastolic heart murmur can be detected. Arrhythmias (40–70%) and pyrexia (70%) are also common findings (Sisson and Thomas, 1984; MacDonald, 2010).
Cats diagnosed with infective endocarditis often present with congestive heart failure, a substantial proportion of these have clinical signs suggestive of arterial thromboembolism (Malik et al, 1999; Kovacevic et al, 2002; Palerme et al, 2016) or an inflammatory arthritis (Palerme et al, 2016).
Acute congestive heart failure is the most common consequence of endocarditis in dogs, especially if the aortic valve is affected (Sykes et al, 2006). Immune-mediated diseases, thromboembolic episodes, arrhythmias and septic polyarthritis have also been reported in both cats and dogs (MacDonald, 2010; Palerme et al, 2016; Reagan et al, 2022).
Methods of diagnosis
The criteria for diagnosing infective endocarditis in dogs and cats are presented in Tables 1 and 2 respectively. These criteria were based on modified Duke criteria used in human medicine (Baddour et al, 2005).
Table 1. Suggested criteria for diagnosis of infective endocarditis in dogs
| Major criteria | Minor criteria |
|---|---|
|
|
Table 2. Suggested criteria for diagnosis of infective endocarditis in cats
| Major criteria | Minor criteria |
|---|---|
Positive echocardiographic findings:
|
|
A definitive diagnosis for both cats and dogs can be made if two major or one major and two minor criteria are met, or there is a pathology of the valve confirmed.
If one major and one minor or three minor criteria are met, diagnosis of infective endocarditis is classified as possible.
If a strong alternative explanation of clinical signs is made or clinical signs resolve with fewer than 4 days of treatment, the diagnosis of infective endocarditis is rejected (Bonagura and Twedt, 2009).
Echocardiography
Vegetative lesions on the mitral and/or aortic valve may be detected using two-dimensional echocardiography. Typical locations of valve vegetations are on the edge and on the low-pressure side of a regurgitant valve. This corresponds to the left ventricular outflow tract side of the aortic valve, and the atrial side of the mitral valve. Small lesions may be difficult to detect and distinguish from myxomatous mitral valve disease.
Vegetations may appear as irregular, thickened, hyperechogenic areas of valve leaflets or as highly mobile, prolapsing masses on leaflet edges, often referred to as oscillating lesions (Figure 2). Valvular insufficiency is identified as a turbulent regurgitant jet on colour flow Doppler interrogation of the affected valve (Lombard and Buergelt, 1983; Elwood et al, 1993; Miller et al, 2004; MacDonald, 2010).
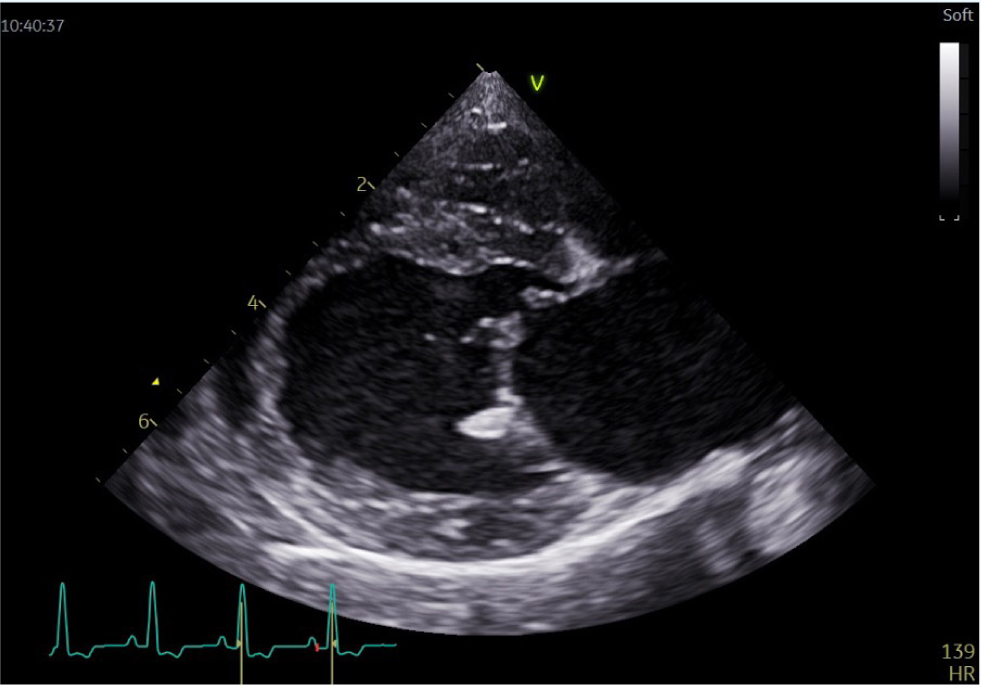
Echocardiography can also detect secondary changes in cardiac size. Left atrial and ventricular enlargement may be present secondary to mitral or aortic endocarditis causing valvular insufficiency. In acute infective endocarditis, left atrium and ventricle size may be normal (Lombard and Buergelt, 1983).
In human medicine, the detection rate for vegetations by transthoracic echocardiography in patients with a clinical suspicion of endocarditis averages approximately 50%. Studies comparing sensitivity and specificity of transthoracic echocardiography and transoesophageal echocardiography confirmed higher sensitivity and specificity of transoesophageal echocardiography (Erbel et al, 1988; Shively et al, 1991; Shapiro et al, 1994; Evangelista and Gonzales-Alujas, 2004).
The use of transoesophageal echocardiography has resulted in more detailed imaging and, therefore, suspected findings of infective endocarditis are no longer considered minor criteria (Mügge et al, 1989). Transoesophageal echocardiography could be considered a useful tool in diagnosing infective endocarditis in veterinary medicine, although there are no studies confirming its accuracy in diagnosing infective endocarditis in small animals yet. Possible limitations of transoesophageal echocardiography would be the need to anaesthetise potentially unstable patients, high cost and limited availability of this procedure.
Thoracic radiography
Thoracic X-rays are obtained in case of presence of congestive heart failure. It has been reported that 14.2–50% of dogs with infective endocarditis develop congestive heart failure (MacDonald et al, 2004; Reagan et al, 2022). Acute congestive heart failure occurs in the absence of left atrial enlargement in 75% of cases, which makes radiographic interpretation challenging (MacDonald et al, 2004).
Radiography may also help to exclude any non-cardiac thoracic disease such as haematogenous pneumonia.
Electrocardiography
Supraventricular and ventricular arrhythmias may be present. The electrocardiographic evidence of chamber enlargement could be observed in patients with infective endocarditis, causing chamber dilatation and hypertrophy. In one study, 62% of dogs with aortic infective endocarditis were reported to have ventricular arrhythmias (Sisson and Thomas, 1984).
In rare cases, an aggressive lesion may invade the intraventricular septum or cause coronary artery embolisation and result in atrioventricular node or bundle–branch damage, causing high grade or third-degree atrioventricular block (Romito et al, 2021). Left bundle–branch block may be seen more often because of intraventricular conduction delays (Miller et al, 2004; MacDonald, 2010).
Laboratory data
Haematology can be used to reveal leucocytosis, and in one study this was observed in 89% of dogs with infective endocarditis (Sykes et al, 2006). Anaemia was noted in 58% of dogs in a recently published retrospective study (Reagan et al, 2022). In another study, seven out of thirteen cats had an inflammatory neutrophilia and six were anaemic (Palerme et al, 2016).
Serum biochemistry often reveals azotaemia, metabolic acidosis, hypoalbuminaemia (95% of dogs) and elevated levels of liver enzymes (MacDonald, 2010). The most common serum chemistry findings in cats were increased blood urea nitrogen, hypoalbuminaemia and hyperbilirubinaemia (Palerme et al, 2016).
Positive blood culture may indicate the presence of disease as one of the major criteria presented in modified Duke criteria. Three or four samples, from different peripheral veins, should be obtained 30–90 minutes apart using an aseptic technique. The use of special tubes at a controlled temperature is also important and should be discussed with the laboratory before obtaining the blood samples.
Bartonella spp. are rarely cultured from dogs and cats, as they are an intracellular bacterium. The blood serum may be tested to measure antibody titres. PCR-based detection of Bartonella spp. DNA from blood or cardiac valve on necropsy is currently recommended (Breitschwerdt et al, 1995). Dogs with PCR evidence of Bartonella in one study were highly seroreactive to Bartonella spp. and all titres were higher than 1:1024 (MacDonald et al, 2004).
The use of a combinational approach of pre-enrichment culture with Bartonella alpha-proteobacteria growth medium (BAPGM) and PCR may assist in the diagnostic confirmation of Bartonellosis in small animals (Duncan et al, 2007).
In one study using enrichment blood culture in BAPGM followed by PCR amplification and DNA sequence confirmation, Bartonella henselae bacteraemia was confirmed in four out of five cats tested, while conventional blood cultures from all tested cats were negative for bacterial growth (Palerme et al, 2016).
The study by Kilkenny et al (2021) suggested that serum troponin-I concentration (cTnI) may also be considered a useful diagnostic test. The cTnI was used to diagnose dogs with infective endocarditis when compared to control groups of dogs with myxomatous mitral valve disease and dogs with systemic inflammatory disease. A cut-off value of 0.625 ng/ml had 100% specificity in that study sample.
C-reactive protein is a useful tool in human medicine for monitoring patients with infective endocarditis and predicting prognosis (McCartney et al, 1988; Verhagen et al, 2008). As far as the authors are aware, the use of C-reactive protein for monitoring patients with infective endocarditis, or for prognostic purposes, has not been evaluated in dogs. However, it has been used to support the diagnosis of infective endocarditis in a dog in a recent case report (Bae et al, 2022).
Haemoglobinuria, haematuria and proteinuria may also be present. Proteinuria was detected in 84% of dogs (Reagan et al, 2022). Urinalysis, culture and sensitivity should be obtained to identify the bacteria and choose appropriate treatment. Urine protein:creatinine ratio should be tested to assess renal protein loss, which is common in patients with infective endocarditis (Reagan et al, 2022).
Necropsy findings
The postmortem examination may identify raised, roughened, plaque-like valvular lesions (mitral and aortic valves are most commonly affected; Figure 1) and changes secondary to embolisation. The kidney and spleen are the most frequent sites for infarction (Figure 4), but the heart, brain, intestine and other large organs may be also affected (Manzi, 2004). Myocardial infarction caused by embolic occlusion of the coronary artery is a known cause of death (Swanwick and Williams, 1982).
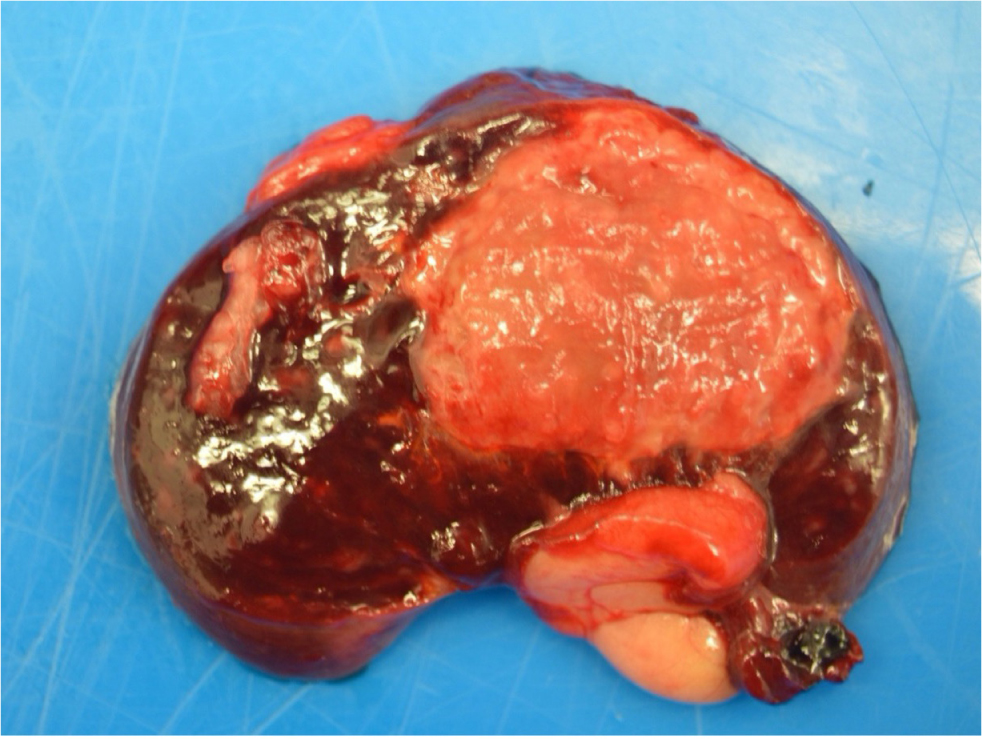
Treatment
Long-term treatment (8–12 weeks) is needed with broad-spectrum antibiotics, ideally including 1 week of intravenous administration to eradicate the infective microorganisms (MacDonald, 2010). Selection of appropriate antibiotics, based on culture and sensitivity results whenever possible, is crucial, although broad-spectrum antibiotic therapy (fluoroquinolones or aminoglycosides combined with beta-lactams) is recommended while blood culture results are pending. Aminoglycosides should be used with caution especially in combination with furosemide because of its nephrotoxicity (Noli and Morris, 2011). In cases with positive blood or urine cultures, the cultures should be repeated 1–2 weeks from starting antibiotics and 1–2 weeks after antibiotic therapy is finished (MacDonald, 2010).
Thromboembolic disease was reported in 45 out of 113 dogs in a recent retrospective study, and was associated with increased mortality (Reagan et al, 2022). Administration of antithrombotic medications in the same study resulted in prolonged survival. The authors would also consider antithrombotic treatment in cats with infective endocarditis, based on a case report showing a successful treatment outcome in a feline patient (Perez et al, 2010). An echocardiogram should be repeated 1–2 weeks after starting therapy and after treatment is finished. Depending on the changes visible on echocardiography, it may need to be performed more often. Euthanasia should be considered when the blood culture results reveal that multi-resistant bacteria is present and poses a risk to other animals and humans. Congestive heart failure, if present, should be managed appropriately.
Prognosis
Overall, prognosis is poor, with various studies finding different factor correlations with survival rates, including the valve that is affected, infective agent, severity of secondary changes and the accuracy and initiation time of treatment. In one study, dogs with infective endocarditis of the aortic valve caused by Bartonella spp. had a grave prognosis, with a median survival time of 3 days (MacDonald et al, 2004). In another study, median survival time was 54 days and dogs with infective endocarditis secondary to Bartonella spp. infection were more likely to develop congestive heart failure, rarely had mitral valve involvement, and had shorter survival times (Sykes et al, 2006). The most recent retrospective study on infective endocarditis in dogs showed 41.6% survival beyond one month after diagnosis. Median survival time was 72 days, neither the valve affected nor the bacterial organism identified correlated with the outcome (Reagan et al, 2022).
In feline infective endocarditis, the aortic and mitral valves are often equally involved, although the affected valve does not impact prognosis. Overall, the prognosis for feline infective endocarditis is grave (Palerme et al, 2016).
KEY POINTS
- Non-specific clinical signs and difficulty of diagnosis may be a reason why infective endocarditis is not commonly detected.
- The mitral and aortic valves are most commonly affected, and proliferative or erosive lesions often result in valvular insufficiency.
- Pyrexia, arrhythmias, lethargy, lameness, anorexia, increased respiratory rate and effort, weakness, collapse episodes and presence of a new heart murmur are common clinical signs of infective endocarditis.
- Conventional blood cultures may be negative for bacterial growth in cases with Bartonella henselae infective endocarditis.
- Serum troponin-I concentration is considered a useful diagnostic test in the diagnosis of infective endocarditis.
- Administration of antithrombotic medications may prolong survival in patients with infective endocarditis.

