Pulmonary hypertension is defined as an abnormal increase in pressure within the pulmonary artery. The gold standard method of diagnosing pulmonary hypertension is directly assessing pulmonary arterial pressure (PAP), obtained by right heart catheterisation. According to the human literature, pulmonary hypertension can be diagnosed if mean PAP is ≥25 mmHg at rest (Hoeper et al, 2013). Increased PAP is an abnormal haemodynamic state that can be associated with numerous, diverse disorders which in human patients include diseases such as congenital systemic-to-pulmonary shunts, idiopathic pulmonary fibrosis, chronic thromboembolism or chronic lung disease (Simonneau et al, 2019).
Pulmonary blood flow in healthy dogs
The systemic circulation consists of veins, venules, arteries, arterioles and capillaries, and is a high-pressure system. There is a significant pressure difference between the arterial and venous systems. The inlet is the aorta with high blood pressure of approximately 98–120 mmHg and the outlet is the venae cavae, with pressure of approximately 2–6 mmHg for a typical dog at rest. The pressure difference between the arterial and venous systems is a direct cause of blood flow (Klein et al, 2007).
On the other hand, the pulmonary vasculature is a low-pressure, low resistance and high capacitance system (Klein et al, 2013). The normal pulmonary vasculature is composed of thin-walled veins, venules, arteries, arterioles and capillaries. In healthy animals, blood is ejected from the right ventricle during systole into the pulmonary trunk, and flows into the right and left pulmonary artery. Then, through a dense network of pulmonary capillaries, it reaches the alveolar–capillary interface in order to allow gas exchange. Rapid filling of the vasculature, with a large volume of deoxygenated blood, is possible as there are large numbers of pulmonary capillaries. Following oxygenation, blood is collected by pulmonary venules that later unite to form pulmonary veins. According to a computed tomography (CT) and endocast study in dogs, there is great variation in the number of the more peripheral pulmonary veins. The confluence of the pulmonary veins then form three ostia at the left atrial entry point (Brewer et al, 2012).
Pathophysiology
Development of pulmonary hypertension can result from three major disturbances in homeostasis: increase in pulmonary blood flow, increase in pulmonary vascular resistance or increase in pulmonary venous pressure (Figure 1) (Reinero et al, 2020).
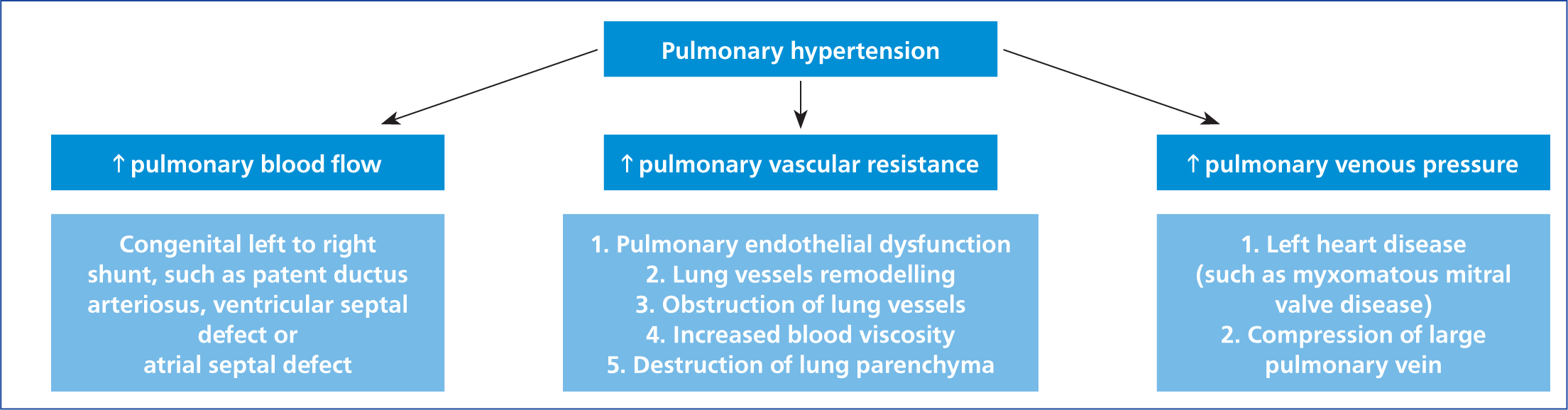
Increased pulmonary blood flow is usually a consequence of congenital left-to-right shunts, caused by intra- or extra-cardiac congenital defects such as atrial septal defect, ventricular septal defect or patent ductus arteriosus. Left-to-right shunting results in the addition of blood from the systemic to the pulmonary circulation. That flow occurs both in systole and diastole through the patent ductus arteriosus or during systole when the septal myocardium is affected by a ventricular septal defect. Left-to-right shunting is a consequence of a normal pressure gradient between the left and right heart (or aorta and pulmonary artery). Significant left-to-right shunting can lead to an increase in PAP combined with increased flow through the pulmonary vasculature, which can result in vascular remodelling and pulmonary hypertension (Kittleson and Kienle, 1998).
Increased pulmonary vascular resistance may be caused by several conditions including pulmonary endothelial dysfunction, pulmonary vascular remodeling, heartworm infestation (Uchide and Saida, 2005), acquired left heart disease (Prosek et al, 2004), perivascular inflammation, vascular luminal obstruction (Reinero et al, 2019) and lung parenchymal destruction, such as occurs in chronic bronchitis (Rozanski, 2014).
Increased pulmonary venous pressure, in most cases, is a direct result of left heart disease, that causes either left ventricular systolic or diastolic dysfunction. Left heart diseases that can increase pulmonary venous pressures include myxomatous mitral valve disease, hypertrophic cardiomyopathy and mitral valve stenosis (Moller and Hoffman, 2012).
Disease severity and clinical classification of pulmonary hypertension
The recent American College of Veterinary Internal Medicine (ACVIM) consensus statement on canine pulmonary hypertension has proposed a classification system (Table 1) based on the human reference system with the addition of one extra group (Simonneau et al, 2019; Reinero et al, 2020). This system divides animals affected by pulmonary hypertension into one of seven groups based on the underlying pathology. For example, a dog with pulmonary hypertension caused by Dirofilaria immitis infection would be in group six.
Table 1. Proposed classification of pulmonary hypertension in dogs1
| Pulmonary arterial hypertension | Congenital cardiac shunts: atrial septal defect, ventricular septal defect, patent ductus arteriosus |
| Idiopathic | |
| Drug and toxin induced | |
| Pulmonary veno-occlusive disease or pulmonary capillary haemangiomatosis | |
| Pulmonary hypertension caused by left heart disease | Acquired disease such as myxomatous mitral valve disease or dilated cardiomyopathy |
| Congenital disease such as mitral valve stenosis, mitral valve dysplasia | |
| Pulmonary hypertension secondary to respiratory disease, hypoxia or both | Interstitial lung disease, such as fibrosis |
| Chronic obstruction, such as tracheal collapse or bronchomalacia | |
| Pulmonary emboli, thrombi or thromboemboli | |
| Parasitic disease (Dirofilaria immitis or Angiostrongylus vasorum infection) | |
| Pulmonary hypertension with multifactorial or unclear mechanisms | Disorders having clear evidence of two or more underlying American College of Veterinary Internal Medicine group 1–5 pathologies contributing to pulmonary hypertension |
| Other disorders with unclear mechanisms | |
| Masses compressing the pulmonary arteries | |
Canine pulmonary hypertension can also be classified depending on severity (mild, moderate or severe), although this has been recently discouraged (Reinero et al, 2020). This classification system is based on echocardiographic findings that provide an estimate of PAP, which may be highly dependent on inter- and intra-operator variability. Traditionally, PAP is estimated from the tricuspid regurgitation velocity which, using the modified Bernoulli equation, gives an estimate of the pulmonic transvalvular pressure gradient, in the absence of right ventricle outflow tract obstruction.
Dogs affected and clinical presentation
Pulmonary hypertension usually occurs in dogs as either a primary vascular lung disorder or as a complication of other respiratory, cardiac or systemic diseases (Ettinger et al, 2017). Increased pulmonary venous pressure, in most cases, is a direct result of left heart disease. Examples of left heart diseases that can increase pulmonary venous pressures include myxomatous mitral valve disease, cardiomyopathy and mitral valve dysplasia. In young dogs, a cardiac shunt (atrial septal defect, ventricular septal defect, patent ductus arteriosus) or a developmental lung disease might be expected. In older, small breed patients with a left apical systolic heart murmur the cause of pulmonary hypertension is often myxomatous mitral valve disease (Serres et al, 2007); other older dogs might have an acquired lung disease, such as idiopathic pulmonary fibrosis. Laboured respiration, respiratory distress, and exercise intolerance are remarkable clinical signs frequently associated with and highly suggestive of pulmonary hypertension, as well as episodes of exertional syncope without another identifiable cause (Campbell, 2007).
There is evidence that suggests a breed predisposition of developing pulmonary hypertension secondary to interstitial lung disease for West Highland White Terriers (Schober and Baade, 2006) and Pekingese dogs (Kyranis et al, 2018). Overall, the population of dogs reported with pulmonary hypertension usually consists of smaller breeds, typically middle-aged to older, which correlates with the predisposition of the latter for myxomatous mitral valve disease and chronic pulmonary conditions (Pyle et al, 2004; Bach et al, 2006).
The one most commonly reported finding is a cough, although it is more likely associated with underlying respiratory disease (Johnson et al, 1999). The assessment of probability of pulmonary hypertension occurrence can initially be based on history and clinical findings (Table 2). It is also important to remember that many other conditions can present with similar clinical signs and the clinician must maintain an open mind, evaluating for other possibilities.
Table 2. Clinical findings suggestive of pulmonary hypertension in dogs1
| Findings strongly suggesting pulmonary hypertension | Findings possibly suggesting pulmonary hypertension |
|---|---|
| Syncope (especially with exertion or activity) without another identifiable cause | Tachypnoea at rest |
| Respiratory distress at rest, increased respiratory effort at rest | Increased respiratory effort at rest |
| Activity or exercise terminating in respiratory distress | Prolonged postexercise or post-activity tachypnoea |
| Right-sided heart failure (cardiogenic ascites) | Cyanotic or pale mucous membranes |
Findings on physical examination
Depending on the cause of pulmonary hypertension, findings on physical examination might vary. Heart auscultation may reveal a heart murmur localised to the mitral valve, tricuspid valve or both; a diastolic heart murmur of pulmonary regurgitation is occasionally observed in severe pulmonary hypertension cases (Masuyama et al, 1986). When pulmonary hypertension develops in congenital cardiac shunting diseases the continuous murmur of a patent ductus arteriosus or a holo- or pansystolic ventricular septal defect murmur might not be heard. Often, dogs with right-to-left shunting cardiac disease will have no murmur at all because of the low pressure differences between chambers. In severe cases of pulmonary hypertension, a right-sided systolic heart murmur from tricuspid valve regurgitation (Ohad et al, 2013) might be auscultated and signs of right-sided congestive heart failure can be seen (distended abdomen caused by hepato- or splenomegaly, ascites or distention and pulsation of jugular veins). Cyanosis may be present, most likely caused by primary pulmonary disease or secondary to right-to-left shunting of blood; it also can be observed in severe cases of pulmonary fibrosis. Patients affected by pulmonary hypertension will show changes in lung auscultation such as reduced lung sounds, wheezes and crackles. Auscultation of crackles may indicate interstitial lung disease, severe bronchomalacia, pulmonary oedema or the presence of exudate (Reinero, 2019).
Diagnostic investigations
The main aim of performing diagnostic evaluation in dogs suspected of having pulmonary hypertension is to confirm the diagnosis and identify a predisposing cause, so that appropriate treatment can be initiated. Another aim is to quantify the severity of the disease, because this will guide treatment decisions and aid in prognostication. Right heart catheterisation is the gold standard for evaluating the severity of pulmonary hypertension (Hoeper et al, 2013). The procedure consists of placing a catheter in the pulmonary artery, via peripheral venous access. As the catheter is advanced, right ventricle and PAP is recorded. Unfortunately, the procedure is not widely available in general veterinary practices and may be considered unacceptably invasive, especially in haemodynamically compromised patients (Kellihan and Stepien, 2010).
Minimum database blood work (routine biochemistry and haematology) can be performed in cases suspected of having pulmonary hypertension, however, they do not reveal findings specific for pulmonary hypertension; sometimes polycythaemia may be seen as a result of chronic hypoxia. Specific blood tests would include assessing for parasitic diseases such as Dirofilaria immitis and Angiostrongylus vasorum which are common causes of pulmonary hypertension. A number of diagnostic tests can be used to identify the underlying cause and echocardiography can be used to determine severity.
Electrocardiography
Electrocardiography is quite often normal in patients with pulmonary hypertension and is a relatively insensitive and non-specific diagnostic test. Electrocardiography findings that may be identified in dogs with pulmonary hypertension include a shift of the mean electrical axis to the right (right axis deviation), as well as signs of right atrial (tall P wave) and right ventricular (deep S waves and occasionally deep Q waves) enlargement. Sometimes right bundle–branch block morphology may look similar to right axis deviation (Tilley, 1985). Supraventricular or ventricular arrhythmias associated with heart disease, bradyarrhythmias and atrioventricular block associated with increased parasympathetic tone as a consequence of pulmonary disease, might also be present (Kellihan and Stepien, 2010).
Echocardiography and point-of-care ultrasound
Point-of-care ultrasound may be beneficial for patients with a history of persistent cough, lethargy, dyspnoea, ascites or syncope, especially when the patient is dyspnoeic and presented in an emergency setting. Although it does not replace detailed echocardiographic examination it may bring practitioners a step closer to a correct diagnosis.
Point-of-care ultrasound examination of the heart and thorax is quickly performed (Lisciandro et al, 2008). It can show major changes within heart anatomy, such as severe dilation of the right atrium and/or right ventricle, right ventricle hypertrophy and flattening of the interventricular septum (especially during systole) (Figures 2 and 3). Point-of-care ultrasound examination of the abdomen may indicate right-sided heart disease and findings may include: distended, not collapsing caudal vena cava, hepatic venous distension, ascites or gallbladder wall oedema. Abdominal ultrasound findings may be supportive of a pulmonary hypertension diagnosis, but they have poor utility for discriminating dogs with and without pulmonary hypertension, based on ultrasounds findings alone (Vientós-Plotts et al, 2019).
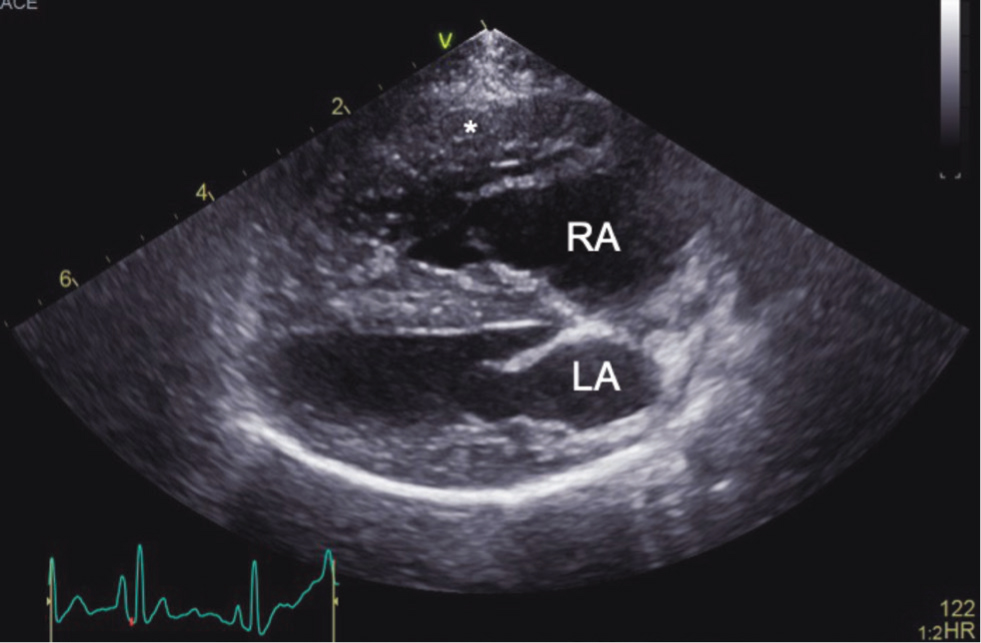
Advanced transthoracic echocardiography is a standard diagnostic procedure for identifying pulmonary hypertension in veterinary patients and should be considered in any dog where the disease is suspected. Using multiple modalities, such as two-dimensional imaging, spectral Doppler and tissue Doppler examination, sufficient information is usually collated to confirm or exclude a diagnosis of pulmonary hypertension. Full echocardiography examination gives details about structural and haemodynamic changes within the heart, as well as severity (Table 3 and 4). There are a number of different echocardiographic parameters that help to identify and classify pulmonary hypertension, or are suggestive of pulmonary hypertension. Some of these include:
- Two-dimensional echocardiography confirms point-of-care ultrasound findings, such as right ventricle hypertrophy (eccentric or concentric), septal flattening and pulmonary trunk dilation and enlargement, which may be observed in severe pulmonary hypertension cases. Sometimes, underfilling or decreased size of the left ventricle is also detected (Reinero et al, 2020).
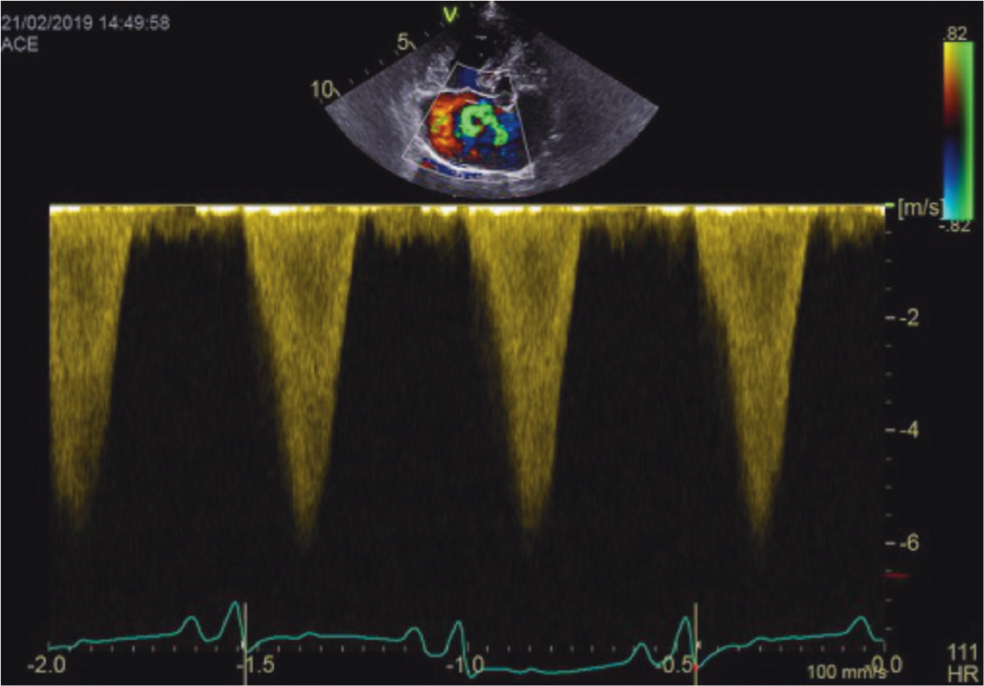
- Tricuspid regurgitation provides a quantitative assessment of systolic PAP and may be used as a tool to grade the severity of pulmonary hypertension. The presence of tricuspid regurgitation is easily confirmed by a colour flow Doppler. Use of continuous-wave Doppler detects peak tricuspid regurgitation (Figure 3). According to the latest advice, the velocity of tricuspid regurgitation should exceed 3.4 m/s, which (using the modified Bernoulli equation) gives a transpulmonary pressure gradient of >46 mmHg is highly suggestive of moderate pulmonary hypertension (Reinero et al, 2020). It should be noted that when tricuspid regurgitation is identified and velocity is high, the echocardiographer should exclude right ventricle outflow tract obstruction, for example pulmonic stenosis, before diagnosing pulmonary hypertension.
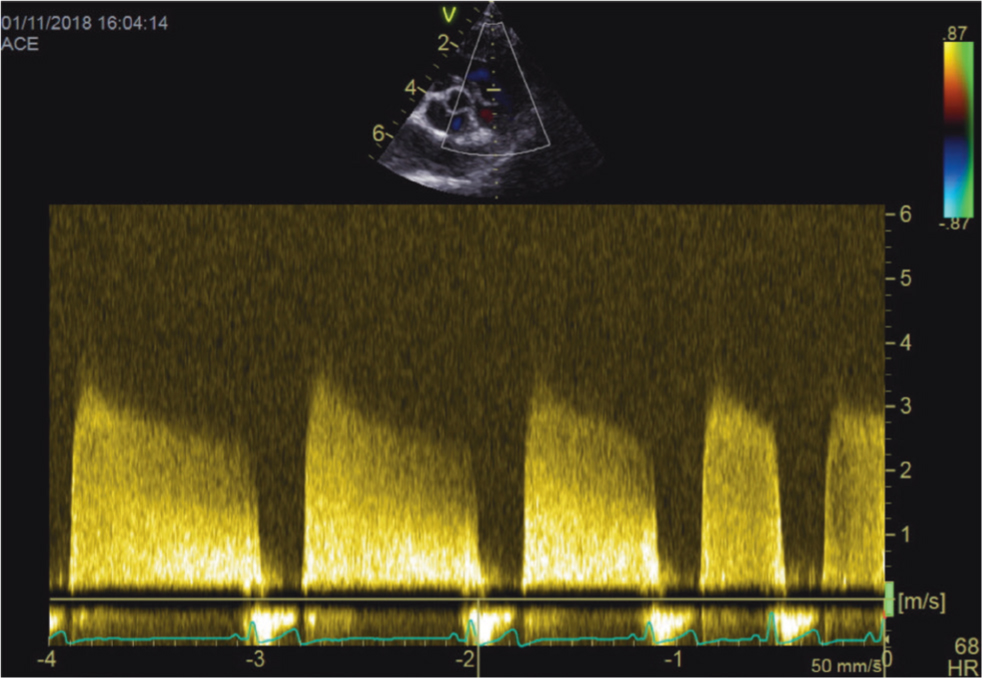
- Pulmonic regurgitation allows for the quantitative assessment of diastolic PAP (Figure 4). It is especially helpful in diagnosing pulmonary hypertension if tricuspid regurgitation is not present. A pulmonic regurgitation velocity of 2.2 m/s or more or a gradient of 19 mmHg or higher is highly suggestive of diastolic pulmonary hypertension (Kellum and Stepien, 2007).
- Tricuspid annular plane systolic excursion is measured as the displacement of the lateral part of the tricuspid valve annulus toward the heart apex during systole. Tricuspid annular plane systolic excursion remains generally decreased and below the reference interval for most dogs with severe pulmonary hypertension (Pariaut et al, 2012).
- Pulmonary artery systolic flow profiles are used to assess severity of pulmonary hypertension. They are obtained by measuring the pulmonary artery blood flow with pulse-wave Doppler immediately after the pulmonic valve. There are three types. Type I represents normal flow, type II represents mild to moderate pulmonary hypertension and type III represents severe pulmonary hypertension (Kellum and Stepien, 2007).
- Right ventricular tissue Doppler imaging consists of measuring right ventricle myocardium movement. Detailed Doppler interrogation remains challenging in patients with severe dyspnoea. Nevertheless, tissue Doppler assessment of the right ventricle may be considered supportive of pulmonary hypertension diagnosis, but no great correlation has been detected (Baron Toaldo et al, 2016).
- Right ventricular systolic time intervals. Acceleration time and ejection time are obtained from echocardiographic and electrocardiographic findings associated with the pulmonic outflow velocities (right ventricle ejection). They may be supportive of a diagnosis of pulmonary hypertension diagnosis especially when other clinical findings supporting pulmonary, hypertension are present in the absence of a tricuspid regurgitation jet. Acceleration time of ≤45 m/s and an acceleration: ejection ≤0.25 were 100% specific to diagnose pulmonary hypertension, wheras an acceleration time of ≤64 m/s and an acceleration:ejection ≤0.42 almost certainly excludes pulmonary hypertension (Schober and Baade, 2006).
- Right pulmonary artery distensibility index is calculated as the difference in diameter of the right pulmonary artery in systole and diastole measured by M-mode echocardiography. The systolic dimension of the pulmonary artery is measured at the maximum diameter (usually at the T wave) and diastolic diameter at its smallest dimension (at the Q wave). A right pulmonary artery distensibility index value lower than 35% is indicative of pulmonary hypertension and can be used to assess the severity of hypertension. A right pulmonary artery distensibility index between 28–35% is correlated with mild pulmonary hypertension, 23–27% with moderate pulmonary hypertension, and correlates with severe pulmonary hypertension if 22% or less (Venco et al, 2014). Right pulmonary artery distensibility index is particularly useful if tricuspid regurgitation is absent or difficult to measure (Visser et al, 2016).
Table 3. Anatomical sites of echocardiographic signs of pulmonary hypertension1
| Right ventricle |
|
| Pulmonary artery |
|
| Right atrium and caudal vena cava |
|
Table 4. Echocardiographic probability of pulmonary hypertension1
| Peak tricuspid regurgitation velocity (m/s) | Number of different anatomical sites with echocardiographic signs of pulmonary hypertension | Probability of pulmonary hypertension |
|---|---|---|
| ≤3.0 or not measurable | 0 or 1 | Low |
| ≤3.0 or not measurable | 2 | Intermediate |
| 3.0–3.4 | 0 or 1 | Intermediate |
| >3.4 | 0 | Intermediate |
| ≤3.0 or not measurable | 3 | High |
| 3.0–3.4 | ≥2 | High |
| >3.4 | ≥1 | High |
Radiography
Thoracic radiography does not provide enough information alone to diagnose pulmonary hypertension (Kellihan and Stepien, 2010), although radiographic findings that may be suggestive or supportive for initial diagnosis of pulmonary hypertension may be obtained. They include pulmonary artery enlargement, right heart enlargement, pulmonary arterial tortuosity and pulmonary infiltrates. Quite often thoracic radiographs show findings consistent with underlying disease such as patent ductus arteriosis or lung fibrosis (Kellihan and Stepien, 2010). Radiography, or some form of thoracic imaging, should always be performed in dogs with suspected or proven pulmonary hypertension.
Computed tomography
There are still limited criteria for detection of pulmonary hypertension with CT in dogs. CT angiography can be used to evaluate pulmonary vasculature, e.g. in an event of thromboembolism, and is also often combined with high resolution CT to assess the lung parenchyma. This investigation should be performed carefully and involve use of angiographic pressure injector, bolus tracking and cautious interpretation (Goggs et al, 2014). CT remains a very useful tool for identification of underlying disease such as interstitial lung fibrosis, chronic bronchitis or pulmonary metastatic nodules, rather than severity of pulmonary hypertension. Echocardiography still remains a standard tool in evaluation and diagnosis of pulmonary hypertension in canine patients (Sutherland-Smith et al, 2018).
Cardiac biomarkers
One of the ventricular natriuretic peptides known as N-terminal pro B-type natriuretic peptide (NT-proBNP) is released by heart ventricular myocardium in the event of mechanical stress, primarily in response to ventricular myocyte stretch. Some research shows that levels of NT-proBNP concentration are significantly higher in dogs with pulmonary hypertension as a result of pulmonary vasculature abnormalities than in dogs with respiratory disease without pulmonary hypertension. Data suggest NT-proBNP may be useful to predict the severity of estimated pulmonary hypertension (Kellihan et al, 2011).
Treatment of pulmonary hypertension
The main approach is to treat the underlying cause of pulmonary hypertension and this must be considered before treating the pulmonary hypertension. Indeed, the recent ACVIM consensus statement advises against the use of sildenafil as a firstline treatment in such cases (Reinero et al, 2020).
There is no one definitive treatment for pulmonary hypertension itself. Most peer-reviewed articles examining canine pulmonary hypertension have reviewed the phosphodiesterase-5 inhibitor sildenafil (Bach et al, 2006; Kellihan et al, 2015) as a treatment of choice. These studies suggest improvement in clinical signs (Kellum and Stepien, 2007), quality of life (Kellum and Stepien, 2007; Brown et al, 2010), exercise tolerance (Brown et al, 2010), and decrease of echocardiographically estimated PAP, (Bach et al, 2006; Brown et al, 2010; Ueda et al, 2019), although a reduction in tricuspid regurgitation velocity has not been identified in all publications (Kellum and Stepien, 2007).
Sildenafil acts as a pulmonary artery vasodilator and is often considered the treatment of choice for dogs with pulmonary hypertension secondary to pulmonary disease. In some cases, especially in dogs with left heart disease or left heart failure, there is a potential risk of developing pulmonary oedema after sildenafil use (Reinero et al, 2020), especially in the case of congenital disease and/or intracardiac shunts. Sildenafil may increase right heart cardiac output, acutely increasing pulmonary venous return to the left atrium. Increase in left atrium pressure, followed by increase in pulmonary venous and capillary pressures, may result in pulmonary oedema. Appropriate management of congestive heart failure may reduce pulmonary venous and, therefore, PAP sufficiently without the need to include a drug like sildenafil. In those patients in which sildenafil is initiated, close monitoring for development of pulmonary oedema is highly recommended. The goal would be to start therapy on a conservative dose, sildenafil 0.5 mg/kg orally every 8 hours in a hospital environment, followed by monitoring sleeping or resting respiratory rates and ruling out pulmonary oedema, such as performing thoracic radiography, if these increase. Sildenafil when not tolerated orally can be used rectally (Yang et al, 2018). The reader is directed to the ACVIM consensus statement for detailed treatment strategies for the different groups of pulmonary hypertension (Reinero et al, 2020).
Recently Tadalafil, which is another phosphodiesterase-5 inhibitor, has been used in clinical studies evaluating options for the treatment of pulmonary hypertension (Serres et al, 2006; Jaffey et al, 2019). It may be considered superior to sildenafil because of its once-daily administration, improved compliance and sometimes lower cost of therapy. Regardless, studies have shown no significant difference in clinical outcome, when comparing sildenafil to tadalafil (Jaffey et al, 2019). Alternative or adjunct pulmonary hypertension therapies are presented in Table 5 (Reinero et al, 2020).
Table 5. Unproven alternative or adjunct therapies that might be considered for use in dogs with pulmonary hypertension
| Pimobendan |
|
| Tyrosine kinase inhibitors (such as toceranib, imatinib) |
|
| L-arginine |
|
There are also several management strategies, yet untested in randomised clinical trials, which seem highly beneficial, especially in dogs with high probability of suffering from pulmonary hypertension. They consist of: exercise restriction, up to date vaccination as prevention of contagious respiratory pathogens, ongoing antiparasitic treatment (such as Dirofilaria immitis and Angiostrongylus vasorum prevention), avoidance of pregnancy (possibility of transmission of genetic contribution and potential to exacerbate pulmonary hypertension), avoidance of high altitude and air travel, avoidance of non-essential wellness procedures (such as dental cleanings) and elective surgery, requiring general anaesthesia (Reinero et al, 2020).
As stated before, treatment strategies depend highly on the cause and chronicity of pulmonary hypertension and they should be aimed at addressing the primary disease.
Conclusions
Pulmonary hypertension is identified relatively infrequently in clinical practice and remains a challenging condition to diagnose. It may be associated with congenital or acquired disease. Acquired causes of pulmonary hypertension in dogs often are a result of myxomatous mitral valve disease and idiopathic pulmonary fibrosis. Congenital causes are usually associated with congenital heart defects with shunting. Diagnosis of pulmonary hypertension may be suspected based on history (dyspnoea, exercise intolerance, syncope), clinical signs (predominantly right-sided heart murmur and cough) and breed predisposition. Definitive diagnosis requires echocardiographic examination. Following diagnosis of pulmonary hypertension, treatment options and prognosis depend upon the aetiology and severity of the condition.
KEY POINTS
- Pulmonary hypertension can be caused by congenital or acquired disease in dogs.
- The most common causes of pulmonary hypertension in young dogs are congenital heart disease with congenital heart disease with shunting (ventricular septal defect, patent ductus arteriosus); whereas in old, small breed dogs they are myxomatous mitral valve disease and interstitial lung disease, especially in West Highland White Terriers and Pekingese dogs.
- Pulmonary hypertension should be considered as a differential diagnosis for dogs with a history of some or all of the following: respiratory signs, syncope, collapse, exercise intolerance, heart murmur, ascites.
- Echocardiography remains the most commonly used tool for diagnosing and staging the severity of pulmonary hypertension.
- Treatment and prognosis depend on the underlying cause of pulmonary hypertension.


