This article summarises the surgical and non-surgical options for desexing dogs, using standardised terminology by defining the meaning of commonly used terms. Desexing is a sex-neutral term that refers to any procedure that causes permanent or temporary loss of fertility. Desexing is frequently performed via surgical removal of the gonads (testes or ovaries) which is referred to as gonadectomy, another sex-neutral term. The common use of terms such as neutering and castration by some authors and practitioners as a sex-specific term for male gonadectomy is well understood, although others have used them as sex-neutral terms for gonadectomy in both sexes. Other terms, such as sterilisation and spaying, can cause confusion among practitioners and their clients (Table 1).
Table 1. Terms that may cause confusion when describing methods to prevent reproduction
| Term | Sex-neutral meaning | Sex-specific meaning |
|---|---|---|
| Desexing | Any procedure that causes permanent or temporary loss of fertility | |
| Gonadectomy | Removal of the gonads | |
| Neutering or castration | Gonadectomy | Male gonadectomy or orchiectomy |
| Sterilisation | Gonadectomy or other method that induces infertility without gonadectomy | Female gonadectomy* |
| Spaying | Female gonadectomy* |
Desexing has been historically recommended to help with population control, disease prevention and behaviour modification, although prevalence rates of desexing vary by country (Table 2) and can be very low in countries such as Germany and the Nordic countries where it goes against animal welfare legislation unless there is a clear medical indication (Sallander et al, 2001; Kuhne, 2012; Jitpean et al, 2012; Wongsaengchan and McKeegan, 2019). For consistency and clarity, desexing and the terms included in Tables 3 and 4 will be used throughout this review. The effects of desexing on the production of hormones have implications for potential health problems which may develop over the lifetime of a dog. There is a growing body of literature on the disease risks associated with whether a dog is intact or desexed and by which method it has been desexed. This will be discussed in the next article. Before considering how various methods of desexing affect dogs, we must first consider normal reproductive physiology in intact dogs.
Table 2. Prevalence of desexing by country reported as % of dogs
| Country | Prevalence of desexing | Reference |
|---|---|---|
| USA | 64–78% | Trevejo et al (2011) |
| UK | 54% | Diesel et al (2010) |
| Ireland | 47% | Downes et al (2009) |
| Sweden | 10% | Egenvall et al (1999) |
Table 3. Surgical methods of desexing
| Sex | Term or method | Procedure or outcome | Notes | |
|---|---|---|---|---|
| Males | Male gonadectomy | Orchiectomy (orchidectomy) | Surgical removal of testes | Most common |
| Other surgical methods | Vasectomy | Surgical transection of the vas deferens using one of several available techniques | Does not result in any changes in circulating testosterone levels, libido or male-specific behaviours | |
| Females | Female gonadectomy | Ovariohysterectomy* | Surgical removal of ovaries and uterus | Recommended for older dogs; most common in the UK and north America for younger dogs |
| Ovariectomy (oophorectomy)* | Surgical removal of ovaries | More common in western Europe for younger dogs | ||
| Other surgical methods | Hysterectomy* | Surgical removal of uterus | ||
| Salpingectomy* | Surgical removal of Fallopian tubes | |||
Table 4. Hormonal methods of desexing: summary of advantages and disadvantages for synthetic analogues of progesterone and exogenous testosterone, gonadotropin-releasing hormone (GnRH) agonists and antagonists
| Advantages | Disadvantages | |
|---|---|---|
| Progestational and androgenic drugs | Proven suppression of oestrus and fertility in both sexes, depending on the drug used | Unacceptable side effects |
| Some approved veterinary drugs have regulatory approval and are available in some markets although this varies greatly | Timing of administration within the oestrous cycle determines effectiveness, duration and side effects | |
| Consistent veterinary monitoring required as a result of significant and sometimes life-threatening side effects | ||
| Maximum duration of effectiveness is unpredictable | ||
| GnRH agonists | Proven reversible suppression of fertility in males and females; when the drug is discontinued, reproduction should resume within a reasonable period of time | A commercially viable product may need to have at least 6–12 months of efficacy for convenience (longer duration will be desired for many pets and unowned animals) |
| Suppresses sexual behaviour such that females do not come into oestrus during treatment and males behave as castrates | Must be administered repeatedly to maintain effects | |
| Active compounds available as generic compounds manufactured under current good manufacturing practice | No patent protection for common active compounds | |
| Demonstrated to be effective in a variety of formulations, including implants, depot injections and microspheres | Slow onset of activity (generally a few weeks) and variable duration of treatment effects | |
| May initially induce oestrus in females | ||
| GnRH antagonists | Proven reversible suppression of fertility in males and females for short periods; when the drug is discontinued, reproduction should resume within a reasonable period of time | No depot or long-acting formulations of peptide antagonists have been developed or licensed for use in animals |
| Suppresses sexual behaviour such that females do not come into oestrus during treatment and males behave as castrates | Must be administered repeatedly to maintain effects | |
| Effective within a short period after treatment initiation (hours) | Some of the first-generation peptide antagonists may cause histamine release in dogs | |
| May be less expensive to manufacture and may be given orally (as non-peptide antagonists) | There are no publications about research on non-peptide antagonists in dogs |
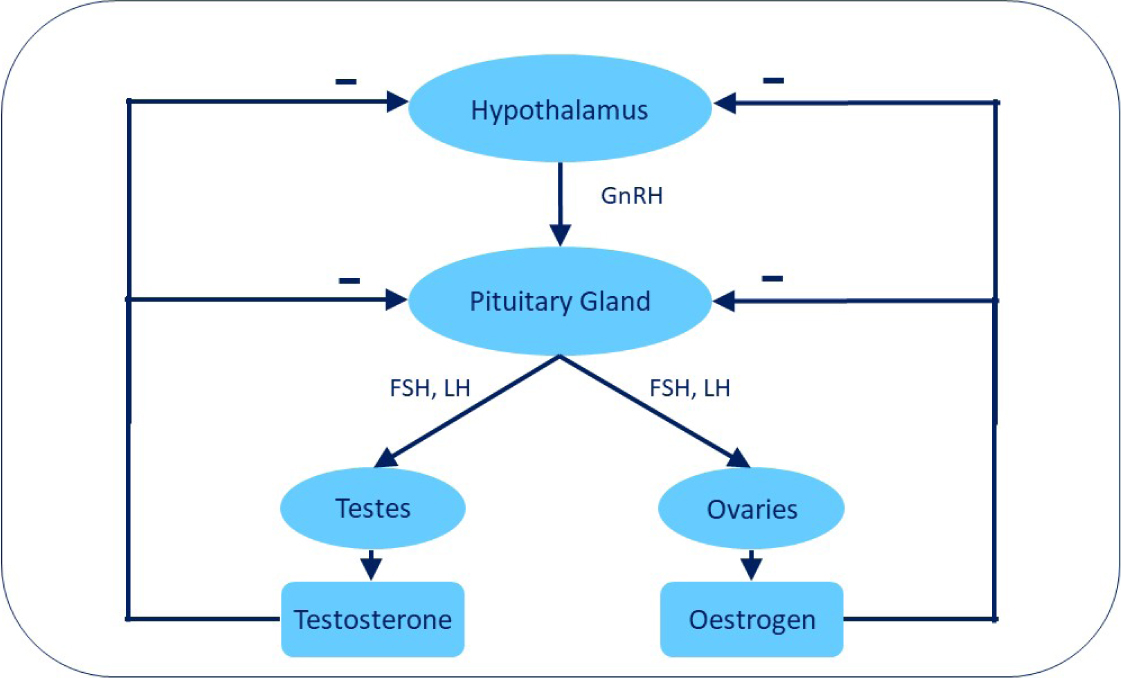
Gonadotropin-releasing hormone (GnRH) is normally secreted in a pulsatile fashion by the hypothalamus and it acts on target cells with GnRH receptors in the pituitary gland. This results in episodic production of the main gonadotropins (follicle-stimulating hormone (FSH) and luteinising hormone (LH)) by the pituitary gland; FSH and LH induce ovarian follicular development and ovulation in the bitch, and testicular development, androgen production and spermatogenesis in the male. In an intact dog, the gonads (ovaries or testes) secrete sex hormones (oestrogen or testosterone) and, when these gonadal hormones reach a certain concentration, there is self-regulation through negative feedback which downregulates further secretion of GnRH, FSH and LH (Figure 1). Thus, the hypothalamic–pituitary–gonadal axis is regulated via intricate feedback mechanisms. GnRH is released in pulses by the hypothalamus which is critical for normal reproduction and fertility (Clark, 2019).
Surgical desexing options
Surgical methods of gonadectomy are the most common method for desexing in both owned and unowned dogs, with orchiectomy the most common method of desexing male dogs. While several surgical desexing methods not involving orchiectomy have been proposed for male dogs, they have not been widely adopted in practice.
Vasectomy, as the surgical transection or ligation of the vas deferens, leads to infertility as a result of azoospermia without affecting circulating testosterone levels (De Souza et al, 2006). Methods include open (Clinton et al, 1972; Rubin and Maplesden, 1977) and endoscopic vasectomy (Silva et al, 1993), as well as experimental methods including implantation of an intravasal filtering device (Chen et al, 2010) and non-scalpel methods involving ultrasound (Roberts et al, 2002), laser surgery (Cilip et al, 2010, 2011), or electrocoagulation of the vas deferens (Rao et al, 1980). Specific complications of vasectomy may include testicular degeneration (Whyte et al, 1997) and the formation of spermatocoeles (Perez-Marin et al, 2006) or sperm granulomas (Mayenco Aguirre et al, 1996; Perez-Marin et al, 2006), although these appear to be rare in dogs.
Other methods in dogs include the use of a Burdizzo castrator to crush the spermatic cord, which was evaluated in the USA but is likely to require anaesthesia and/or pain relief (Ortega-Pacheco et al, 2006) or an elastrator, although this is considered unethical because of animal welfare concerns (Rothenburger, 2012).
Surgical methods of desexing in females may refer to ovariohysterectomy, ovariectomy, salpingectomy or hysterectomy with or without an ovarian autograft (one form of an ovary-sparing spay, Table 3). The use of ovarian autografting, with the portal vein drainage area as the transplant site, as an alternative to oophorectomy in cats and dogs may be a practical technique for preventing oestrus while avoiding the eunuchoid syndrome observed in ovariectomised dogs. Another ovary-sparing or partial spay involves removing the uterus and cervix while leaving one or both ovaries intact although careful placement of the uterine ligatures at the cervix is needed to avoid the complication of a stump pyometra (Brent and Kutzler, 2018).
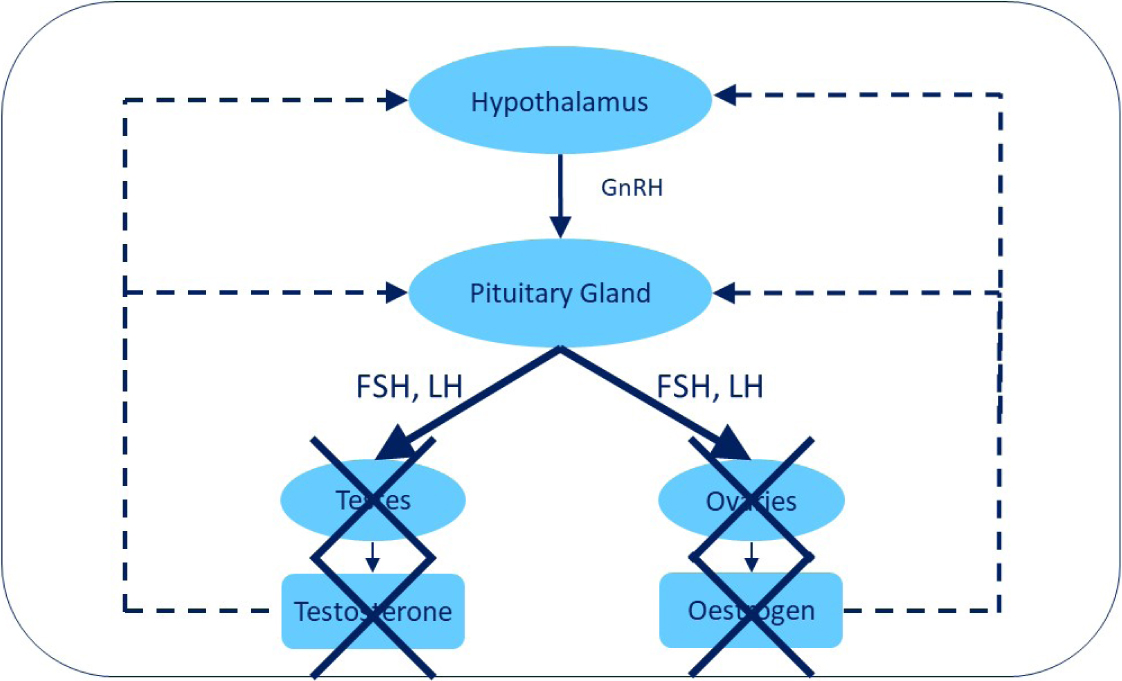
Short-term complications of gonadectomy are rare and are mostly limited to the usual non-specific surgical risks including anaesthetic complications, swelling, haemorrhage, haematoma formation and infection (Howe, 2006; RCVS Knowledge Clinical Audit Report, 2019). With a dog that has undergone surgical removal of the gonads, there are no sex hormones produced by the gonads and no negative feedback, which results in increased secretion of LH and FSH (Figure 2). The effects of surgical gonadectomy in elevating LH and FSH levels have implications for potential health problems which may develop over the lifetime of dogs of both sexes. This will be discussed in the second part of this article.
Non-surgical desexing options
Non-surgical desexing methods include three broad categories: hormonal, immunological, and chemical or physical methods (Tables 4–6; Box 1).
Table 5. Immunological methods of desexing: summary of advantages and disadvantages for gonadotropin-releasing hormone (GnRH), zona pellucida, luteinising hormone (LH) receptor, egg (including zona pellucida) and sperm protein vaccines
| Advantages | Disadvantages | |
|---|---|---|
| GnRH vaccines | Proven reversible suppression of fertility in males and females for short periods; when the vaccination boosters are discontinued, reproduction should resume | Immune response may be inconsistent in individual animals; may need multiple boosters to develop and maintain effect |
| Suppresses sexual behaviour such that females do not come into oestrus during treatment and males behave as castrates | Might cause injection site reactions depending on the formulation | |
| Under certain circumstances, may be possible to achieve longer duration of effect desired by many pet owners and for unowned and ‘community’ cats and dogs | Difficult to predict when reproduction would resume after cessation of treatment, as this will depend on the gradual decrease in circulating antibodies to GnRH | |
| Slow onset of activity (may be more than 2–3 months) as animal mounts immune response | ||
| Zona pellucida vaccines | Proven reversible suppression of fertility in females in some studies; efficacy shown in equids, some ruminants, pinnipeds and elephants but not yet in carnivores; when the vaccination boosters are discontinued, reproduction should resume | Immune response may be inconsistent in individual animals; may need multiple boosters to develop and maintain effect; response varies by species |
| Might cause injection site reactions depending on the formulation | ||
| Difficult to predict when reproduction would resume after cessation of treatment, as this will depend on the gradual decrease in circulating antibodies to zona pellucida | ||
| Ineffective in males | ||
| Will not affect sexual behaviour; animals will still come into oestrus and have normal cycles | ||
| Difficult to purify the zona pellucida under good manufacturing practice conditions | ||
| Slow onset of activity (may be more than 2–3 months) as animal mounts an immune response | ||
| May cause inflammation of the ovary | ||
| Not effective in males | ||
| Egg and sperm proteins | Reversible suppression of fertility in females in some studies; when the vaccination boosters are discontinued, reproduction should resume although this may depend on the antigen used | Immune response may be inconsistent in individual animals and may vary depending on the preparation of the antigen; may need multiple boosters to develop and maintain effect |
| Defined recombinant proteins easier to manufacture under good manufacturing practice | Requires the development of an IgA response in the female reproductive tract, which is difficult to assess experimentally when compared to a serum IgG response | |
| Might cause injection site reactions depending on the formulation | ||
| Difficult to predict when reproduction would resume after cessation of treatment, as this will depend on the gradual decrease in circulating antibodies | ||
| May require multiple boosters to maintain effect | ||
| Will not affect sexual behaviour; animals will still come into oestrus and have normal cycles | ||
| Slow onset of activity (may be more than 2–3 months) as animal mounts an immune response | ||
| Not effective in males | ||
| LH receptor vaccines | Reversible suppression of fertility in females in some studies; when the vaccination boosters are discontinued, reproduction should resume although this may depend on the antigen used | Immune response may be inconsistent in individual animals and may vary depending on the preparation of the antigen |
| Suppresses sexual behaviour such that females do not come into oestrus during treatment | Might cause injection site reactions depending on the formulation | |
| Difficult to predict when reproduction would resume after cessation of treatment, as this will depend on the gradual decrease in circulating antibodies to LH receptors | ||
| Difficult to manufacture under good manufacturing practice without defining recombinant antigen | ||
| Not shown to be effective in males |
Table 6. Chemical/physical methods of desexing: summary of advantages and disadvantages for chemical sterilants such as zinc gluconate or calcium chloride with or without dimethyl sulfoxide (DMSO) solutions for injection into gonads (usually testes)
| Advantages | Disadvantages |
|---|---|
| Permanent sterility | Irreversible |
| Low cost | Potential for improper administration and related side effects |
| Convenience | Side effects may necessitate surgery and require provisions for follow up |
| Ease of use | Continued testosterone production with zinc gluconate use although at a reduced level |
| Surgery and anaesthesia not required | Sedation strongly recommended |
Box 1.The non-surgical desexing market: owned dogs vs population controlA report by the Alliance for Contraception in Cats & Dogs (2013) looked at the different outcomes for two products used to induce infertility in male dogs: a GnRH agonist as a contraceptive implant, deslorelin acetate (Suprelorin) and an intratesticular sterilant, zinc gluconate (Zeuterin, zinc gluconate, Ark Sciences Inc). One is approved and has found a large market in the UK, European Union, Australia and New Zealand, while the other was approved by the Food and Drug Administration in the USA but is no longer on the market. The Alliance for Contraception in Cats & Dogs (2013) pointed out that the use of non-surgical desexing methods has generated a lot of discussion about market considerations, including what drives acceptance.There are two very different markets for reproduction control that non-surgical veterinary pharmaceuticals must appeal to: the private market which includes individual pet owners and veterinary practices and the not for profit market which includes shelters and animal welfare organisations. This raises the question of whether it is possible to serve both the not for and for profit sectors and, if so, what are the key factors that will drive success?
Hormonal methods
The use of synthetic analogues of progesterone (progestins or progestogens) has been shown to readily suppress oestrus in females. They include megestrol acetate (Burke and Reynolds, 1975), medroxyprogesterone, and proligestone (Selman et al, 1995) as well as competitive steroid hormone inhibitors such as cyproterone acetate (Li and Humpel, 1990). Exogenous sex steroid hormones have variable effects on oestrus which depend on the timing of administration during the oestrus cycle. Temporary postponement of oestrus will occur when treatment is started shortly before the onset of pro-oestrus, while prolonged postponement of oestrus will occur when treatment is started in anoestrus and oestrus will be suppressed when treatment is started after the onset of pro-oestrus. To be effective at preventing pregnancy, they must be given on an ongoing basis and carry some significant risks, particularly with extended use. There are several possible use- and dose-dependent side effects including pyometra (Cox, 1970). These hormones have less reliable effects on male fertility and libido (England, 1997). While exogenous testosterone has also been used to successfully suppress fertility in both male and female dogs, the undesirable androgenic side effects make it impractical for clinical use (England, 1997).
GnRH is a decapeptide; the GnRH agonists and antagonists are short peptide analogues of GnRH produced by amino acid substitutions of endogenous GnRH to create greater potency and longer duration of effectiveness. GnRH agonists are further developed than antagonists for dogs and cats to induce reversible infertility. An effective GnRH agonist mimics the effect of endogenous GnRH by binding to and causing down-regulation (suppressed response) of the GnRH receptors in the pituitary gland. GnRH must be recognised by these receptors in the pituitary cells to have a hormonal effect; as a result, the continuous administration of a GnRH agonist suppresses the effect of GnRH. This then suppresses both fertility and hormonal-driven sex-specific behaviours. In a dog implanted with a long-acting slow release GnRH agonist, there is ultimately a complete down-regulation of the hypothalamic–pituitary axis with no gonadotropins or sexual hormones produced (Figure 3).
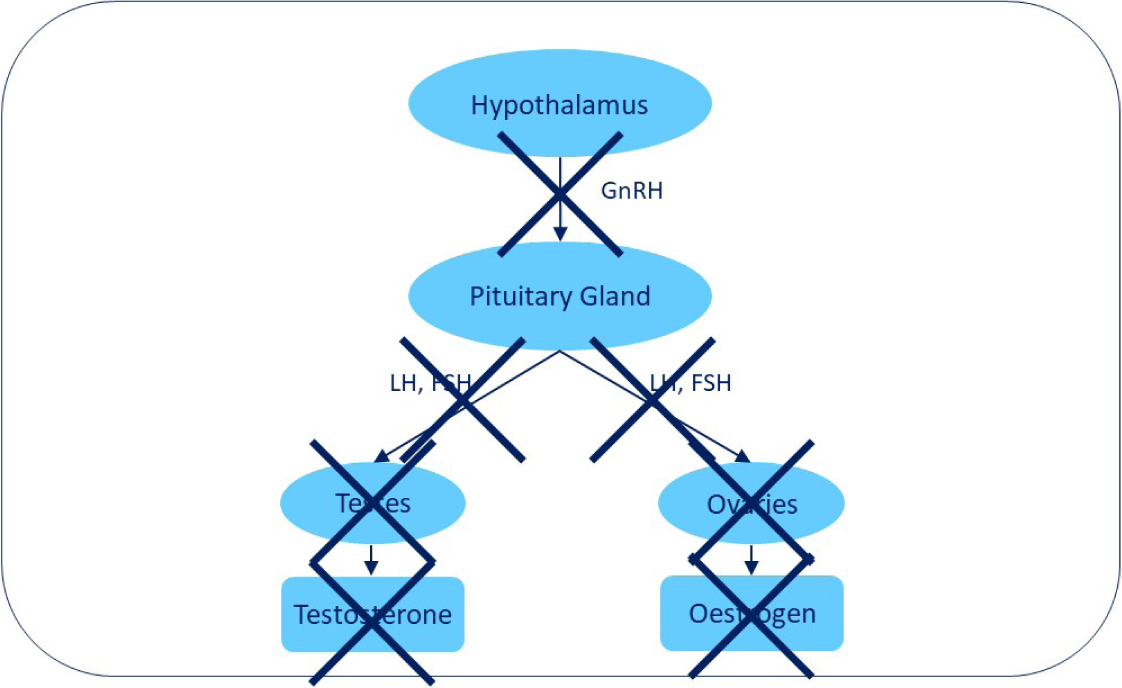
Continuous low-dose administration of a GnRH analogue such as deslorelin acetate in a sustained-release implant postpones oestrus in bitches and suppresses reproductive function in dogs, causing reversible long-term reproductive control in male and female domestic dogs (Trigg et al, 2001). Deslorelin is released slowly and continuously at low doses over time and this pattern of release suppresses fertility. The prolonged and continuous stimulation of pituitary GnRH receptors by deslorelin leads to desensitisation of the receptors; this, in turn, suppresses production of FSH and LH which ultimately results in a suppression of testosterone in males and of oestrogen and progesterone in females. However, before the suppression occurs, the initial exposure to deslorelin acts on the pituitary to increase FSH and LH secretion into the bloodstream. This temporary stimulation of the pituitary gland occurs until the GnRH receptors are down-regulated, which can take up to 5–10 days. The temporary increase in FSH and LH will cause an increase in sexual hormones in both sexes and in females this may induce oestrus before causing suppression, depending on timing of implantation within the oestrus cycle; it is possible for a female dog to become pregnant during this oestrus.
Deslorelin acetate, marketed as Suprelorin (deslorelin acetate, Virbac Ltd), is considered to be a GnRH superagonist. It has been approved and is available in multiple countries for reversible contraception in male dogs. The effect in dogs continues for 6 months in the case of the 4.7 mg implant and 12 months in the case of the 9.4 mg implant, after which implantation can be repeated if necessary. While deslorelin is being released, an implanted dog will remain infertile and behave as if castrated. It generally takes 2–3 weeks after implantation for testosterone levels to be reduced and about 6 weeks to become infertile. In male dogs, suppression of testosterone caused by deslorelin suppresses fertility by stopping sperm production, decreasing seminal fluid and in some dogs reducing libido and sexual behaviours. The prostate gland will also decrease in size, similar to the effects seen from surgical castration. In females, fertility is suppressed by inhibiting ovulation. Although not approved for use in cats or female dogs, it has been used to suppress fertility and hormone-driven behaviours in female dogs and domestic cats of both sexes for more than 15 years, as well as in many other mammalian species. The lack of permanence of GnRH agonists may be viewed as an advantage or disadvantage, depending on the circumstances of use.
GnRH antagonists prevent fertility by blocking GnRH receptor in the pituitary gland. They have the advantage of not eliciting an initial gonadotropin surge which is sometimes seen with GnRH agonists although they also have other disadvantages such as cost and method of administration of the peptide antagonists which may make them less attractive than their GnRH agonist counterparts. There has been limited research to date regarding the use of peptide GnRH antagonists in cats and dogs (Gobello, 2012) and there are no publications describing research on non-peptide GnRH antagonists in dogs. Phytoestrogens have also been proposed as a possible method of inducing infertility in male dogs, although they have not been used in clinical practice (Perez-Rivero et al, 2009).
Immunological methods
Over 40 years ago, research was carried out to test the hypothesis that if an animal could be treated in such a way as to stimulate an immune response to GnRH, the GnRH antibodies would interfere with the action of GnRH and this could result in infertility (Sad et al, 1993). However, as GnRH is a small decapeptide that is normally present in all mammals, it is not recognised as foreign by the immune system, making it a challenge to develop a suitable vaccine to elicit immune suppression of GnRH. Other reproductive vaccines have focused on eliciting an immune response to some component of the egg or sperm which could theoretically cause infertility by interfering with the fertilisation of the egg, sperm transport and/or binding of sperm to the egg. This approach to fertility control will only work in females (Alliance for Contraception in Cats & Dogs, 2013). Another approach has been to use LH receptor protein as a vaccine adjuvant to create antibodies that can block the action of LH by occupying the receptor and blocking its interaction with LH (Singh, 1985). Since LH receptors are not recognised as foreign, the immune system must be misled into mounting an immune response, creating challenges similar to those encountered in trying to generate an immune response to GnRH or the zona pellucida (Liu and Ball, 2002). Vaccination against GnRH or LH receptors would suppress both fertility and sexual behaviour.
Suppression of fertility is expected to be gradual, likely taking some time after vaccination since the effect depends on the vaccinated animal mounting an immune response. Similarly, the effect will be expected to wear off slowly, resulting in a gradual resumption of fertility over several months or even years, depending on the formulation, and this reversibility is likely to be variable among individual animals (Alliance for Contraception in Cats & Dogs, 2013). Unfortunately, there is still no commercial vaccine available for use as a contraceptive in companion animals. Major technical obstacles include inconsistency of the immune response (efficacy), need for multiple injections to maintain results, occurrence of injection site reactions as a result of use of certain adjuvants, inconsistency in the duration of effect among treated animals, and the difficulty and expense of doing large-scale, multiyear studies (Alliance for Contraception in Cats & Dogs, 2013). Nonetheless, since reproductive vaccines have the potential to be effective in both genders and most mammals, research continues.
Chemical or physical methods
Chemical or physical methods of desexing have involved the injection of a chemical agent, such as calcium chloride in alcohol as a necrotising agent, into the gonads to cause permanent infertility. The most common of these has been the use of zinc gluconate as an intratesticular sterilant for male dogs (Alliance for Contraception in Cats & Dogs, 2016). Currently, there are no viable non-surgical options directly targeting the female ovaries (Alliance for Contraception in Cats & Dogs, 2013). More recent research has been carried out that focused on targeting gonadal support cells using an antibody to guide a nanoparticle carrying a cytotoxin (nanocomplex) to specific cells found in the gonads. As these support cells are necessary for germ cells to develop and survive, the hypothesis was that destroying them using a nanocomplex would lead to sterilisation. Preliminary data from a pilot study suggest that this approach could safely suppress reproduction, although further research is needed to understand the extent of suppression and the safety of the approach (Alliance for Contraception in Cats & Dogs, 2013).
Discussion
There are several options available for desexing dogs, each of which has advantages and disadvantages with respect to effectiveness and safety. Despite several decades of promotion and extensive use of desexing as a means of population control, published evidence does not support the effectiveness of gonadectomy in controlling shelter and companion dog populations (Samuelson, 1973; Anonymous, 1976; Carter, 1990; Moulton et al, 1991; Olson et al, 1991; Frank and Carlisle-Frank, 2007; Looney et al, 2008; Diesel, 2010; Bushby et al, 2015; Downes et al, 2015; Griffin et al, 2016; Hiby et al, 2017; Root Kustritz, 2018). However, there is evidence to support the use of desexing to control free-roaming dog populations, which also has the advantage of reducing risks to human health from reduced bite injuries and exposure to zoonotic diseases (Reece and Chawla, 2006; Totton et al, 2010, 2011; Reece et al, 2013; Vargas-Pino et al, 2013; Yoak et al, 2014). Vasectomy may be a potentially more effective means of population control than orchiectomy in free-roaming or feral dog populations because it does not result in any changes in testosterone levels, libido, roaming or other male-specific behaviours (Barnett, 1985; Molento, 2004; Fox, 2005). Vasectomised males should continue to compete for available fertile females, resulting in reduced rates of successful impregnation, although this hypothesis has yet to be tested. Part of this is likely to be a result of the absence of sex-specific considerations when carrying out desexing procedures and studies of their effect. While ecologists accept that the population dynamics of mammals support the view that reproductive rates in a population are largely dependent on the availability of fertile females, the practice of reducing female dog reproduction needs further exploration (Nassar and Mosier, 1980; Looney et al, 2008; Hogasen et al, 2013; Massei and Miller, 2013; Bushby et al, 2015; Yoak et al, 2016).
Conclusions
The evidence for a beneficial effect of desexing on population control is stronger for female dogs than in males. Other benefits of desexing on disease prevention and behaviour modification show significant variation among breeds and sexes. More research is needed to clarify these differences and to arrive at individualised evidence-based recommendations for clinical practice. This will be discussed in the second part of this article, along with the various health risks that have been associated with desexing.
KEY POINTS
- There are surgical and non-surgical options available for desexing dogs and each method has advantages and disadvantages.
- Surgical methods, usually as gonadectomy, have been recommended for population control, disease prevention and behaviour modification, with variable results.
- A gonadotropin-releasing hormone agonist as an hormonal method of inducing reversible infertility in dogs offers a non-surgical option that may have health benefits over and above those of simply controlling reproduction.
- There is much variation among breeds and sexes as to the benefits of the various methods of desexing and more research is needed to facilitate individualised evidence-based recommendations for clinical practice.

