Myxomatous mitral valve disease (MMVD) manifests as degeneration of the atrioventricular valve of the left side of the heart. It is characterised by regurgitant blood flow travelling from the left ventricle back into the left atrium during ventricular contraction. This disease is the most commonly acquired heart disease in dogs, affecting approximately 1/30 dogs attending primary care practices in the UK (Mattin et al, 2015). As a degenerative disease, it is more prevalent in older dogs, with studies reporting that approximately 30% of dogs over 10 years of age are affected (Detweiler et al, 1961; Beardow and Buchanan, 1993).
This disease can be seen in any breed, but the highest prevalence is among small-to medium-sized dog breeds such as Cavalier King Charles Spaniels, Yorkshire Terriers, Dachshunds and miniature Poodles, with higher incidences observed in male dogs (Thrusfield et al, 1985; Beardow and Buchanan, 1993). The aetiology of the disease is not completely established, although a hereditary link is strongly suspected in some breeds (Swenson et al, 1996; Olsen et al, 1999). The disease has a long subclinical course, which may or may not progress to congestive heart failure (Borgarelli and Buchanan, 2012).
General anaesthesia for dogs with MMVD can be more challenging than for normal dogs. Successful anaesthetic management requires knowledge of the pathophysiology of the disease and pre-anaesthetic, induction and maintenance drug pharmacology, as well as possible adverse effects of anaesthesia.
Pathophysiology
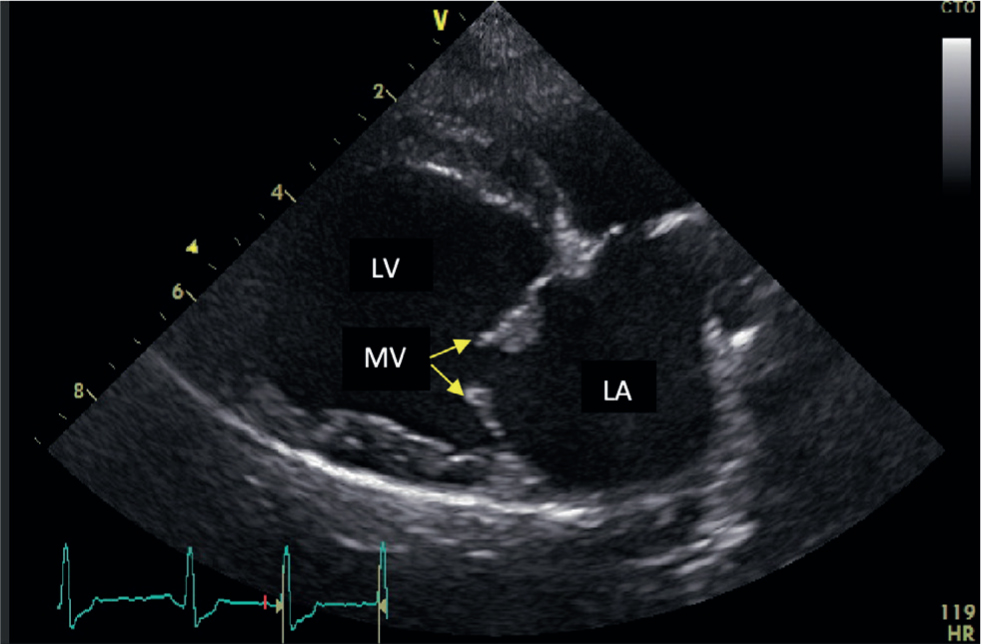
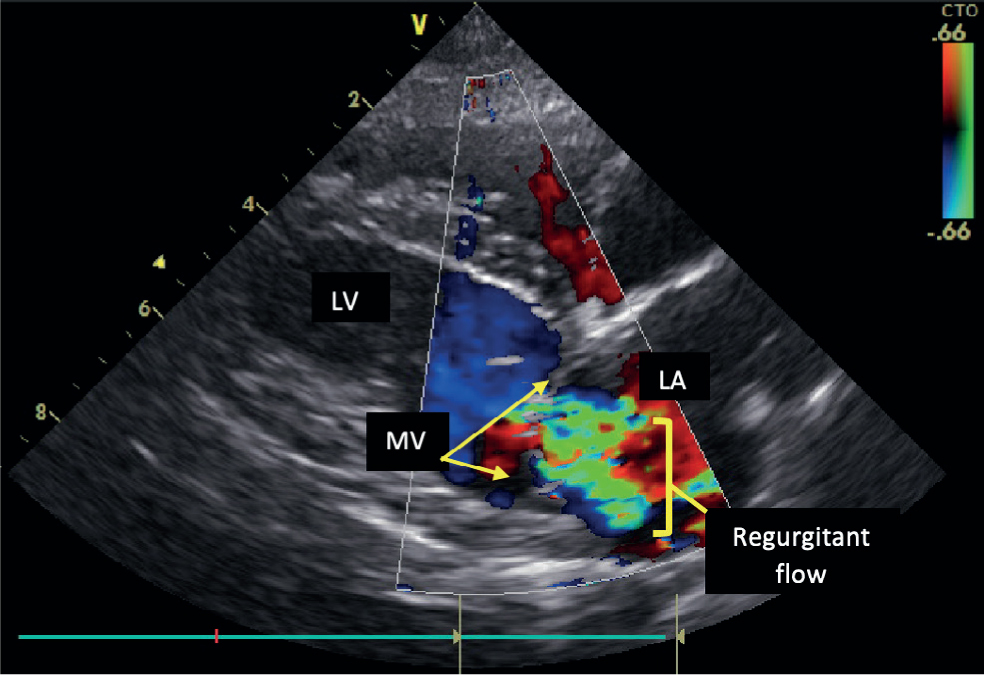
This disease is progressive and degenerative, causing distortion of the mitral valve structure and consequent disruption to the normal haemodynamic function of the heart. In a normal heart, the two mitral valve leaflets are thin and are attached to the papillary muscles by the chordae tendinae. In cases of MMVD, nodular distortion of the valve leaflets and thickening of the chordae tendinae occur (Figure 1). At any stage during disease progression, consequences of this pathology can include mitral valve prolapse, possible chordae tendinae rupture and malcoaptation of the mitral valve leaflets (Pedersen et al, 1999). This results in regurgitation of blood from the left ventricle back into the left atrium during ventricular contraction (Figure 2). In advanced cases of MMVD, this regurgitant fraction can be as high as 88% of the total stroke volume (Kittleson and Brown, 2003).
Cardiac output is defined as the volume of blood the heart pumps throughout the circulatory system each minute; it is the product of heart rate and stroke volume (Duke-Novakovski et al, 2016) (Figure 3). As MMVD progresses, cardiac output is reduced as increasing regurgitation causes declining forward stroke volume.

This results in activation of compensatory mechanisms, including the renin-angiotensin-aldosterone system and sympathetic nervous system, to maintain cardiac ouput and systemic blood pressure via fluid retention, increased heart rate and vasoconstriction. However, ultimately these mechanisms become maladaptive, leading to atrial and ventricular remodelling and pulmonary oedema (Fox, 2012).
Staging of myxomatous mitral valve disease
Auscultation of an adult-onset left apical systolic murmur is suggestive of MMVD in dogs (Ljungvall et al, 2014). However, to confirm MMVD and accurately stage the severity, further diagnostics involving radiographs, echocardiography and cardiac biomarker assays can be performed (Duke-Novakovski et al, 2016). The American College of Veterinary Internal Medicine (ACVIM) have produced consensus guidelines for the appropriate staging of dogs with MMVD (Keene et al, 2019) (Table 1).
Table 1. American College of Veterinary Internal Medicine guidelines on the staging of myxomatous mitral valve disease
| Stage | Characteristics |
|---|---|
| A | Dogs with no identifiable structural disorders of the heart but are at increased risk of their development (such as Cavalier King Charles Spaniels) |
| B1 | Dogs with structural heart disease (for example, murmur auscultation) that have no evidence of cardiac remodelling |
| B2 | Dogs with structural heart disease and evidence of cardiac remodelling |
| C | Dogs currently in, or with past clinical signs, of congestive heart failure |
| D | Dogs in end-stage congestive heart failure with clinical signs refractive to standard treatments |
Adapted from Keene et al (2019)
A common presentation to primary care practitioners is that of an elderly dog with a heart murmur requiring a general anaesthetic for a procedure, such as dentistry or mass removal. Sometimes, this murmur will be an incidental finding during a pre-anaesthetic screening and on other occasions, the dog will have a previous diagnosis of MMVD. Most commonly in primary care practice, general anaesthesia will be performed in dogs with no history of heart failure (stage B1 or B2). Dogs in stage B1 will generally tolerate standard anaesthetic protocols well and carry only a mildly increased risk of cardiac complications under general anaesthesia. Dogs with stage B2 cover a wide spectrum of disease progression and carry variable risk of cardiac complications, depending on disease severity.
Dogs currently in congestive heart failure require further stabilisation before considering a general anaesthetic. Dogs with past clinical signs of congestive heart failure (stage C) carry an increased risk of cardiac-related complications. Dogs in congestive heart failure, refractory to treatment (stage D), have a very high risk of complications and general anaesthesia is generally to be avoided. When performing a general anaesthetic in dogs with MMVD, it is important to understand the haemodynamic changes in order to minimise intra-anaesthetic complications.
Challenges of anaesthetic management
The challenge in anaesthetising dogs with MMVD is to maintain cardiac output by maximising stroke volume and minimising regurgitant flow. To achieve this, there are three main goals to consider (Duke-Novakovski et al, 2016):
- The maintenance of a high-normal heart rate
- The maintenance of adequate but not excessive cardiac preload
- The provision of mild arteriodilation.
During general anaesthesia it is important to maintain cardiac output, in order to ensure adequate tissue perfusion and oxygen delivery. It is important to maintain a heart rate close to the baseline rate obtained during the pre-anaesthetic examination of the conscious patient or, as a rough guide, at 100–120 beats per minute, to compensate for the reduced stroke volume and to maintain cardiac output. Bradycardia should be avoided because not only does it directly decrease cardiac output, but it also results in increased preload. Cardiac preload describes the stretching of the cardiomyocytes of the left ventricle at the end of diastole and is related to the volume of blood present within the left ventricle. A decrease in preload (such as low venous blood pressure or tachycardia) results in a decrease in stroke volume and further reduction in cardiac output. However, a significant increase in preload (such as intravenous fluid overload or bradycardia) can result in backwards failure of the heart, leading to an increase in left atrium filling pressure and consequent increased pulmonary venous pressure and possible development of pulmonary oedema (Duke-Novakovski et al, 2016). Afterload refers to the pressure that has to be overcome for stroke volume to be ejected into the aorta. A reduction in this pressure favours forward stroke volume and decreases regurgitant flow. Therefore, mild vasodilation is desirable to promote forward stroke volume and optimise cardiac output.
Pre-anaesthetic assessment
Dogs with MMVD pose haemodynamic challenges to the primary practitioner and maintaining a stable general anaesthetic can be difficult. However, there are steps that can be taken to better prepare and successfully manage animals with this disease.
Assessment and planning before general anaesthesia is an important part of every anaesthetic protocol. Appropriate history taking before planning the anaesthetic is essential. The focus should be on identifying any risk factors for worsening MMVD such as coughing, tachypnoea, exercise intolerance, syncope and/or collapse. If any of these signs have been observed by the owner, further investigations are warranted before a general anaesthetic is performed. The physical examination should be thorough, focusing particularly on the cardiovascular and respiratory system. Pre-anaesthetic screening with basic echocardiography to assess cardiac chamber size can be very valuable when performed by an experienced veterinarian (see further reading). Pre-anaesthetic blood work, including haematology, biochemistry and electrolytes, is good practice in geriatric dogs over 7 years old (Joubert, 2007). The correction of any significant abnormalities, if possible, should be undertaken before anaesthesia.
Current medications
Dogs with stage B2 MMVD are commonly treated with pimobendan and possibly angiotensin converting enzyme inhibitors (ACE-inhibitors). Stage C dogs are always on an additional diuretic, such as furosemide or torsemide (Keene et al, 2019). These medications need to be considered when formulating an anaesthetic plan. It is generally recommended that pimobendan be continued at the usual dose. Its effects as a positive inotrope and vasodilator assist in maintaining cardiac contractility while simultaneously providing mild vasodilation. It is recommended to discontinue ACE-inhibitors at least 24 hours before general anaesthesia, because they can potentially cause a profound, non-responsive hypotension in response to the vasodilatory effects of inhalational anaesthetic agents (Coleman et al, 2016). Excessive vasodilation can reduce arterial blood pressure, resulting in poor tissue perfusion.
Pre-anaesthetic medication considerations
During hospitalisation, it is important to keep dogs with compromised cardiovascular function as calm as possible. Excitement or anxiety-inducing environments can result in increased activation of the sympathetic nervous system, triggering release of catecholamines and leading to an increase in heart rate, cardiac contractility and vasoconstriction (Höglund et al, 2012). Alterations in these cardiovascular parameters can cause a previously stable animal with stage B2 MMVD to decompensate into congestive heart failure (stage C).
Alpha-2-adrenergic receptor agonists, such as medetomidine and dexmedetomidine, should be avoided in dogs with MMVD. The peripheral vasoconstriction caused by these drugs leads to significant increases in cardiac afterload and a baroreceptor-mediated reflex bradycardia (Vainio and Palmu, 1989; Ko et al, 1996; Pypendop and Verstegen, 1998). In dogs with MMVD, this has a threefold effect on cardiac output. An increase in afterload results in both a decrease in stroke volume and an increase in regurgitant flow, which can exacerbate atrial stretch. Finally, bradycardia directly reduces cardiac output (Figure 3). These compounding effects can result in reduced cardiac output and tissue perfusion, as well as backwards failure of the heart and pulmonary oedema.
Opioids are an important part of pre-anaesthetic medication protocols in dogs with MMVD, as they provide analgesia and display minimal cardiovascular effects at clinical doses (Imai et al, 1994). However, opioids display dose-dependent bradycardia as a result of the increase in parasympathetic activity (vagal tone) to the heart. Atropine or glycopyrrolate should be available in case of significant bradycardia. Glycopyrrolate may be preferred over atropine as there is potential for atropine to cause extreme tachycardia and increase cardiac oxygen demand (Westenskow et al, 1981).
The phenothiazine acepromazine is a useful pre-anaesthetic agent in cases of MMVD. Acepromazine's sedative action is mediated at dopamine receptors, while also causing vasodilation through inhibition of alpha-1 adrenergic receptors (Farver et al, 1986). At clinically low doses, mild vasodilation assists in reducing afterload, lessening regurgitant flow. Higher doses should be avoided as there is a risk of significant vasodilation resulting in hypotension.
Induction of anaesthesia
Pre-oxygenation is important in all patients undergoing a general anaesthetic. As most pre-anaesthetic and induction agents are dose-dependent respiratory depressants (Duke-Novakovski et al, 2016), the goal is to fill the functional residual capacity of the lungs with 100% oxygen. This effectively provides an extra reserve of oxygen, which delays the onset of haemoglobin desaturation and hypoxaemia in the event of apnoea or difficult airway management. It is recommended to pre-oxygenate the animal for 3–5 minutes using a tight-fitting face mask, if this does not cause distress, otherwise flow-by oxygen can be used (McNally et al, 2009).
Alfaxalone and propofol are both licenced options for the induction of anaesthesia in dogs. Some studies have shown there may be benefits to using alfaxalone on account of its propensity to increase heart rate and maintain arterial blood pressure compared to propofol (Muir et al, 2008; Okushima et al, 2015). However, other studies have shown that there is no clinical significance between the two induction agents (White and Yates, 2017). It is recommended that the administration of induction agents should be done slowly, as it has been shown to reduce the dose required to achieve adequate anaesthetic depth, thereby limiting their dose-dependent cardiovascular effects (Raillard et al, 2018). This is particularly important in animals with reduced cardiac output, where the anaesthetic effects may be delayed, potentially ‘fooling’ the anaesthetist into overdosing them.
Benzodiazepines, such as midazolam, may be a good choice for animals with MMVD. They have minimal, if any, effects on heart rate, myocardial contractility or vascular tone (Farver et al, 1986). When used alone as a pre-medication agent, their sedative effects are unreliable and can unpredictably cause paradoxical excitation. However, when used in combination after an initial dose of propofol (Hopkins et al, 2014; Robinson and Borer-Weir, 2013) or alfaxalone (Miller et al, 2019) as a co-induction agent, midazolam was shown to significantly reduce the dose of induction agent required to achieve intubation. However, despite this dose-sparing effect, no significant effect on cardiovascular parameters has been observed (Hopkins et al, 2014; Kropf and Hughes, 2018; Miller et al, 2019).
It is important to ensure an adequate depth of anaesthesia before tracheal intubation, to avoid stimulation of the sympathetic nervous system and an increase in circulating catecholamines (Duke-Novakovski et al, 2016; Lindgren et al, 1993).
Adjunctive analgesia
It is important to consider the incorporation of local and regional techniques, along with systemic analgesia, to improve intra-operative stability and post-operative pain management (Grubb and Lobprise, 2020). Painful or nociceptive stimuli activate the sympathetic nervous system, triggering an increase in heart rate as well as peripheral vasoconstriction. By reducing intraoperative nociception, the animal is more likely to maintain a stable plane of anaesthesia and have an improved recovery. Another important benefit of a multimodal approach is the reduction in the minimum alveolar concentration of inhalant anaesthetics required to achieve an adequate plane of anaesthesia (Snyder et al, 2016). As a result, anaesthetic safety is improved by reducing the dose-dependent cardiorespiratory effects of these agents. There are many local and regional anaesthetic techniques that can be used, but these are outside the scope of this review (Duke-Novakovski et al, 2016).
A constate rate infusion may also be considered for the provision of analgesia during invasive procedures. Constate rate infusions of lidocaine have been shown to be both minimum alveolar concentration-sparing for isofluorane (Valverde et al, 2004) and beneficial in reducing the amount of intraoperative analgesia required in dogs undergoing general anaesthesia for surgery (Ortega and Cruz, 2011).
Non-steroidal anti-inflammatory drugs, such as meloxicam, can be used as part of a multimodal analgesia plan in dogs with MMVD. However, their use should be avoided for dogs in congestive heart failure (Duke-Novakovski et al, 2016). As with cardiovascularly normal patients, contraindications for non-steroidal anti-inflammatory drug use in dogs that are not in congestive heart failure include hypotension, hypovolaemia, renal or hepatic insufficiency and a previous adverse response.
Maintenance of anaesthesia
Isoflurane is the mainstay of inhalation anaesthetic maintenance in the UK and is known to elicit dose-dependent hypotension caused by its vasodilatory and negative inotropic effects (Hettrick et al, 1996; Goya et al, 2018). Therefore, it is important to minimise the amount of isoflurane and similar drugs used during anaesthesia. This issue highlights the importance of multimodal and balanced anaesthetic protocols in minimising the minimum alveolar concentration to achieve an adequate anaesthetic plane.
Animals with MMVD are less tolerant of high intravenous fluid administration rates (increases preload), so a rate of 2–5 ml/kg/hr with crystalloid solution is reasonable in most stable dogs to maintain metabolic needs, while minimising the risk of cardiac overload and precipitation of heart failure (Cooke and Snyder, 1998). The American Animal Hospital Association recommends reducing the anaesthetic fluid rate by 25% every hour until maintenance rates are reached, provided the animal remains stable (American Animal Hospital Association, 2021).
Monitoring of anaesthesia
Anaesthetic monitoring is crucial for all veterinary patients. It has been shown that monitoring of anaesthesia decreases the risk of anaesthetic-related death (Brodbelt et al, 2008). Multi-parameter anaesthetic monitors are useful to provide an overall assessment of the animal's vital parameters, although the authors recognise these may not always be available in primary care practice. At a minimum, it is recommended to monitor the pulse rate and quality, capillary refill time, blood haemoglobin saturation (SpO2), non-invasive blood pressure and temperature throughout any general anaesthetic. An electrocardiograph and capnography are also very useful if available.
The postoperative recovery period is critical for all animals with approximately 47% of anaesthetic-related deaths occurring during this period (Brodbelt et al, 2008). In dogs with MMVD, it is important to monitor SpO2 and provide supplemental oxygen if needed, as well as continuously monitoring the heart rate, blood pressure and temperature. Stress minimisation is still important and if necessary, light sedation can be provided if the dog is experiencing a stressful recovery.
Conclusions
General anaesthetic management of dogs with MMVD can be challenging and there is no ideal protocol that works for every case. However, with a basic understanding of the pathophysiology of the disease and the hurdles this can pose to successful anaesthetic management, safety during general anaesthesia can be greatly improved.
KEY POINTS
- Myxomatous mitral valve disease is the most common acquired cardiac disease seen in primary practice.
- It is a degenerative disease that results in regurgitant blood flow through the mitral valve and subsequently reduced cardiac output.
- Knowledge of the pathophysiology of the disease and the pharmacology of agents used in general anaesthesia can improve the safety of anaesthetic management.
- Alpha-2 adreno-receptor agonists, high concentrations of inhalant anaesthetics and high intravenous fluid rates should be avoided.
- Angiotensin converting enzyme inhibitors should be discontinued 24 hours before general anaesthesia.
- It is important to maintain a high normal heart rate, maintain cardiac preload and provide mild vasodilation to support cardiac output and minimise regurgitant flow in dogs with myxomatous mitral valve disease.


