The aim of any anaesthetic is to induce anaesthesia safely and keep the patient stable and asleep throughout the procedure. To do this successfully, the anaesthetist wants to minimise risk factors wherever possible. Brodbelt (2009) thoroughly studied and published the mortality rates and the reasons for death in various species. While his studies showed that mortality from anaesthesia is 0.17% in dogs and 0.24% in cats, this percentage is higher in rabbits, namely 1.39%. This means that every 72nd rabbit out of 100 died during anaesthesia or within the following 48 hours; 6% died during induction, 30% during maintenance, and 64% in the postoperative phase. Over 60% of the postoperative deaths happened in the first 3 hours after the procedure. The study of Clark and Hall (1990) showed a higher mortality rate with 1 out of every 28 anaesthetised rabbit dying. Both studies show that the risk of anaesthesia is much higher in rabbits than it is in dogs and cats. This is important considering the fact that the rabbit is the third most commonly kept pet animal after dogs and cats (Brodbelt, 2009).
Various factors contribute to the deaths seen during anaesthesia, such as age, body condition, reason for the anaesthetic, underlying diseases, urgency of the surgery reducing the time for perioperative tests, the type and length of the surgery, the type of anaesthetic drugs used, the type of anaesthetic technique used (e.g. intubation vs mask), and the degree of hypothermia occurring during surgery (Brodbelt et al, 2008). Not all parameters can be influenced or controlled, but several can, including hypothermia.
Body temperature is internally regulated in mammals, with metabolic functions declining if the body temperature changes in either direction from the optimal temperature. Anaesthetics inhibit the central heat regulation system, allowing the body temperature to change without control (Caro et al, 2013; Grint, 2013; Mosing, 2016). In patients with a decreased bodyweight, this effect is intensified (Hawkins and Pascoe, 2012; Caro et al, 2013; Grint, 2013). Hypothermia slows the metabolism, so relative drug overdose can develop more easily. A 1–2 °C intraoperative body temperature drop can have a negative effect on blood clotting, increases blood loss by 20% in humans, while also leading to a slower recovery and prolonged hospitalisation (Mosing, 2016).
In animals, hypothermia can cause clotting problems, prolonged recovery and vasoconstriction, which may decrease the blood and oxygen supply of the surgical site (Hawkins and Pascoe, 2012; Caro et al, 2013; Grint, 2013; Mosing, 2016). Hypothermia can also cause cardiopulmonary functional impairment, arrhythmia, and decreases in heart and respiratory rate (Hawkins and Pascoe, 2012; Grint, 2013). Hypothermia suppresses the immune system and increases the risk of postoperative infection (Mosing, 2016). For animals with hypothermia, because of the weak peripheral perfusion, any injections given subcutaneously have a prolonged absorption time and the metabolism of the medication is slower (Grint, 2013).
Intraoperative hypothermia in rabbits is very common. Because of the increase of mortality and morbidity in hypothermia, the monitoring of core body temperature and the reduction of any body temperature decrease is important (Hawkins and Pascoe, 2012; Grint, 2013). It is easier and less risky to prevent hypothermia than to treat it, so prevention is advised (Grint, 2013).
Heat loss from the body can be caused by a number of reasons. Quite often a temperature drop can be seen during premedication, because of peripheral vasodilatation and muscle relaxation caused by the various medications used in anaesthesia (Grint, 2013; Mosing, 2016). After premedication, the patients may be put into a cold environment and wait for a long time for the procedure, which can lead to a large temperature drop (Redondo et al, 2012a, 2012b). Because the biggest difference in temperature between the patient and the surroundings is at the beginning of anaesthesia, the biggest heat loss happens at that time. The cold air of the surroundings cools the outer layers of the animal through the skin and the blood flow under the skin heats the molecules of the air, thus decreasing the body temperature further. Age also becomes a factor, with older patients being more prone to hypothermia because of the decreased ability to physically compensate for heat loss (Stone et al, 1981). Thus, providing a warm and stable environment during premedication is essential, along with regular monitoring and reduced delay between premedication and surgery.
During surgical preparation, fur must be clipped from the surgical area. The aim is to clip the least amount and still maintain sterility of the surgical site. Fur is a good insulator. When cleaning the surgical areas, it is also essential to avoid getting the neighbouring areas wet to minimise heat loss through evaporation (Grint, 2013; Mosing, 2016).
During preparation and surgery, contact with a cold surgical table can also cause major heat loss. Placing insulation between the table and the patient, along with active heating, can reduce heat loss (Mosing, 2016). Non-surgical areas, like limbs, can be covered by blankets, cloths and bubble wrap (Grint, 2013; Mosing, 2016). Covering small animals during surgery can make monitoring of respiration hard, especially if not intubated (Hawkins and Pascoe, 2012). Using transparent drapes, which allows visualisation of the thorax, can eliminate this problem. Active heating can be achieved with heating lamps, heating blankets and heating pads. Bottles or gloves filled with uncontrolled high temperature water can cause burn wounds, and after they cool down, can increase hypothermia (Hawkins and Pascoe, 2012; Grint, 2013; Mosing, 2016).
Large volumes of cold or room temperature intravenous infusions can also cause a temperature decrease in patients, so giving warmed fluids is advised (Tausk et al, 1976; Grint, 2013). However, caution must be taken to ensure that the fluid is not of a higher temperature than the patient, otherwise this may cause overheating. Any lavage of the body cavity during surgery should also only occur with warmed fluids to avoid excessive cooling (Mosing, 2016). Reduction of surgical time and the duration the body cavity is open to the surrounding cooler environment will also reduce the intraoperative drop in body temperature that can occur (Tausk et al, 1976; Hawkins and Pascoe, 2012; Mosing, 2016). The cold temperature of surgical rooms can also contribute to hypothermia (Tausk et al, 1976). The study by Morris (1971) showed that room temperatures under 21 °C leads to hypothermia occurring more often. For smaller patients, such as cats, this effect is more pronounced, and in another study it was found that the effect was seen with a room temperature below 26 °C (Steinbacher et al, 2010). This effect can be explained by the fact that the nearer the room temperature is to that of the patient, the less the difference in temperature gradient and so the slower any hypothermia will develop (Grint, 2013). The room temperature also has to be balanced against that which is optimal for the staff undertaking the work.
Stone et al (1981) monitored patients at an ambient temperature of 21 ± 0.4 °C and found that inhaled humid 37 °C heated air is effective to prevent hypothermia. A linear body temperature increase was seen in hypothermic patients after inhaling warm and humid air. This also helped to reduce the risk of hypothermia, even when patients were in cooler surgical rooms. However, it was found that the temperature of the humid anaesthesia gas cannot be increased above 43.3 °C without causing thermal damage in the airways (Klein and Graves, 1974). The humid heated anaesthesia gas is delivered to the patient via the anaesthetic circuit during the maintenance of inhalational anaesthesia (Grint, 2013). Heating systems can be installed in most anaesthesia circuits, which can help decrease hypothermia, but it does potentially increase the dead space (the volume of a breath that does not participate in gas exchange) of the system (Hawkins and Pascoe, 2012; Grint, 2013). Thus, an external heating method would be best to avoid this.
Aim
The aim of the study was to determine a cost-effective, simple and easy reproducible way to decrease heat loss in everyday veterinary or field practice when using inhalational anaesthesia.
Methods
A total of 30 rabbits were identified and selected for the study, with all the animals having owners arriving at the hospital for various surgical procedures. Consent was given to be involved in the study. The age, sex and weight of the animals were noted before any procedures were undertaken. Apart from the surgical procedures, no other medical concerns were noted. Premedication was performed via intramuscular injection with a combination of either dexmedetomidine-hydrochloride (Dexdomitor 0.5 mg/ml, Orion Pharma, Finland), midazolam (Dormicum 5 mg/ml oldatos injekció, AEGIS, Hungary), ketamin (CP-Ketamin 10% injekció A.U.V., Produlab Pharma B.V., Netherlands), and butorphanol (Alvegesic vet. 10 mg/ml, Alvetra u. Werfft GmbH, Austria) or buprenorphine (Bupaq 0.3 mg/ml, Richter Pharma AG, Austria). As our cohort involved a clinical caseload, the exact amount of medication given was always determined by the veterinarian considering the health status of the rabbit and the type of the surgery to be undertaken. All animals were anaesthetised using inhalational anaesthesia via a facemask and a T-piece circuit. The circuit was checked for leakage before every surgery. Isoflurane was applied with 100% oxygen at a flowrate of 1.5 litre/minute. The concentration of isoflurane at the beginning of surgery was 5 v/v%, which later was decreased to 2.5 v/v% during the surgery. The patients were placed on a heated table on towels and the temperature of the surgery room was 20 ± 1 °C throughout.
The rabbits were randomly assigned into three groups of 10. Two groups inhaled the warmed gas mixture, while the third control group inhaled room temperature gas mixture. The two study groups differed by the length of the inhalation circuit being warmed. This was 60 cm in the first group and 80 cm in the second group. The inhalational breathing tube was immersed in a 40 ± 1 °C water chamber. The temperature of the water in the chamber heating the circuit was measured to ensure it remained constant. The temperature was taken using a paediatric/neonatal reusable oesophageal/rectal temperature probe connected to a monitoring device (MINDRAY iMEC-8 Vet Portable Multi-Parameter Veterinary Monitor, Mindray Medical International Ltd. Shenzhen, China) (Figure 1).
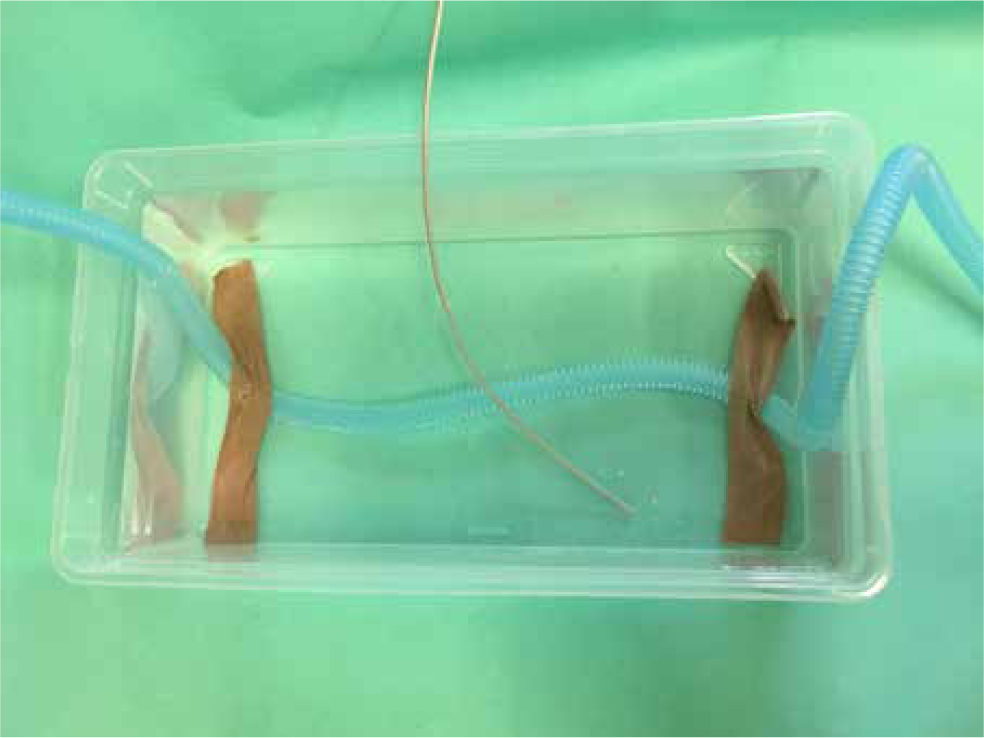
The veterinary assistant monitoring both patient and monitoring equipment checked the temperature at least every 3 minutes and informed the veterinarian if the temperature of the water started to decline. If the water temperature was decreasing, a small volume of hot water was added and the water mixed to ensure even distribution and thus an even temperature in all parts of the chamber. It was found that a small volume of hot water added two to three times adequately stabilised the temperature for the length of the surgeries. After adding water to the chamber and stabilising the temperature, any excess volume above the original volume marked on the side of the bath container was removed to ensure the length of the circuit covered in the chamber remained the same.
The rectal temperature of the anaesthetised animal was measured regularly, using another thermometer of the same make. Rectal temperature measurements were taken every 10 minutes, from induction with isoflurane anaesthesia until recovery (40 minutes). Any changes in temperature of the rabbit were compared to the starting temperature at the beginning of the surgery.
Statistical analysis
The Statistical Package for the Social Sciences (version 22.0) for Windows (SPSS, Inc., Chicago, IL USA) was used for statistical analysis and graphic representation produced with Microsoft Excel.
Results
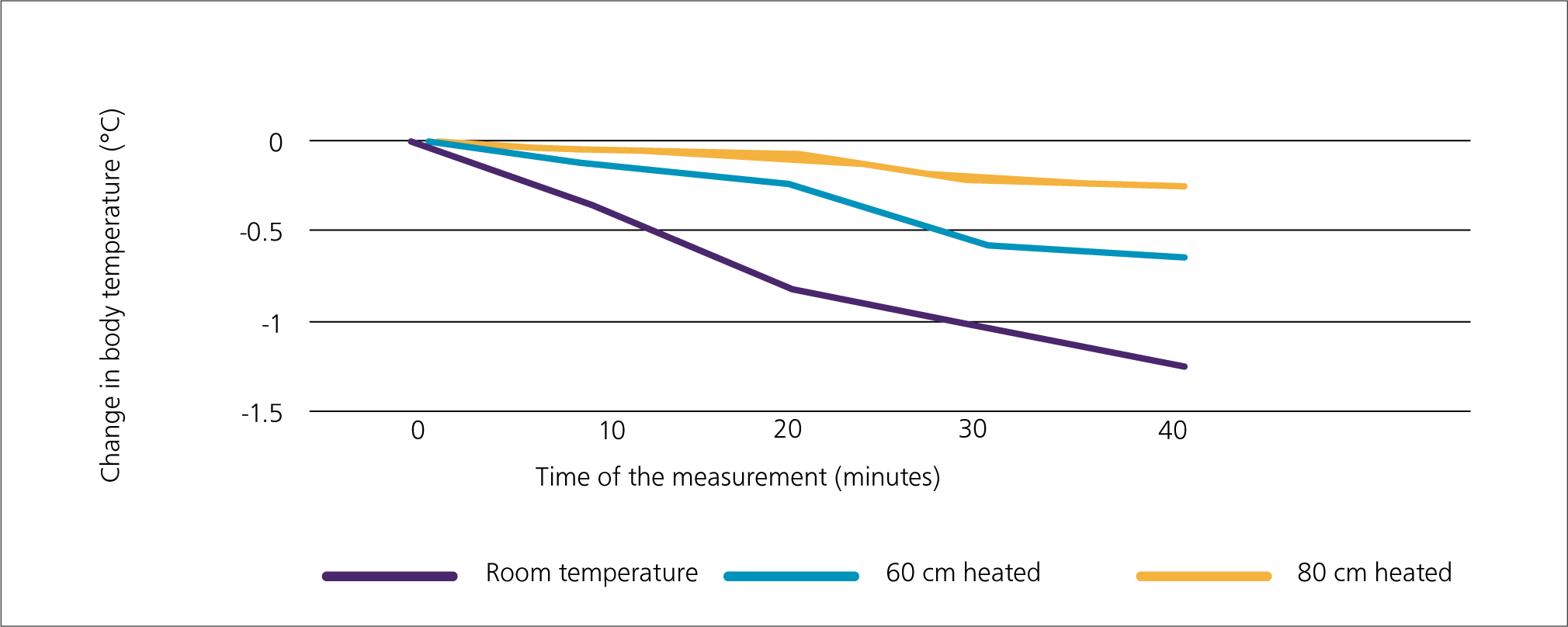
The degree of heat loss was much larger when using the anaesthesia gas mixture at room temperature than using anaesthesia gas mixtures passing through the heated circuit.
By the 40th minute, an average temperature drop of −1.28 °C could be seen in rabbits inhaling room temperature gas mixture. However, when heating 60 cm of the inhalation circuit, this drop was only −0.65 °C and when heating 80 cm of the inhalation circuit it was −0.27 °C. The results are shown in Figure 2.
Discussion
Less than 10% of heat loss in dogs happens through the airways, even when breathing cold dry air (Mosing, 2016). This may be influenced by endotracheal intubation and the larger body mass of canines. Visualisation of the trachea in dogs is easier than in rabbits, and often intubation of rabbits is not performed. Although V-gels are also available, facemasks are still used in many practices out of choice, with the whole head of the animal being exposed to the cold anaesthetic mixture. Because of this, and to reflect availability across many nations and areas of the world, it was elected to use facemasks in this study, even though intubation or V-gels would be preferable to facemasks. A ‘triple’ injectable premedication/induction combination was also chosen, as again in many countries this is still the main method used, with drugs such as alfaxalone not yet licenced or available.
To reduce morbidity and mortality during anaesthesia, the maintenance of the core body temperature is important in rabbits regardless of the length of the procedure (Hawkins and Pascoe, 2012). The measurement of core body temperature can happen through the oesophagus or rectally. Rabbits are obligate nasal breathers and do not aspirate because of the position of the epiglottis meeting the soft palate. However, when applying a thermometer into the oesophagus, this anatomical position is changed (e.g. by intubation) and may increase the aspiration risk if the oesophagus is not guarded. As our rabbits were masked, we thus elected to take rectal temperatures during the study.
There are various heating and humidifying products on the market, but all are relatively expensive or require specific setups. The technique used here is simple to produce, is cheap, and has proved to be an effective way to prevent or reduce hypothermia in rabbits and guinea pigs during anaesthesia (Nógrádi et al, 2019). Humidifying the inhalation gas does reduce the heat loss of the patient, but because the breathing circuit is reused it has the potential to cause an ideal environment for bacterial growth. Another problem with humidifying is that when done incorrectly, humid air at temperatures above 43.3 °C can cause burn wounds in the airways (Klein and Graves, 1974). Heating and humidifying the circuit would have meant a more complicated and costly system, which defeated one of the goals of this study. The 40 °C water bath used in this study does not damage the skin and airways of the patients, reduces heat loss under anaesthesia, is easy to produce, and can be applied to any simple circuit in any situation (as long as you can heat water and maintain the water temperature).
The heating and humidifying systems that can be built into circuits also increase dead space, which, in case of anaesthesia of small animals such as rabbits, is not ideal. Increased dead space can cause the CO2 levels to rise in small mammals, via rebreathing of gas mixes as a result of their smaller tidal volumes. Some systems used in paediatric medicine would be fit for rabbit anaesthesia (Grint, 2013), but the high price of these devices stops them from being accessible for most veterinary practices. The method used for this study does not increase the dead space of the circuit and the circuit chosen is one readily available in most practices.
During anaesthesia, the prevention of hypothermia should be the primary goal, rather than treating it afterwards (Grint, 2013). If hypothermia does start to occur, reducing the difference between the core body temperature and the temperature of the outer layers or the room should help reduce the speed of its onset and effects. Peripheral vasodilatation can be seen in most cases during anaesthesia, which means heating the patient and increasing the core body temperature to an ideal level can be more effective. The danger of hypothermia is higher in older patients because the circulation time is prolonged and compensatory skills decrease. Thus this needs to be especially factored in with these patients from the start of the procedure to the end. The job of the veterinarian does not end when the maintenance of anaesthesia is over and when the animal is taken off of the anaesthesia circuit. The temperature must also be measured during recovery to maintain the physiological values (Grint, 2013).
Conclusions
Given these results, the authors suggest that using heated inhalational anaesthesia in small patients is essential, especially in cold environments, or when piped gases may come from outside stores. Heating anaesthesia gas, combined with other methods mentioned (e.g. heated tables, insulated drapes, smaller shaved areas), is effective in helping to reduce hypothermia and the harmful, sometimes lethal, consequences if it is not addressed. The method of warming the anaesthesia inhalation circuit, with a warmed surrounding liquid, is easy, cheap and reproducible.
KEY POINTS
- Without support heat during anaesthesia, a rabbit's core temperature will drop, leading to hypothermia and complications.
- One of the main factors affecting the core temperature of the anaesthetised patient is the temperature of the inhaled anaesthetic gas mixture, along with the type of circuit used. In most rabbit anaesthetics, this will be a T-piece circuit.
- With the variability in room air temperature, the drop in core temperature can vary, being worse if the inhaled gas is colder.
- By heating a section of circuit, and thus mimicking the idea of Lac and Bain circuits, the inhaled gas mix in the T-piece circuit is heated to a more consistent temperature.
- The longer the piece of circuit heated at a steady temperature, the lower the drop in core body temperature and thus the less risk to the anaesthetised patient.

