The kidneys are complex multifunctional organs which control multiple different functions, all of which can be affected by anaesthesia. The main functions are regulation of water balance (blood volume, osmolarity (Na+) and blood pressure), regulation of electrolyte balance (sodium, potassium, calcium and phosphate), regulation of blood pH (acid (H+), excretion, bicarbonate, reabsorption) and gluconeogenesis. The kidneys also excrete waste products and toxins and eliminate drugs via filtration or secretion into urine. In addition to the functions previously mentioned, the kidneys play an important role in endocrine functions. These include the control of red blood cell production by synthesis of erythropoietin, renin production and 1,25-dihydroxycholecalciferol, the active form of vitamin D (Hall, 2015).
Renal physiology
In order to fulfil all its functions, each kidney filters a large amount of water and solutes from the blood, reabsorbing the substances needed and secreting the waste products into the convoluted tubules (Schroeder, 2014).
High renal blood flow is necessary to maintain glomerular filtration, reabsorption and secretion. Therefore, the kidneys receive between 20 and 25% of the cardiac output, which is a high percentage considering their size. As a result, urine is produced at a rate of around 1–2 ml/kg/h. Autoregulation of renal blood flow and glomerular filtration rate is achieved by changing renal vascular resistance as arterial pressure changes. This is accomplished by maintaining constant blood flow across a range of mean arterial pressure from 60 to 150 mmHg (Stoelting, 1987) (Figure 1). A change in systemic arterial pressure, such as hypotension caused by hypovolaemia, circulatory shock or some anaesthetic drugs, can lead to autoregulatory responses in the kidneys. This occurs because of the two internal autoregulatory mechanisms that work without outside influence; the myogenic mechanism and the tubuloglomerular feedback mechanism. When perfusion pressure decreases, the afferent arteriole of the glomerulus senses a reduction in vascular resistance. The myogenic mechanism responds by relaxing the smooth muscle in the arteriole, increasing its vessel diameter. Consequently, vascular resistance is reduced, which keeps the blood flow constant. The opposite occurs when perfusion pressure increases. The tubuloglomerular feedback mechanism is more complex. The juxtaglomerular apparatus senses the concentration of sodium chloride in the distal convoluted tubule reaching the macula densa cells and adjusts the diameter of the afferent arteriole accordingly. Autoregulation ensures that these critical organs receive adequate blood flow and oxygen (Hall, 2015).
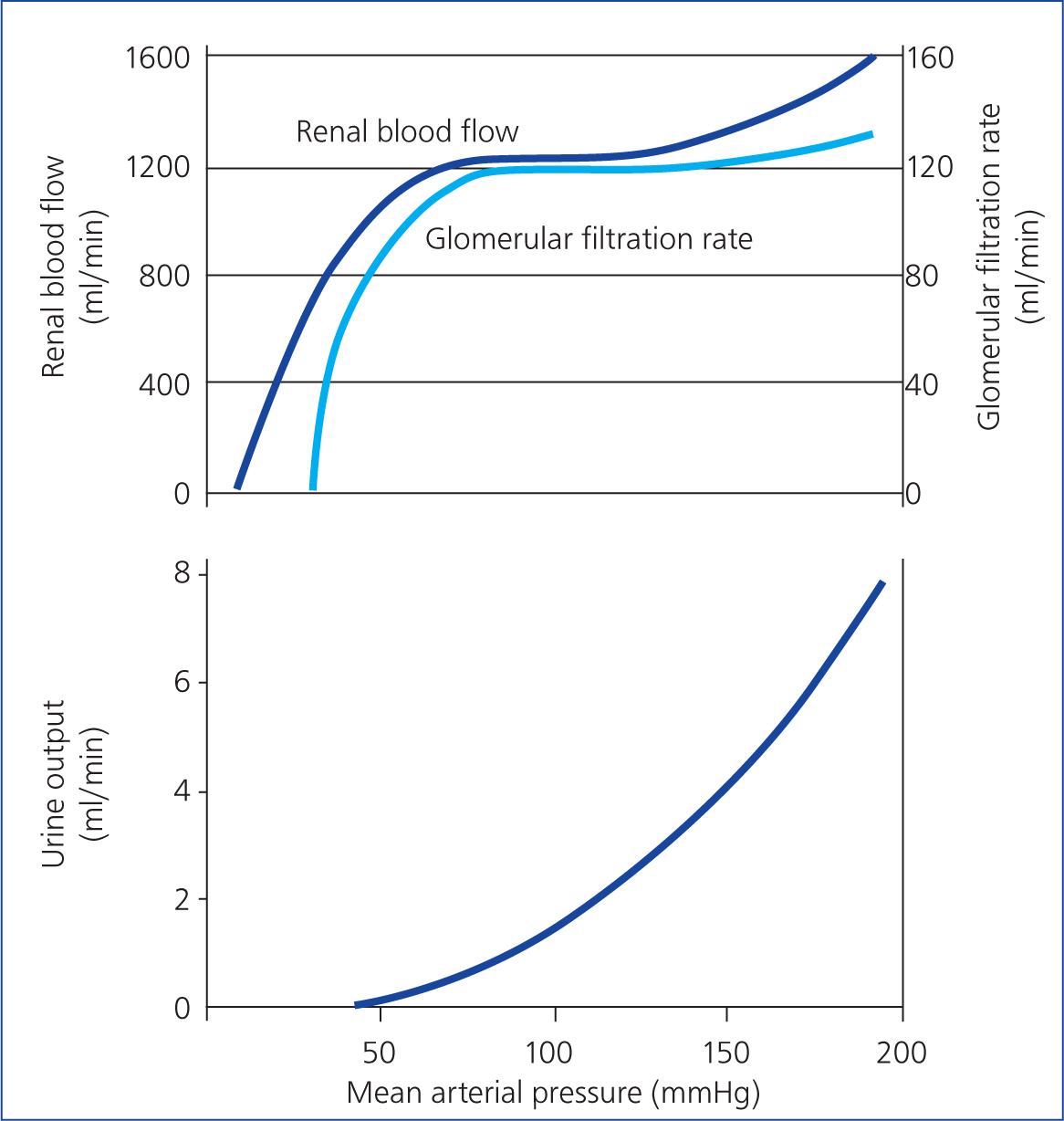
Glomerular filtration rate is a measure of renal function and is used clinically to assess the degree of renal failure. It estimates how much blood passes through the glomeruli each minute. This is achieved by measuring the rate of clearance of inulin (not to be confused with insulin), a small exogenous polysaccharide that is freely filtered at the glomerulus, and not reabsorbed or secreted along the tubule. The glomerular filtration rate is expressed as millilitres of glomerular filtrate, formed per minute per kilogram of bodyweight. This is around 2.9 ml/kg/min in dogs (Laroute et al, 1999) and 3.22 ml/kg/min in cats (Miyamoto, 2001). The autoregulation necessary to maintain glomerular filtration rate can be compromised during anaesthesia, especially in a patient with pre-existing chronic kidney disease. The reduction in renal blood flow and perfusion pressure can alter the glomerular filtration rate and oxygen delivery into the kidney leading to renal ischaemia. Urine output is not autoregulated, but there is a linear relationship between urine output and mean arterial pressure (when pressure is >50 mmHg) (Table 1). Animals with pre-existing renal pathology have much less autoregulatory capacity. Therefore, they may be more likely to decompensate during anaesthesia and surgery, leading to worsening renal disease (Rioja, 2016).
Table 1. Staging of chronic kidney disease in dogs and cats
| Stage 1 No azotaemia (Normal creatinine) | Stage 2 Mild azotaemia (Normal or mildly elevated creatinine) | Stage 3 Moderate azotaemia | Stage 4 Severe azotaemia | ||
|---|---|---|---|---|---|
| Creatinine in mg/dl Stage based on stable creatinine | Canine | <1.4 (125 µmol/litre) | 1.4–2.8 (125–250 µmol/litre) | 2.9–5.0 (251–440 µmol/litre) | >5.0 (440 µmol/litre) |
| Feline | <1.6 (140 µmol/litre) | 1.6–2.8 (140–250 µmol/litre) | 2.9–5.0 (251–440 µmol/litre) | >5.0 (440 µmol/litre) | |
| IDEXX SDMA test (µ/dl) Stage based on stable SDMA | Canine | <18 | 18–35 | 36–54 | >54 |
| Feline | <18 | 18–25 | 26–38 | >38 | |
| UPC ratio Substage based on proteinuria | Canine | Non-proteinuric <0.2 Borderline proteinuric 0.2–0.5 Proteinuric >0.5 | |||
| Feline | Non-proteinuric <0.2 Borderline proteinuric 0.2–0.4 Proteinuric >0.4 | ||||
| Systolic blood pressure in mmHg Substage based on blood pressure | Normotensive <140 Prehypertensive 140–159 Hypertensive 160–179 Severely hypertensive ≥180 | ||||
SDMA Symmetric dimethylarginine
Note: In the case of staging discrepancy between creatinine and SDMA, consider patient muscle mass and retesting both in 2–4 weeks. If values are persistently discordant, consider assigning the patient to the higher stage. See www.iris-kidney.com for more detailed staging, therapeutic and management guidelines.
Adapted from International Renal Interest Society (IRIS) guidelines (2019)Pathophysiology of chronic kidney disease
Chronic kidney disease is defined as the structural or functional disease of one or both kidneys that has been present for a long time, generally 3 months or longer (Polzin, 2011).
It is especially common in elderly cats but can also affect younger cats and geriatric dogs (Bartges, 2012). Chronic kidney disease progression is believed to be the result of an ongoing insult or to be secondary to compensatory changes stemming from different familial, congenital or acquired conditions. These changes occur in the glomerulus, tubulointerstitium or in both nephron subunits.
The International Renal Interest Society (IRIS) has published classification guidelines for staging renal disease, on a scale of 1–4 (Table 1). This is based on renal function tests, such as creatinine and symmetric dimethylarginine (SDMA) concentrations, proteinuria or urine protein:creatinine (UPC) ratio, and systolic blood pressure. This classification helps to adequately treat and monitor patients with chronic kidney disease.
When renal failure occurs, nitrogenous waste products such as ammonia, urea, uric acid and creatinine start to accumulate in the blood. If the levels of these are mildly increased, it is called azotaemia, whereas if the levels are largely increased, causing clinical signs, it is called uraemia. Azotaemia is characterised by increased levels of urea and creatinine, with no obvious clinical signs of disease in shown in patients. Patients do not show clinical signs until 75% of renal function has been lost. Uraemia or uraemic syndrome is defined as increased levels of both urea and creatinine, with some of the associated clinical signs summarised in Table 2 (Schroeder, 2014).
Table 2. Physiological abnormalities associated with chronic kidney disease
| ↑BUN | Respiratory rate |
| ↑Creatinine | Hypertension |
| ↑ or ↓K+ | Anaemia |
| ↑Mg | Uraemic coagulopathy |
| ↓Ca2+ | Hypoalbuminaemia |
| ↑P | Dehydration |
| Metabolic acidosis | Nausea, vomiting |
| Polyuria | Lethargy |
| Polydipsia | Dysphagia |
| Anorexia | Weight loss |
| Halitosis | Weakness |
Systemic abnormalities, such as reduction in erythropoietin and calcitriol production, hyperparathyroidism, osteodystrophy and gastritis may also be present in patients with chronic kidney disease.
In some situations, if a patient's condition deteriorates and becomes decompensated, it may become acute kidney injury, which is a common condition in patients diagnosed with chronic kidney disease, if they become decompensated secondary to an insult (low blood pressure, dehydration, certain drugs) (Ross, 2011; Langston and Eatroff, 2015). Dogs and cats presenting with acute kidney injury can suffer anuria or oliguria (urine output <0.5 ml/kg/h), while patients with chronic kidney disease present polyuria (urine output >2 ml/kg/h) (Ross, 2011).
Good stabilisation and management of the underlying conditions, before the administration of anaesthesia, are key to successful management of chronic kidney disease (Schroeder, 2014).
Evaluation and stabilisation pre-anaesthetic
Patients diagnosed with chronic kidney disease must be stabilised before anaesthesia for any elective procedure. The anaesthetic evaluation should include a complete history and clinical examination, with blood pressure measurement and blood tests, including electrolytes and blood gas analysis.
The main points to take into account regarding pre-anaesthetic stabilisation are as follows:
- Evaluation and correction of the hydration status. Patients with decompensated or end-stage chronic kidney disease are commonly presented with severe dehydration and consequent water volume depletion. A balanced polyionic fluid solution (Hartmann's or lactated Ringers) is generally adequate to replace water deficits. In some situations if electrolytes have to be replaced, specific electrolyte supplementation may be necessary. Fluid therapy should be administered at rates directed towards normalisation of hydration status and improvement in acid–base and electrolyte disorders. However, if large volume of fluids are administered, the patient may be at greater risk of developing volume overload. Signs of volume overload (increased respiratory rate, heart rate and blood pressure; pulmonary oedema, peripheral oedema, chemosis, serous nasal discharge, increased skin turgor, gallop rhythm in cats and pleural effusion) should be monitored (Rudloff, 2015). Water should not be withheld, as these patients normally present with polyuria and polydipsia.
- Assessment of electrolytes. Potassium is the most common electrolyte to be imbalanced in cases of chronic kidney disease. Low potassium levels can be found in these patients. This should be corrected by fluid supplementation with potassium chloride. Note that the rate of potassium administration should not be higher than 0.5 mmol/kg/h.
- If hyperkalaemia (K+>5.5 mEq/litre), the most common alteration, is present, administration of Hartmann's solution (if not contraindicated for other reasons) will dilute the potassium levels in blood. The traditional use of 0.9% NaCl is discouraged because of the high concentration of sodium and chloride. Administration of 0.9% NaCl could lead to metabolic acidosis, which may potentially worsen any pre-existing metabolic acidosis. Hartmann's solution contains a low concentration of potassium, which would be insufficient to raise the plasma levels of potassium. However, the addition of a buffer in Hartmann's solution makes it the ideal solution to administer in patients at risk of developing metabolic acidosis. If hyperkalaemia is associated with bradycardia or atrial standstill, it is considered to be life-threatening and further emergency treatment must be initiated (Table 3).
- Patients with marked anaemia may need a perioperative blood transfusion. However, chronic anaemia is better tolerated than acute anaemia and lower haematocrit values may be accepted. The general clinical condition and any other comorbidities should be considered when making this decision. The recommended therapies vary greatly depending on the cause, severity and progression of the anaemia. The reader is referred to Giger (2015) for further knowledge of how to treat anaemia in specific disease conditions.
- Adjustment of the blood pressure should ideally be done before anaesthesia. Hypertension is a common finding in patients with chronic kidney disease. Angiotensin converting enzyme inhibitors (benazepril or enalapril), and calcium channel blockers (amlodipine) are used to control the hypertension. Although there is no consensus, with many contradictions in the veterinary literature, the withdrawal of these drugs 24 hours before anaesthesia may be considered for non-controlled patients (Coleman et al, 2016). These drugs may cause hypotension which, combined with the vasodilatory effects of certain anaesthetic drugs, can be detrimental for non-controlled patients with chronic kidney disease. However, well controlled patients may benefit from the effect of these drugs in controlling hypertension.
- Protection of the gastrointestinal tract should be considered in animals with uraemic gastritis or vomiting. If this is the case, an antagonist of the histamine receptors, such as ranitidine or famotidine, or proton pump inhibitors like omeprazole, can be administered. A reduction in the dose should be considered as these drugs are excreted renally. If vomiting is persistent, administration of maropitant or metoclopramide may be an option (Rioja, 2016).
- Other concurrent diseases may be present at the time of the anaesthesia. Hypertrophic cardiomyopathy or hyperthyroidism are common concurrent diseases in cats with chronic kidney disease. An in-depth analysis of the treatment of these conditions is beyond the scope of this article. However, these conditions must be treated and stabilised before any anaesthetic procedure is planned.
Table 3. Treatment of life-threatening hyperkalaemia
| Drug | Dosage | Mechanism of action | Onset of action |
|---|---|---|---|
| 10% calcium gluconate | 0.5 to 1.5 ml/kg intravenous (IV) slowly over 5 to 10 minutes with electrocardiogram monitoring | Increases threshold voltage but will not lower serum potassium | 3 to 5 minutes |
| Sodium bicarbonate | 1 to 2 mEq/kg/IV slowly over 15 minutes | Increases extracellular pH, allowing for potassium to move intracellularly | 15 minutes or longer |
| 25% dextrose | 0.7 to 1 g/kg/IV over 3 to 5 minutes | Allows for translocation of potassium into the intracellular space | <1 hour |
| 25% dextrose with insulin | Regular insulin at 0.5 U/kg IV with IV dextrose at 2 g/U of insulin administered | As above | 15 to 30 minutes |
| Terbutaline | 0.01 mg/kg IV slowly | Stimulates Na+/K+-ATPase to cause translocation of potassium into the cell | 20 to 40 minutes |
Elective surgeries should be postponed until the animal is stable enough to undergo the anaesthetic and the procedure where possible.
Anaesthetic management
Premedication
Reduction of the stress response and increasing the animal's comfort is one of the main aims. The administration of an opioid is one of the safest options available for animals with chronic kidney disease, as they have relatively fewer effects on the cardiovascular system. However, pain may activate the stress response and significantly reduce renal blood flow (Schroeder, 2014). A significant reduction in the inhalation anaesthetic concentration and its side effects (vasodilation and reduction in contractility) can be achieved with the appropriate selection of an opioid (Machado et al, 2006)
The choice of opioid should be made based on the expected degree of pain for the procedure being performed. A full mu agonist, such as methadone (0.2–0.3 mg/kg) intramuscular (IM)or intravenous (IV), or fentanyl (5–10/kg/h IV), should be considered for procedures likely to cause moderate to severe pain. When only sedation is needed and the procedure is not painful, butorphanol (0.2–0.3 mg/kg IV) can be used as it will provide a greater degree of sedation (Trimble et al, 2018).
Alpha2 adrenoreceptor agonists (medetomidine or dexmedetomidine) are commonly used to achieve a predictable degree of sedation. The cardiovascular side effects, described as initial peripheral vasoconstriction, and hypertension, followed by reflex bradycardia and pronounced decrease in cardiac output, should make practitioners particularly alert when using alpha2 adrenoreceptor agonists (Murrell and Hellebrekers, 2005). Contradictory effects upon the renal blood flow and glomerular filtration rate have been documented after the use of alpha2 adrenoreceptor agonists in dogs and rats (Lawrence et al, 1996; Marangoni et al, 2007). As supporting evidence is not clear enough, the administration of alpha2 agonists should be reserved for hydrated and well controlled cases, using low doses (µg/kg IM or IV) or half dose when using dexmedetomidine (medetomidine 1–5 µg/kg), alongside the monitoring of blood pressure. The alpha2 adrenoreceptor antagonist atipamezole (2.5 times the µg/kg dose of medetomidine IM in cats or 5 times the µg/kg dose of medetomidine IM in dogs) should be available when using either medetomidine or dexmedetomidine in case any associated complications occur (Zatroch et al, 2019).
Acepromazine is a mild sedative used as part of premedication in dogs and cats. This drug has been widely used despite the fact that it can cause vasodilation, potentially leading to hypotension, in animals affected by chronic kidney disease (Boström et al, 2003). Acepromazine has been reported to maintain normal renal blood flow and glomerular filtration rate in dogs and cats during anaesthesia, despite causing hypotension (Boström et al, 2003). Acepromazine has no antagonist, its duration of action is 6–8 hours, it decreases the temperature and it has been reported to reduce the haematocrit by 5%, which could be contraindicated in patients with anaemia (Mosallanejad et al, 2006). Special attention should be paid to the hydration status of the patient before the administration of acepromazine (0.005–0.02 mg/kg IM or IV), in order to mitigate its vasodilatory effects. Acepromazine is also contraindicated in cats with hypertrophic cardiomyopathy.
Benzodiazepines combined with opioids are the safest option available for pre-anaesthetic medication of animals with chronic kidney disease. Benzodiazepines, such as diazepam (0.3–0.5 mg/kg IV, never intramuscularly) or midazolam (0.2–0.5 mg/kg IM or IV), cause minimal cardiovascular depression and may decrease the requirement of other anaesthetic agents, although this has not been associated with clinically detectable improvement in cardiovascular changes (Robinson and Borer-Weir, 2015; Miller et al, 2019). However, benzodiazepines may lead to paradoxical excitation in young or healthy dogs and cats, especially if administered intravenously (Ilkiw et al, 1996; Simon et al, 2014). For this reason they can be used during the induction period, discussed later on the text. Generally, benzodiazepines administered intramuscularly (diazepam intravenously) can be considered in dogs and cats with chronic kidney disease.
Induction
An intravenous catheter must be placed and secured before induction of anaesthesia. Preoxygenation is always recommended, especially in anaemic animals. High oxygen flow (100 ml/kg/min) should be provided via a mask for 3–5 minutes before induction (McNally et al, 2009). A flow-by technique to preoxygenate can be used if the animal is stressed by the use of a face mask. However, the desaturation time is longer in patients receiving oxygen via a mask, compared with the flow-by technique (Ambros et al, 2018).
During the induction period, the aim should be to reduce the cardiovascular depression produced by the induction agents used. The use of coinduction drugs may allow a dose reduction of the induction agent, thus preserving cardiac output and renal blood flow. Some examples of available coinduction agents include benzodiazepines, if not administered as part of premedication, such as midazolam or diazepam (0.2–0.5 mg/kg IV) or opioids such as fentanyl (3–5 µg/kg IV), administered 1 minute before the induction agent choice. Coinduction with midazolam (0.4 mg/kg IV) has significant alfaxalone-sparing effects with no clinically detectable cardiovascular changes, but apnoea was common after induction (Miller et al, 2019). This highlights the importance of preoxygenation mentioned above.
Propofol and alfaxalone are the most commonly used drugs for induction of anaesthesia in small animals. Induction of anaesthesia may be achieved by using alfaxalone (0.5–3 mg/kg IV) or propofol (1–4 mg/kg IV) (Chang et al, 2011). The authors suggest performing a dose titration to effect induction, that is, administration of small bolus (1 mg/kg IV) and reassessment of palpebral reflex combined with jaw tone, in order to allow endotracheal intubation. Reassessment and administration of further boluses (1 mg/kg IV) should be performed every 30 seconds until endotracheal intubation can be achieved.
Maintenance
Inhalation anaesthesia may be used for maintenance of anaesthesia in animals with chronic kidney disease. Volatile anaesthetics, such as isoflurane or sevoflurane, are mainly eliminated by the respiratory system. Administration of 100% oxygen is recommended to ensure adequate oxygenation levels.
All of the volatile anaesthetics lead to dose-dependent vasodilation and hypotension and a reduction in renal blood flow and glomerular filtration rate. Therefore, they should be titrated to effect (Flood and Shafer, 2015). Multimodal balanced techniques are well known to have a minimum alveolar concentration sparing effect. Hence, a reduction in the amount of inhalation agent can be achieved. Opioid administration, benzodiazepines infusions (such as midazolam 0.15–0.6 mg/kg/h) (Seddighi et al, 2011) and locoregional techniques can be used to allow the delivery of lower volatile agent concentration, therefore reducing the associated vasodilation (Steffey et al, 2017). This is also known as partial intravenous anaesthesia technique.
Total intravenous anaesthesia techniques may be an alternative to inhalation anaesthesia. Propofol (0.2–0.4 mg/kg/min) or alfaxalone (0.07–0.11 mg/kg/min) (Herbert et al, 2013) infusions can be used to maintain a good plane of anaesthesia. When using total intravenous anaesthesia techniques, preservative-free preparations should be used to avoid the neurological side effects associated with some preservatives like benzyl alcohol (Griffenhagen et al, 2015). Unfortunately, there is currently no preservative-free alfaxalone available in the market, so its use is generally not recommended for longer than 1 hour. Similar to volatile anaesthetics, these agents can be combined with an opioid, such as the aforementioned benzodiazepine infusions or locoregional techniques, to decrease the anaesthetic requirements (Rioja, 2016).
Cats have a decreased ability, compared to dogs, to metabolise drugs which undergo glucuronidation, which is necessary for the elimination of many drugs. Propofol has been reported to induce injury to feline red blood cells after repeated daily administration, leading to Heinz body formation (Andress et al, 1995). Feline haemoglobin seems to be particularly susceptible to phenolic compounds (Boothe, 1990). Propofol, a phenolic compound, and not its preservative, could be the cause of oxidative injury to feline red blood cells. Further investigation is needed to finally elucidate whether the feline red blood cells are injured because of the total dose administered or by repeated daily administration. Caution should be taken when administering propofol in cats on consecutive days.
Recovery
During the postoperative period, urine output and urine specific gravity should ideally be measured. A quick abdominal ultrasound may be performed to subjectively evaluate the urinary bladder filling, if a urinary catheter is not indicated or available. Weighing the incontinence pads would be another option to estimate urine output. Blood tests may be considered, especially for measurement of renal parameters if the animal is not clinically stable. A study evaluating the effects of general anaesthesia on renal function in healthy dogs reported changes in renal parameters 48 hours after anaesthesia (Lobetti and Lambrechts, 2000). However, blood analysis should be done after careful consideration, especially in anaemic patients as this could be detrimental. As the anaesthetic period may have resulted in an insult to the kidney and further progression of the disease, performing a follow up after anaesthesia in animals with chronic kidney disease is advisable.
As mentioned previously, reducing the stress response and increasing an animal's comfort should be one of the main aims. Thus, analgesia should be continued throughout the postoperative period.
Fluid therapy should be administered in the postoperative period, unless contraindicated, until the animal can maintain its fluid balance with oral intake. The type of fluid therapy administered should be chosen with a view to restoring the fluid imbalance. By using maintenance fluids, the kidney will not have to excrete the excess sodium or chloride load administered with replacement fluids. In patients with chronic kidney disease the excretion of sodium or chloride may be impaired, which may lead to electrolyte imbalances and acid–base disorders.
Analgesia
Pain management is paramount to avoid excessive activation of the stress response and multimodal analgesia is recommended. Multimodal analgesia refers to the practice of combining multiple analgesic drug classes or techniques to target different points along the pain pathway. Locoregional techniques should be used wherever possible. The most common local anaesthetic drugs for local blocks (lidocaine, bupivacaine, ropivacaine, mepivacaine) are metabolised in the liver and their metabolites are excreted by the kidneys. Those metabolites may accumulate in patients with renal disease and contribute to toxicity, as reported in humans (Collinsworth et al, 1975). Caution should be taken especially when using lidocaine intravenously as a repeated bolus or infusion. Furthermore, lidocaine has been reported to be potentially toxic in cats, especially when administered intravenously. Therefore, its use via infusions or intravenously is not recommended in cats (Pypendop and Ilkiw, 2005).
Neuraxial (epidural and intrathecal) administration of a local anaesthetic is associated with peripheral vasodilation and potential hypotension. However, this has been shown not to worsen preoperative renal dysfunction in dogs (Steagall et al, 2017). Epidural administration of preservative–free morphine may provide excellent analgesia for 12–24 hours. Urinary retention after epidural administration of morphine has been evaluated clinically and experimentally in dogs (Peterson et al, 2014). Although the rate was found to be low (5%), urinary retention should still be monitored when administering morphine epidurally.
The use of non-steroidal anti-inflammatory drugs (NSAIDs) in patients with chronic kidney disease is controversial. During periods of hypotension or low blood flow, cyclooxygenase-2 (COX-2) is upregulated and produces prostaglandins, which cause vasodilation within the kidney, increasing glomerular filtration rate. Prostaglandins are only involved if decreased renal blood flow or hypotension are present (Clark-Price and Grauer, 2017). The administration of NSAIDs will inhibit the formation of prostaglandins, which may potentially affect the maintenance of renal perfusion during anaesthesia, especially in patients where renal blood flow is impaired. The use of NSAIDs in animals with decompensated chronic kidney disease or acute kidney injury, or animals being treated with diuretics, is not recommended (Boström et al, 2006). However, NSAID administration could be considered for postoperative pain relief in well controlled cases (IRIS stage 1–2), if the animal has been normotensive during the anaesthesia and their hydration status is under control. Meloxicam has been reported to produce minimal effects on renal function in healthy dogs undergoing general anaesthetic (Boström et al, 2006).
If treating chronic pain, NSAIDs may be administered if the patient is well compensated and the owner has been informed of any possible side effects and how to recognise them. These patients should also be monitored regularly for any decline in renal function (Gowan et al, 2011). Grapiprant (Galliprant; Elanco) (2 mg/kg once a day orally) is a novel drug designed for pain and inflammation in dogs. This new drug is an EP4 prostaglandin receptor antagonist. Prostaglandin E2 is a prostanoid responsible for regulating pain and inflammation and it binds to the EP4 receptor. By inhibiting only the EP4 receptor, the rest of the haemostatic functions of Prostaglandin E2 (including maintenance of renal flow, among others) are better maintained (Nagahisa and Okamura, 2017). No studies have been published demonstrating the efficacy of treatment with grapiprant for pain caused by osteoarthrosis in cats.
Monitoring and fluid therapy
Blood pressure monitoring is highly advisable. The mean arterial pressure must be maintained at a minimum of 60 and 70 mmHg to ensure adequate renal perfusion. However, higher limits may be considered (70–80 mmHg) in animals with concurrent systemic hypertension. Autoregulation is effective only if the mean arterial pressure is 80–150 mmHg. Mean arterial pressure is generally 20–30 mmHg less than systolic arterial pressure. If a Doppler device is used, a systolic arterial pressure above 90 mmHg would be a sensible lower threshold value in dogs and cats.
The use of electrocardiogram allows for the assessment of rhythm and identification of possible arrhythmias, especially those present in animals suffering hyperkalaemia (Tag and Day, 2008).
The monitoring of oxygen saturation (SpO2%) of haemoglobin in arterial blood by using pulse oximetry may help to ensure adequate oxygenation in our patients. Caution should be taken in anaemic animals, where SpO2 can display normal levels but the oxygen content would not be adequate due to the low haemoglobin concentration (Chan et al, 2013).
Capnometry provides real-time information on respiratory rate, end-tidal carbon dioxide (ETCO2). Capnography also offers a graphical representation of continuous carbon dioxide levels throughout the respiratory cycle. Hypercarbia (ETCO2 >45 mmHg) should be avoided as this may lead to respiratory acidosis, which can worsen acidaemia in cases of coexisting metabolic acidosis (Haskins, 2017). However, it is common practice for anaesthetists to allow a controlled high ETCO2 (<60 mmHg) in anaesthetised small animals, if oxygenation levels and blood pressure are appropriate. This concept is termed as permissive hypercapnia.
Temperature should be monitored continuously. Hypothermia should be avoided, as it has many adverse effects, especially on renal function, such as decreases in metabolic rate, increases in blood viscosity, disorders in coagulation capacity and depression of central nervous system activity, all of which may lead to delayed recovery. Mild hypothermia leads to an increase in urine production because of increased renal blood flow, secondary to peripheral vasoconstriction. Moderate and severe hypothermia leads to a reduction in renal blood flow following low cardiac output and hypovolemia from the earlier diuresis (Reuler, 1978). Postoperative shivering should be avoided as it may increase the animal's oxygen demand (Buggy and Crossley, 2000). If shivering occurs, gradual rewarming should be initiated, alongside oxygen supplementation, to meet the possible increased oxygen demand. Pethidine, an opioid agonist at both the mu and kappa receptors, may be useful in reducing the shivering threshold (Dabir et al, 2011). Further studies are needed to clarify the mechanism of action of its anti-shivering effects.
Placing a urinary catheter may be considered to monitor the urine output in animals with chronic kidney disease, significant coexisting disease, where volume status is questionable, or if preoperative fluid resuscitation was insufficient. It is important to highlight that urinary catheterisation may increase discomfort and risk of urinary infection. For those reasons, urinary catheterisation should be considered carefully. Urine output should be maintained 1–2 ml/kg/h, as mentioned before.
Intravenous fluids should be administered during anaesthesia based on the requirements of the individual. The administration of a extracellular fluid replacement such as Hartmann's or lactated Ringer's solution (2–4 ml/kg/h), titrated upon the requirement of the patients, is recommended (Rioja, 2016).
Troubleshooting hypotension: the most common complication
Hypotension is the most important complication especially in patients with chronic kidney disease. The criteria for intraoperative hypotension varies among studies. Currently the human recommendation is to allow blood pressure change of +/-10% from the baseline (Futier et al, 2017). In veterinary patients diagnosed with hypertension before anaesthesia, maintenance of a mean arterial pressure of above 70–80 mmHg may be necessary to preserve autoregulation and renal perfusion. All animals should be monitored, paying particular attention to their blood pressure. Hypotension should be treated promptly as follows:
- Decrease the amount of inhalational anaesthesia as much as possible, always ensuring a good plane of anaesthesia.
- If the hypotension is associated with bradycardia causing a low cardiac output, administration of an anticholinergic such as glycopyrrolate (0.005–0.01 mg/kg IV) or atropine (0.01–0.02 mg/kg IV) may be considered. If bradycardia-induced hypotension is associated with the administration of an alpha2 adrenoreceptor agonist, consider administration of atipamezole (2.5 times the µg/kg dose of medetomidine IM in cats or 5 times the µg/kg dose of medetomidine IM in dogs).
- A crystalloid bolus (10–20 ml/kg in dogs and 5–10 ml/kg in cats, over 10 minutes) can be administered in patients with chronic kidney disease. Further boluses will be titrated based on clinical signs associated with volume depletion (tachycardia in dogs; and tachycardia/bradycardia in cats). If anaemia is present, minimisation of haemodilution should be considered in order to avoid a reduction in oxygen delivery. Oxygen delivery in humans may remain normal in contempt of haemoglobin concentration as low as 5 g/dl (caused by isovolaemic haemodilution), because of the compensatory improvement in cardiac output and oxygen extraction (Weiskopf et al, 1998). Blood products, such as packed red blood cells or whole blood, may be considered in anaemic patients if necessary.
- If, despite volume replacement, hypotension still persists, administration of positive inotropic agents could be indicated. Dopamine (1–10 µg/kg/min) or dobutamine (5–10 µg/kg/min) may be used intravenously. Dobutamine stimulates beta1 adrenoreceptors, leading to increased inotropic and chronotropic effects, whereas dopamine stimulates beta1 adrenoreceptors at low doses (1–5 µg/kg/min) and alpha1 adrenoreceptors at higher doses (>5 µg/kg/min), leading to an increase in systemic vascular resistance.
- Administration of vasopressors should be done with caution as they can reduce the renal blood flow. Noradrenaline or phenylephrine could be used if the alternatives have failed (0.1–0.5 µg/kg/min IV). Hypotension should be taken into account as one of the most common complications in patients with chronic kidney disease. Close monitoring, prompt recognition and treatment are essential to reduce the detrimental effects of hypotension in these patients.
KEY POINTS
- General anaesthesia may have profound effects on the kidneys, especially affecting renal blood flow and glomerular filtration rate.
- Good stabilisation and management of the underlying conditions before anaesthesia are key to successful management of chronic kidney disease.
- Drug choice must be based on the current state of the disease.
- Monitoring, especially blood pressure, is the key to success during anaesthesia in patients with chronic kidney disease.
- During recovery, monitor vital parameters frequently initially. The time can be extended between checks. Renal parameters can be normal up to 48 hours following the anaesthetic procedure.
