Angiostrongylus vasorum continues to pose a significant threat to canid populations worldwide (Koch and Willesen, 2009; Elsheikha et al, 2014). Adult nematodes are commonly observed in the right ventricle and pulmonary vessels of infected animals where they generate an array of clinical signs, in some cases with fatal consequences (Chapman et al, 2004; Oliveira-Júnior et al, 2004; Koch and Willesen, 2009). In the UK, infection continued to spread from established ‘hotspots’ into previously uninfected areas, possibly as a result of climatic change and movement of infected dogs (Morgan et al, 2008, 2009). This makes infection with A. vasorum patchy and unpredictable. Failure to control infection is associated with significant health risks. When there is a high index of clinical suspicion, veterinary clinicians should appreciate the odds of their patients being infected with A. vasorum in order to rule out differential diagnoses and then provide treatment, which can provide health benefits. While many studies have calculated the prevalence of angiostrongylosis in dog populations, only a few have attempted to characterise risk factors.
The use of risk factors or predictors can be invaluable to veterinary practitioners, as it can underpin a sound clinical suspicion and facilitate faster and more accurate diagnosis. Useful information would include identification of at risk breeds, or breed groups, as well as some knowledge regarding the degree of influence that the gender, age and weight of an animal has on likelihood of infection. Secondary to signalment, there is some information available regarding general laboratory findings (Chapman et al, 2004), but these vary greatly between individual cases (Elsheikha et al, 2014). During a consultation, a veterinary practitioner carries out a full clinical examination. Knowledge of the way in which A. vasorum affects each of these parameters may assist practitioners in compiling a list of differential diagnoses. When a diagnosis of A. vasorum has been established it would be useful for practitioners to have additional information regarding treatment strategies. For example, there is no available information detailing the reoccurrence of infection following different treatments, and the time in which this is likely to happen.
Studies have been carried out in various countries hinting at trends in the signalment of presenting animals (Boag et al, 2004; Chapman et al, 2004; Conboy, 2004; Taubert et al, 2009; van Doorn et al, 2009; Morgan et al, 2010). Since studies have begun to report cases of A. vasorum outside of the previously recognised foci (Koch and Willesen, 2009), it has become increasingly important to document risk factors and presenting signs, so that clinicians unaccustomed to this disease succeed in identifying affected dogs before their condition deteriorates.
The aim of this retrospective study was to identify trends in demographic factors (age, gender, breed) and frequency of clinical signs in dogs with A. vasorum. The study determined which signalment (age, gender, breed), historical information and clinical examination findings are associated with naturally occurring A. vasorum in dogs in primary practice in a highly endemic area.
Materials and methods
Study population
The clinical records of 100 dogs, examined between 2003 and 2009, were obtained from private practices predominantly located in the southeast of England. This location was chosen as a known endemic focus for A. vasorum. The clinical cases were included based on a positive diagnosis for angiostrongylosis by Baermann technique and/or other methods such as cytology or radiography. All cases included demonstrated one or more clinical signs suggestive of angiostrongylosis, such as cough, respiratory distress, dyspnoea, tachypnoea, coagulopathy, scleral bleed, lethargy, vomiting, diarrhoea, neurological signs, pale mucous membranes or abdominal pain. Other diagnostic tests (e.g. airway cytology, blood profile, biochemical profile, radiography) were performed at the discretion of both the veterinarian and their client, so, each animal had not undergone a full or identical diagnostic procedure. To enable comparison between dogs diagnosed with angiostrongylosis and the general clinic population, the same veterinary practices were asked to provide the age, gender and breed of a total of 100 dogs presenting with other conditions, including a wide array of clinical conditions as well as routine healthcare appointments, but not suspected of being infected with A. vasorum.
Data collection
This study was carried out in accordance with the Data Protection Act 1998 and with approval from The University of Nottingham School of Veterinary Medicine and Science research ethics committee. No confidential information was accessed, or stored, to compile data for the study. Records were analysed retrospectively according to signalment, historical information and clinical examination findings. Historical information recorded clinical examination findings included vital signs, results of auscultation and all other observations made by the clinician.
Data analysis
Statistical analysis was carried out to test the significance of the potential risk factors. First, the proportion of dogs in each category of age, gender and breed were compared between the infected and uninfected (control) groups using the chi-squared test. Because exact age was not given for dogs <1 year, average age could not be compared precisely between groups; instead, age was divided into <1 year and 1 year or more. After accounting for the proportion of dogs <1 year of age in each group, the average age of older dogs was then compared between groups using the unpaired t-test, without assuming equal variances. Because so many breeds (n=34) were specified among the 200 dogs in the study, individual breeds were amalgamated into breed type for initial analysis, using the seven Kennel Club breed groups (www.thekennelclub.org.uk): gundog, hound, pastoral, terrier, toy, utility and working, as well as cross-breed.
To account for co-variation between age, gender and breed, binary logistic regression was used to determine robust odds ratios for significant risk factors. Breed was included in three nested models, with a separate logistic regression conducted in each case. First, all individual breeds were included, each as a separate level within the analysis: this failed to return a valid regression. Second, breeds over-represented in the lungworm group relative to the control group, assessed as above using chi-squared, were selected for inclusion in the logistic regression at a cut-off below P=0.10. Breeds previously found to have higher than average risk of presenting with angiostrongylosis, i.e. Staffordshire Bull Terrier, Jack Russell Terrier, Springer Spaniel, Cocker Spaniel and Cavalier King Charles Spaniel (Blehaut et al, 2014), were also included; and other breeds combined into an ‘other’ category. Binary logistic regression then proceeded in a stepwise manner, with breeds not significantly associated with higher or lower risk of angiostrongylosis removed in turn until only significant breeds remained (as well as those breeds whose removal would lead to loss of significance for others). At each step, dogs belonging to breeds not retained in the model were re-assigned to the ‘other breeds’ group. Finally, the risk of angiostrongylosis was compared between cross-bred dogs and all pure breeds combined, in a third logistic regression. Age, age category (less than 1 year, or older) and gender were included in the logistic regression starting models along with breed. These analyses were conducted using IBM SPSS version 25.
The predictive ability of clinical signs to discriminate between dogs with and without lungworm disease was examined using area under the receiver operating characteristic (ROC) curve (AUC). This analysis was performed using the statistical software R, version 3.2.5 (The R Foundation for Statistical Computing, Austria).
Results
Demographics
The lungworm dataset contained 51 female and 49 male dogs, of which 32 were intact females (62.7%), 26 were intact males (53%), 19 were spayed females (37.3%) and 23 were neutered males (47%). The weight of dogs in the lungworm group varied from 0.82 to 45 kg. The control group comprised 50 male and 50 female dogs; neutered status and weight were not recorded. Reasons for presentation were diverse, such as vaccination (n=25), lameness (n=7), otitis externa (n=6), surgery (n=4) and 37 other less common conditions. Overall, the dogs represented 33 different breeds, and cross-bred animals, and their age ranged from 9 weeks to 18 years.
Risk factors
The lungworm group contained more dogs <1 year of age (39/100) than did the control group (12/100; chi-squared=19.1, 1 d.f., P<0.0001). After removing dogs <1 year, average age did not differ between groups (lungworm group mean 6.4±SD 4.8; control group 5.2±SD 3.1; F88,61=19.79, t=−1.74, P=0.09). Age remained a significant factor after including breed. Gender was balanced between the two groups and was not significant in any logistic regression.
Among breed groups, cross-bred dogs comprised the lowest number of dogs in the lungworm group and the highest number in the control group (Table 1). In contrast, working breed types were over-represented in the lungworm group. Binary logistic regression confirmed that cross-bred dogs were much less likely to be diagnosed with lungworm infection in this study. Using cross-breeds as the reference, all breed groups were more likely to have lungworm, and the odds ratio for dogs <1 year of age was 3.8 (95% confidence interval (CI) 1.8–8.2). Confidence intervals for odds ratios varied widely, however, and the full model is not presented here, since the effect of breed was further investigated.
Table 1. The frequency of different breed categories diagnosed with lungworm (lungworm group, n=100) and in the non-infected control group (n=100).
| Breed group | Examples | Control group | Lungworm group | Chi-squared | P |
|---|---|---|---|---|---|
| 1: Gundog | Labrador Retriever, Springer Spaniel, Cocker Spaniel | 23 | 33 | 2.50 | 0.11 |
| 2: Hound | Greyhound, Dachsund | 2 | 7 | 3.02 | 0.08 |
| 3: Pastoral | Collie, German Shepherd | 8 | 5 | 0.82 | 0.37 |
| 4: Terrier | Staffordshire Bull Terrier, Yorkshire Terrier | 23 | 23 | 0 | 1.00 |
| 5: Toy | Chihuahua, Cavalier King Charles Spaniel | 7 | 15 | 3.32 | 0.07 |
| 6: Utility | Bulldog, Dalmatian, Shih-Tzu | 4 | 7 | 0.96 | 0.33 |
| 7: Working | Boxer, Doberman | 1 | 8 | 5.82 | 0.02 |
| Cross-bred | – | 32 | 2 | 31.9 | <0.0001 |
Chi-squared was estimated using a 2 × 2 contingency table for each breed separately. See text for logistic regression results. Breed groups are as defined by the UK Kennel Club (www.kennelclub.org.uk). Frequencies significantly different between groups are shown in bold text
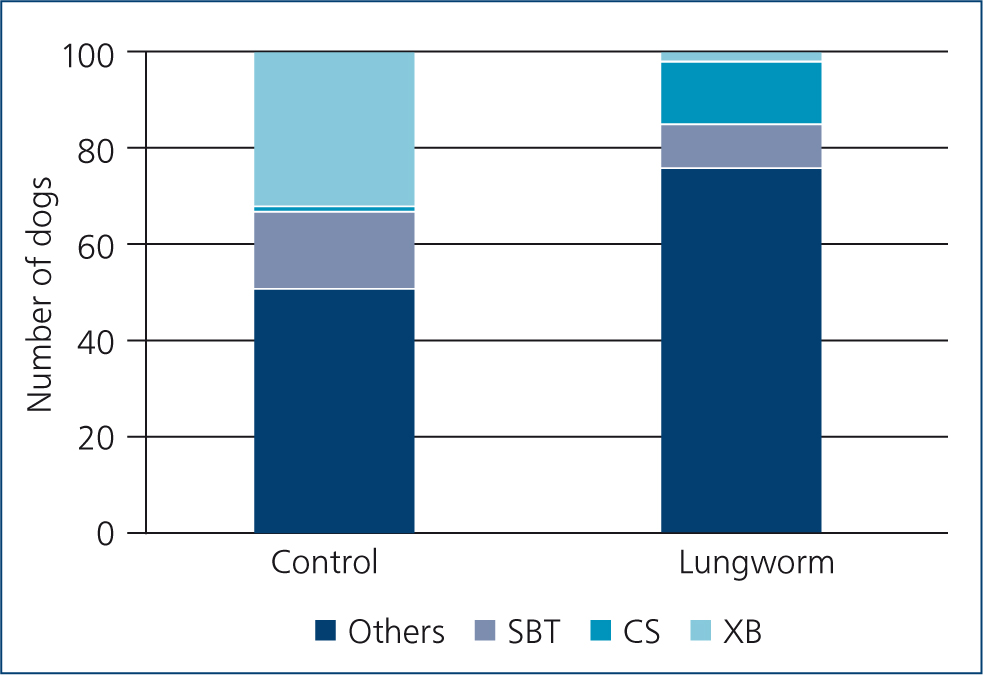
Individual breeds over- or under-represented in the lungworm group are shown in Table 2, with the outcome of subsequent binary logistic regression for those plus breeds previously found to be at higher risk of angiostrongylosis. Results confirmed that cross-bred dogs had significantly lower odds of angiostrongylosis. Staffordshire Bull Terriers also had lower odds, while Cocker Spaniels were nearly nine times more likely to test positive for lungworm, after taking age into account. The proportions of these breeds present in control and lungworm groups are shown in Figure 1. Age category (but not age as a continuous variable) was again significant, with dogs <1 year being 4.2 times more likely than older dogs to test positive. The strong protective effect of cross-breeding was again emphasised in the final logistic regression model (Nagelkerke r2=0.299, Hosmer–Lemeshow chi-squared=0.173, 2 d.f., P=0.92). Compared with cross-bred dogs as the reference value, pure-bred dogs carried an odds ratio of 22.6 (95% CI 5.1–100; Wald 16.919, P<0.001) for a lungworm diagnosis (age odds ratio 3.83, 95% CI 1.8–8.2, Wald 11.83, P=0.001).
Table 2. Breeds present at significantly different frequencies in the lungworm and control groups, or found to be at higher risk of angiostrongylosis, and results of subsequent binary logistic regression (also including gender and age as starting variables), with lungworm diagnosis as the outcome variable
| Breed | N (control group) | N (lungworm group) | Chi-squared | P | Wald statistic | P | OR | CI |
|---|---|---|---|---|---|---|---|---|
| Cross-bred | 32 | 3 | 31.9 | <0.001 | 17.27 | <0.001 | 0.04 | 0.01–0.19 |
| Staffordshire Bull Terrier | 16 | 9 | 2.29 | 0.13 | 5.558 | 0.02 | 0.32 | 0.12–0.82 |
| Jack Russell Terrier | 4 | 3 | 0.30 | 0.09 | NS | |||
| Springer Spaniel | 6 | 1 | 3.85 | 0.05 | NS | |||
| Cocker Spaniel | 1 | 13 | 11.14 | <0.0001 | 4.149 | 0.04 | 8.7 | 1.1–70 |
| West Highland White Terrier | 2 | 7 | 3.03 | 0.08 | NS | |||
| Cavalier King Charles Spaniel | 3 | 9 | 3.28 | 0.07 | NS | |||
| Yorkshire Terrier | 0 | 3 | 3.38 | 0.07 | NS | |||
| Dachsund | 0 | 4 | 4.34 | 0.04 | NS | |||
| Boxer | 0 | 6 | 6.35 | 0.01 | NS |
Logistic regression Nagelkerke r2 =0.37, Hosmer–Lemeshow chi-squared=7.34, 4 d.f., P=0.119; breed Wald statistic=26.36, 3 d.f., P<0.001. Age, not listed, was also significant: Wald statistic=12.47, 1 d.f., P<0.001; OR 4.2 (95% CI 1.9–9.4. OR=Odds ratio; CI=confidence interval; NS=not significant. Reference breed for logistic regression was all other breeds combined. Other breeds listed but not associated with lungworm diagnosis were: Labrador Retriever (n=27), Collie (8), Akita (1), Labradoodle (2), Schnauzer (2), Pug (3), German shepherd (4), Doberman (1), Chihuahua (3), Wheaten Terrier (2), Beagle (3), Miniature Poodle (1), Sharpei (1), Hungarian Viszla (1), Chow-chow (1), Dalmatian (2), Huntaway (1), Bichon frize (1), Scottish Terrier (1), Bulldog (2), Husky (1), Bernese Mountain Dog (1) and Golden Retriever (3). The inclusion criterion for inclusion in the logistic regression analysis was P<0.10 in the univariate chi-squared test, or prior reports of significant association with angiostrongylosis (Blehaut et al, 2014; in bold)
Clinical presentation
Certain clinical signs appear to be more commonly associated with A. vasorum infection in dogs, and in some cases breed and gender seem to be influencing factors to some extent (Table 3). Taking account of the more common clinical presentations (cough, coagulopathy, vomiting/diarrhoea and lethargy; Figure 2), gundogs were highly likely to present with all signs, whereas terriers presented less often with a coagulopathy (9%) than with the other signs, and hounds less often with a cough (2%). Lethargy was most frequent among all breed groups, and like those dogs presenting with a cough and vomiting/diarrhoea, showed no association with gender. However, these data suggest that male dogs were more than twice as likely to present with a coagulopathy (69%) than were female dogs (31%). The Baermann method confirmed diagnosis in 65/100 dogs, while 20/100 dogs tested negative using this method, but were diagnosed by other means (e.g. cytology). Also, radiographic changes were observed in 36/100 dogs, and there appeared to be no gender influence associated with this finding (P>0.05). Area under the ROC curve (AUC) was >0.7, which is an acceptable level of discrimination and indicates that a correct diagnosis can be achieved in 70% of the cases by taking the history and clinical signs into consideration (Figure 3).
Table 3. The frequency of clinical findings suggestive of A. vasorum infection by gender and breed of dogs
| Clinical signs | Total (n=100) | Female % | Male % | Gundog % | Hound % | Pastoral % | Terrier % | Toy % | Utility % | Working % | Crossbreed % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cough | 55 | 49 | 51 | 35 | 2 | 7 | 25 | 18 | 7 | 5 | 0 |
| Coagulopathy | 45 | 31 | 69 | 27 | 9 | 4 | 9 | 27 | 9 | 11 | 4 |
| Vomiting/diarrhoea | 33 | 45 | 55 | 27 | 6 | 3 | 24 | 21 | 6 | 9 | 3 |
| Lethargy | 26 | 58 | 42 | 27 | 12 | 12 | 19 | 12 | 8 | 12 | 0 |
| Respiratory distress | 22 | 59 | 41 | 41 | 9 | 5 | 23 | 9 | 0 | 14 | 0 |
| Tracheal sensitivity | 22 | 27 | 73 | 41 | 0 | 5 | 27 | 14 | 9 | 5 | 0 |
| Cardiac abnormality | 17 | 41 | 59 | 24 | 6 | 6 | 35 | 6 | 18 | 6 | 0 |
| Neurological disorder | 16 | 56 | 44 | 25 | 6 | 0 | 19 | 19 | 6 | 25 | 0 |
| Pale mucous membrane | 16 | 50 | 50 | 38 | 6 | 6 | 13 | 19 | 13 | 6 | 0 |
| Dyspnoea | 11 | 55 | 45 | 45 | 9 | 9 | 27 | 0 | 9 | 0 | 0 |
| Abdominal pain | 10 | 60 | 40 | 10 | 20 | 0 | 20 | 20 | 0 | 20 | 10 |
| Tachypnoea | 8 | 50 | 50 | 38 | 25 | 0 | 25 | 0 | 0 | 13 | 0 |
| Enlarged lymph nodes | 7 | 43 | 57 | 29 | 14 | 0 | 14 | 14 | 0 | 29 | 0 |


Discussion
The present study identified risk factors for angiostrongylosis by comparing the demographics of dogs diagnosed with this disease in primary care practices with a control sample of dogs presenting at the same practices with other conditions. Furthermore, the frequency of different presenting signs in relation to demographic characteristics was assessed. Information on risk factors and common presenting signs can help veterinary professionals to identify this disease quickly and instigate prompt treatment to improve prognosis (Elsheikha et al, 2014).
Age was a significant predictor of diagnosed lungworm: specifically, dogs aged less than 1 year were around four times more likely to test positive, than were older dogs. This confirms previous findings by Koch and Willesen (2009) and Chapman et al (2004), in which the inquisitive nature of young animals was suggested as a mechanism for increased transmission from intermediate hosts. It is also possible that a young animal is somewhat immune-deficient, in comparison to an older animal, and this increases the predisposition for infection. Gender was not a significant risk factor, with approximately equal numbers of males and females in both lungworm and control groups, and this finding is also in line with previous studies (e.g. Morgan et al, 2010; Blehaut et al, 2014).
Breed was strongly associated with the chance of testing positive for lungworm. Cocker Spaniels were more likely to be found in the infected than the control group, and both Staffordshire Bull Terriers and cross-bred dogs less likely. Cocker Spaniels were also found to be at greater risk of angiostrongylosis by Blehaut et al (2014); however, Staffordshire Bull Terriers were found by that study to be at higher, not lower, risk. Other breeds found by Blehaut et al (2014) and Chapman et al (2004) to be at higher risk of the disease, i.e. Springer Spaniels, Jack Russell Terriers and Cavalier King Charles Spaniels, were not associated with increased risk in the current study. Previous studies found elevated risk of infection in hunting dogs in Canada (Conboy, 2004), presumably through increased access to infected gastropods, but this was not confirmed in Greece (Papazahariadou et al, 2007), and hunting dogs were not included in the present study.
Most strikingly, the much lower risk associated with cross-bred dogs in the present study has not previously been reported. This might be explained by study design, along with the nature of the participating practices. Blehaut et al (2014) compared 141 angiostrongylosis cases from 111 veterinary practices, with a control population of 2242 dogs. However, both the cases and the control population were drawn from dogs referred to the Queen Mother Hospital for Animals at the Royal Veterinary College, and not directly from participating practices. As in our study, analysis was complicated by the large number of dog breeds, and a strategy was needed to manage this diversity. Blehaut et al (2014) chose to select the 12 most common breeds for risk factor analysis, and these did not include cross-bred dogs. Chapman et al (2004) took a similar approach. Dogs referred to specialist hospitals are unlikely to be truly representative of the general pet dog population, either in demographics, risk of infection, or preventive veterinary care. In other studies that found no association between breed and angiostrongylosis (e.g. Koch and Willesen, 2009; Morgan et al, 2010), the cases and controls were recruited directly from primary care practices.
Greater health and longevity have been ascribed to cross-bred dogs in epidemiological studies (O'Neill et al, 2013), and could be linked to hybrid vigour (Chen, 2010; Nicholas et al, 2016). It seems unlikely that cross-bred dogs have less opportunity than pure-breds to scavenge or otherwise encounter infected gastropod intermediate hosts, or that they are subject to more robust protective anthelmintic treatment regimens. The negative association between cross-bred status and diagnosed A. vasorum infection seems more likely to be related to immunological or physiological factors. It remains possible that cross-breeds were no less likely to present with angiostrongylosis, but a specific diagnosis was not so frequently pursued, but this also seems improbable in primary practice. It is possible that breed predisposition changes in different areas. Further studies could investigate why certain breeds appear to be more predisposed to A. vasorum than others. Perhaps more importantly, it should be noted that perceived breed associations are subject to limitations in study design. Moreover, the health benefits of cross-breeding, already demonstrated for a number of fatal diseases (O'Neill et al, 2013), might extend to a degree of protection against angiostrongylosis.
The four most common clinical signs associated with A. vasorum infection, as identified by this study, were cough, coagulopathy, vomiting or diarrhoea and lethargy (Table 3). Respiratory signs and coagulopathy have a well-recognised association with A. vasorum infection. The present study showed that vomiting/diarrhoea and lethargy are also prominent clinical signs in dogs infected with A. vasorum, in agreement with previous studies (Chapman et al, 2004; Koch and Willesen, 2009; van Doorn et al, 2009; Morgan et al, 2010). These findings suggest that the involvement of body systems, such as the gastrointestinal tract (vomiting/diarrhoea) and musculoskeletal system (lethargy), may be more important than previously thought, although lethargy could also be cardio-pulmonary or neurological in origin. Coagulopathy appeared to be linked with gender in the present study, as male dogs were more than twice as likely (69%) to present with a coagulopathy than female dogs (31%). This is an interesting finding and may be explained by a difference in biological factors between genders; further research is required to confirm this assumption. It is important to note that clinical signs did not necessarily occur simultaneously, and a differential diagnosis of angiostrongylosis should be considered on recognition of each sign, even in isolation.
AUC shows how the true positive and negative rates change as we vary the threshold at which the model identifies a dog in the case/control. If we were correct every time the area under the curve would be 1. This is not realistic in practice and an area under the curve of 0.7 seems an acceptable level. This means that correct diagnosis can be achieved in 70% of clinical cases by taking history and indicates that by considering a combination of diagnostic testing, demographic risk factors and clinical signs, a diagnosis of angiostrongylosis can be correctly reached in most cases. These findings are important clinically, as they provide clues regarding the risk of infection to an individual dog, facilitate improved recognition of infection based upon clinical presentation, determine which variables might impact the management of A. vasorum infection, and will allow implementation of appropriate treatment as well as preventive strategies to combat infection.
Some limitations of this work should be mentioned. The study was confined to a single area, the southeast of England, and thus, findings might not be reliably extrapolated to the dog population in England or the UK. It is possible that factors specific to this location predispose certain animals in favour of others, although this seems unlikely. Also, the nature of this study as a retrospective analysis meant that a few cases in the study were analysed by the same practitioner. This means that interpretation of clinical signs was likely to be subjective in some cases, perhaps dependent on prior knowledge of A. vasorum infection. It is also important to note that diagnostic tests were performed at the discretion of both the veterinarian and their client; hence it was not possible to create a uniform set of results for statistical analysis.
Conclusions
This study has identified age as a risk factor for A. vasorum infection, supporting the theory that age-related differences exist in response to infection. Also, the breed of dog seems to be a risk factor, with Cocker Spaniels relatively predisposed and Staffordshire Bull Terriers and especially cross-breeds less susceptible. Gender was not a significant risk factor for dogs infected with A. vasorum. The clinical presentation of infected dogs allowed re-evaluation of those signs previously thought to be common to infection, and those thought not to be.
These findings suggest that clinicians should maintain a suspicion in younger dogs, even if the faecal results are negative, especially if the clinical signs are likely attributable to A. vasorum because there is a health risk to leaving the animal untreated. This information should prove invaluable to veterinary practitioners by providing clues as to the likelihood of infection in different age groups and breeds of dog and facilitate faster recognition of infection based on clinical presentation and allow implementation of preventive strategies to combat infection. It is the authors' hope that these results will help in the development of interventions to establish appropriate educational measures to enhance the detection of infection and to disrupt A. vasorum transmission to dogs, especially those at a higher risk.
KEY POINTS
- Angiostrongylus vasorum is a cause of potentially fatal disease in dogs and has spread across the UK and Ireland.
- Public awareness of canine lungworm disease caused by A. vasorum combined with increase in prevalence, means that veterinary professionals must be prepared to give risk-based advice.
- Veterinarians should maintain a high degree of clinical suspicion in younger dogs, particularly if the clinical signs are likely attributable to A. vasorum infection.
- Gender does not appear to be a significant risk factor for A. vasorum infection.
- Cross-breed dogs appear to be at lower risk of infection than pure breeds, especially Cocker Spaniels.

