The kidney has multiple functions including regulation of extracellular fluid volume, electrolytes and pH, excretion of waste and toxins, and hormone production. In the last article looking at poisons by organ system, we discuss some poisons that affect the kidney. Some substances cause renal damage as a result of toxic metabolites or interference with normal physiological processes; however, for other substances, the mechanism of renal injury is unknown. A few other potential causes of kidney injury in companion animals are:
- Vitamin D compounds, such as calcipotriol, calcitriol or large doses of vitamin D
- Some antibiotics (rare)
- Some mushrooms
- Wasp and bee stings
- Zinc
Unknown mechanism of kidney injury
Grapes and their dried fruit
Grapes and their dried fruits, sultanas, raisins and currants (Figure 1) are found in a variety of foodstuffs and poisoning has occurred aft er ingestion of the raw fruit (with or without seeds), as well as cooked foods such as fruit cakes (including Christmas cake) and other baked goods. Toxicosis has been reported from grapes grown in the home environment, from wineries and those brought from supermarkets (McKnight, 2005). Grape pomace or marc, which comprise the skins, pulp, seeds and stems of fruit after pressing has also caused renal injury (Lovell and Harvey, 2006).

Mechanisms of toxicity
The toxic mechanism that results in acute kidney injury from grapes and their dried fruit remains unknown, but the lack of a dose response may reflect a component of grapes or their products that is present in varying quantities or an extrinsic compound that is not always present in, or on the grapes (Eubig et al, 2005). Hypotheses have included canine tannin intolerance (Singleton, 2001), contamination of fruits with mycotoxins, pesticides or heavy metals (Gwaltney-Brant et al, 2001), sugar overload leading to shock (Singleton, 2001), idiosyncratic reactions due to enzymatic differences (Mazzaferro et al, 2004) or ingestion of excess vitamin D (Gwaltney-Brant et al, 2001). None of these seem particularly convincing and studies are required to establish the mechanism of toxicity and to determine whether some individual animals or breeds are at increased risk.
Clinical effects
Clinical signs of grape poisoning usually occur within 6 hours of ingestion and are always present within 24 hours. Initially, there is vomiting (in virtually all cases), diarrhoea, hypersalivation, haematemesis, bloody stools, anorexia, tender abdomen, ataxia, weakness and lethargy. Grapes or dried fruit may be present in the vomitus or faeces (Eubig et al, 2005). Then from 24–72 hours, renal failure may develop with oliguria or anuria. Some animals appear dehydrated and may be polydipsic. There may also be evidence of pancreatitis (Eubig et al, 2005), but the mechanism and significance of pancreatic involvement is unclear. Bradycardia, tachycardia, hypo- or hyperthermia, anaemia, leucocytosis, cyanosis, respiratory depression, tremor, rigidity and seizures are reported in some cases.
Laboratory findings in animals with grape toxicosis include raised creatinine and phosphorous (soon after ingestion), urea (24 hours later) and calcium (typically 48–72 hours after ingestion). Blood amylase is elevated in some cases. Proteinuria, glucosuria, microscopic haematuria and, less commonly, crystalluria may be found on urinalysis (Eubig et al, 2005).
Diffuse renal tubular degeneration, particularly of the proximal tubules, is a common finding in renal histopathology (Morrow et al, 2005). In radiographic studies, the renal size and shape remains normal in most cases but there may be evidence of fluid overload (abdominal effusion, pulmonary oedema, pleural effusion) (Mazzaferro et al, 2004).
Treatment
In view of the lack of a known dose response, reported cases of renal failure from a small quantity, the unknown mechanism and lack of knowledge about other issues such as individual risk factors, treatment is recommended following ingestion of any quantity of grapes or grape products in dogs. Gut decontamination should be considered following ingestion of any quantity of grapes, raisins, sultanas or currants (Mazzaferro et al, 2004). Digestion of grapes and dried fruit appears to be slow and gastric decontamination several hours after ingestion can be worthwhile. In addition, raisins are hygroscopic and can double or triple in volume on contact with water (Eshel et al, 1988). Whole grapes and swollen raisins have been recovered in dogs even after they have remained in the stomach overnight (Eubig et al, 2005). As the toxic mechanism has not been established it is not known if activated charcoal is effective but it is recommended and may be beneficial.
There is no way to determine which animals are at risk of renal impairment so aggressive intravenous fluid therapy (twice the normal maintenance rate) for at least 48 hours for rehydration and to support renal function is recommended in all cases. An antiemetic should be given if vomiting is protracted.
Electrolytes and renal function should be monitored every 24 hours for 72 hours in animals with ingestion of grapes or their dried fruit. Monitoring for signs of fluid overload (tachypnoea, chemosis, serous nasal discharge, pulmonary crackles, increased body weight) is also required. Where necessary, use of furosemide or mannitol may be considered to re-establish urine output but efficacy is unproven and there is evidence that tubular necrosis and obstruction of the renal tubules occurs preventing urine flow (Mazzaferro et al, 2004). Dopamine may be used to enhance renal perfusion. Radiographic and ultrasound findings are not consistently abnormal and are unlikely to be useful in confirming acute renal failure from ingestion of grapes or dried fruit (Eubig et al, 2005).
Haemodialysis and peritoneal dialysis have been employed in several cases in dogs reported in the literature (Mazzaferro et al, 2004; Eubig et al, 2005; Stanley and Langston, 2008) although, in practice, they will rarely be available due to cost and accessibility.
Lilies
Lilies are popular garden and house plants. These plants, that is, species of Lilium (Figure 2a) and Hemerocallis (day lily) (Figure 2b), cause acute kidney injury in cats, but not in dogs or rabbits.


All parts of the plant including the pollen, flowers, stem and leaves are toxic and ingestion of less than one leaf (Volmer, 1999) or part of a flower (Hall, 2013) could cause severe poisoning in cats. There are also reports of cats developing renal failure after exposure to only lily pollen (Veterinary Poisons Information Service data).
Mechanisms of toxicity
The toxic principle(s) and mechanism of lily toxicity are unknown (Bennett and Reineke, 2013), but renal failure is due to necrosis of renal tubular epithelial cells. The basement membrane remains intact (Volmer, 1999; 2002) and prompt, aggressive treatment can result in regeneration of tubular epithelial cells (Volmer, 1999). The mechanism of toxicity involves two processes; initially, there is damage to the renal tubular epithelium resulting in polyuric renal failure, which in turn results in severe dehydration (Hall, 2013). Whatever the toxic agent, either something in the plant or a feline-specific metabolite, it appears to be eliminated within 48 hours; this contention is supported by the observation that fluid diuresis for 48 hours prevents lethal renal failure (Fitzgerald, 2010). In a small number of cases, pancreatic degeneration has also been reported in cats after lily exposure (Rumbeiha et al, 2004) and this may be due to a different mechanism.
Clinical effects
Initial signs after ingestion of lilies are due to gastrointestinal irritation and usually start within 1–6 hours, whereas the later effects are caused by uraemia. Initially, there may be hypersalivation, vomiting, anorexia, weakness, lethargy and depression. Vomiting usually resolves within 12 hours but recurs again at 36–48 hours due to uraemia (Hall, 2007).
Polyuria can occur from 12–30 hours, followed by dehydration at 18–30 hours. As renal failure develops, owners may note depression, weakness, anorexia and vomiting (Tefft, 2004). Physical examination will show signs typical of acute kidney injury such as dehydration, oral ulceration, uraemic breath and enlarged, painful kidneys (Tefft, 2004). Seizures can occur in cats with severe renal failure (Rumbeiha et al, 2004).
The main laboratory finding in cats with lily exposure is severe azotaemia. Biochemical changes with rising urea, creatinine, potassium and phosphorus concentrations generally occur from 18–24 hours (Fitzgerald, 2010). The creatinine may be disproportionately elevated compared with the urea concentration (Tefft, 2004; Malik, 2011). There is tubular nephrosis with haematuria, proteinuria, glycosuria, isosthenuria, squamous and epithelial cells in the urine and numerous urine casts.
A mild elevation in liver enzymes may be noted but this may be due to severe anorexia and hepatic stress (Hall, 2013). Pancreatitis, sometimes with only mild elevation of serum amylase, has been reported in a few cases (Langston, 2002; Rumbeiha et al, 2004).
Treatment
Gut decontamination is not required in dogs after ingestion of lilies, but supportive treatment may be required for gastrointestinal signs.
In cats, aggressive treatment is required with the aim of reducing absorption, preventing renal shutdown and enhancing renal perfusion. An emetic and/or activated charcoal can be given if ingestion was recent. If vomiting has already occurred, an anti-emetic and activated charcoal should be given. It is important to wash the cat thoroughly to remove any residual pollen on the coat, face or feet to prevent continuing exposure.
Monitoring of renal function in lily-exposed cats is essential. Hydration status should also be monitored by calculating fluid in and fluid out, which allows appropriate adjustment of the fluid rate as required. Urine analysis will show evidence of renal tubular injury before onset of azotaemia (Malik, 2011). These changes can be observed as early as 12 hours after ingestion (Fitzgerald, 2010; Hall, 2013).
In cats with a low tolerance to fluids, such as those with cardiomyopathy, care should be taken to prevent fluid overload. In these cases, bodyweight and central venous pressure should be monitored, if possible (Tefft, 2004). Rehydration usually corrects metabolic abnormalities, but if severe hyperkalaemia occurs, insulin and dextrose should be given (Tefft, 2004).
Intravenous fluids at twice the normal maintenance rate for at least 48 hours are recommended to maintain urine output (Volmer, 1999). The fluid should be either isotonic saline or a balanced polyionic solution, such as Hartmann's solution (Tefft, 2004).
Once renal impairment has occurred, treatment options are limited and management is supportive. Although haemodialysis and peritoneal dialysis have been successful in some cases (Volmer, 2002; Berg et al, 2007; Hall, 2013), these procedures are rarely available and are too costly in most cases. Other drugs commonly used in the management of renal failure, such as furosemide or mannitol, are not very effective in cats with lilyinduced acute kidney injury (Tefft, 2004; Hall, 2013).
Interference with normal physiological mechanisms
Non-steroidal anti-inflammatory drugs (NSAIDs)
Human NSAIDs, particularly ibuprofen, are readily available and a common cause of enquiries to veterinary poisons centres. Dogs are particularly sensitive to the propionic acid group of NSAIDs (e.g. ibuprofen (Figure 3) and flurbiprofen), and they are prone to develop severe gastric ulceration and intestinal irritation (Adams et al, 1969; Bates and Faber, 2020).
Mechanism of toxicity
NSAIDs competitively inhibit the synthesis of various prostaglandins through reversibly blocking the action of cyclooxygenase (COX) enzymes. There are several isoforms of this enzyme (COX-1 and COX-2), but some NSAIDs such as ibuprofen, flurbiprofen and naproxen are non-specific inhibitors. COX-1 is involved in important physiological functions and is mainly found in the stomach, kidneys, endothelium and platelets.
COX-2 is inducible and responsible for the production of inflammatory mediators. Inhibition and subsequent reduction in concentrations of prostaglandins account for the major effects seen in NSAID toxicosis.
In the kidneys, prostaglandins maintain arteriolar dilatation regulating renal blood flow and the glomerular filtration rate (Lomas and Grauer, 2015). They also have a role in control of renin release and maintenance of water balance. NSAID inhibition of prostaglandin synthesis will result in reduction of renal blood flow and disrupt normal renal function and homeostatic mechanisms. Constriction of renal arterioles may result in renal papillary necrosis. These effects may be more evident in animals that are dehydrated or volume depleted.
In the gastrointestinal tract, prostaglandins have a cytoprotective function as they control production of gastric acid, stimulate secretion of mucous and bicarbonate by the gastric epithelium and maintain mucosal blood flow by causing vasodilatation (Papich, 2008). Deficiency results in increased gastric acid secretion, and reduction in mucous production and mucosal blood flow. These effects and the acidic nature of these drugs increase gastrointestinal irritation (KuKanich et al, 2012) and the risk of ulcer formation.
Clinical signs
NSAIDs cause gastrointestinal, neurological, renal and hepatic signs in overdose. Initial signs of NSAID overdose are vomiting, haematemesis, diarrhoea, abdominal tenderness and anorexia.
Ingestion of a large dose may result in rapid onset of neurological signs (within 1–2 hours) with agitation, hyperactivity, hyperaesthesia, tremors, twitching or seizures and coma. Death can occur following ingestion of a very large dose. Renal failure can occur from 12 hours but can be delayed (3–5 days). There may be protracted vomiting, pyrexia, anaemia, anorexia, polyuria, polydipsia, dehydration and collapse. Laboratory findings include oliguria, azotaemia and elevated creatinine and urea. Hypotensive or dehydrated animals are more at risk from renal effects, as are those with pre-existing renal impairment. Gastric erosion, ulceration and perforation may occur, particularly with flurbiprofen and naproxen. Dark tarry stools may be observed in animals with gastric ulceration. Signs of liver damage (raised liver enzymes, bleeding, jaundice) are uncommon but can occur in severe cases.
Treatment
Treatment of NSAID toxicosis is aimed at preventing or treating gastrointestinal and renal signs. After a large dose, treatment may also be required for neurological signs.
Any animal with chronic ingestion of a human NSAID, even after administration of a low dose or late presentation, should be assessed for gastrointestinal signs, dehydration and kidney injury.
An emetic and activated charcoal can be given if ingestion was recent. If vomiting has already occurred, an anti-emetic and activated charcoal should be given. In animals that have ingested a large quantity, emesis is best avoided owing to rapid onset neurological signs. Animals with dehydration, hypotension or pre-existing renal insufficiency are more at risk of toxic effects.
In all cases where treatment has been necessary, ulcer-healing or ulcer-preventing drugs should be started. Gastroprotectants should be given for 7–14 days or longer depending on clinical condition and the dose ingested (Poppenga and Gwaltney-Brant, 2011).
A recent consensus opinion on gastroprotectants for the management of gastroduodenal ulceration and erosion in cats and dogs states that proton pump inhibitors are superior to H2-receptor antagonists, sucralfate, and misoprostol for most causes of gastroduodenal ulceration and erosion in people, and should be considered as standard of care in dogs and cats (Marks et al, 2018). Twice daily dosing of a proton pump inhibitor, such as omeprazole, is recommended. Misoprostol (a synthetic prostaglandin) can be used for prevention (rather than treatment) of ulcers. Misoprostol should not be used in pregnant animals as it can cause uterine contractions and abortion.
If activated charcoal has been given, parenteral administration is recommended for other drugs or a lapse of 2 hours between the administration of charcoal and oral drugs. Sucralfate is only recommended if ulceration is suspected or confirmed. Therefore, it is not used in animals that present early and do not develop ulceration. If there is severe or persistent vomiting, anti-emetics should be administered as vomiting may result in dehydration and subsequent renal failure.
Renal function should be monitored in an animal with NSAID toxicosis. It is generally not necessary to check baseline renal function in a healthy animal presenting soon after ingestion. The renal function should be checked at 24 and 48 hours post ingestion (Tegzes, 2016), although renal failure may occasionally be delayed for up to 5 days post ingestion. If possible, blood gases and electrolytes should be checked and corrected, especially if there is evidence of renal dysfunction and/or oliguria. Liver toxicity occurs occasionally and routine monitoring is not required except after ingestion of a significant quantity.
Comatose animals should be managed supportively with regular turning. Diazepam or barbiturates may be given if there is any convulsant activity. In severe cases, radiography or gastroscopy may be employed to assess gastric mucosal damage. Perforation (which is not common with ibuprofen) will require surgical intervention. A blood transfusion may be required in animals with gastrointestinal haemorrhage.
In animals with NSAID toxicosis, it is important to ensure adequate hydration and good urine output with monitoring for signs of fluid overload (tachypnoea, chemosis, serous nasal discharge, pulmonary crackles) with monitoring of central venous pressure if possible (otherwise assess by using clinical signs and bodyweight).
If the dose ingested may cause renal impairment or the animal is vomiting, fluid therapy appropriate to its hydration and perfusion status, or at least maintenance intravenous fluids, should be given for a minimum of 24 hours. The duration of therapy should be adjusted depending on the renal parameters.
Toxic metabolites
Ethylene glycol
A common cause of renal failure, particularly in cats, is ethylene glycol (also known as ethanediol) — a common ingredient of antifreeze. Cats and dogs may be exposed by drinking neat antifreeze from a spill but are probably more commonly exposed by drinking the diluted fluid from drained vehicle radiators. Most exposures in cats are not witnessed (VPIS case data).
Mechanisms of toxicity
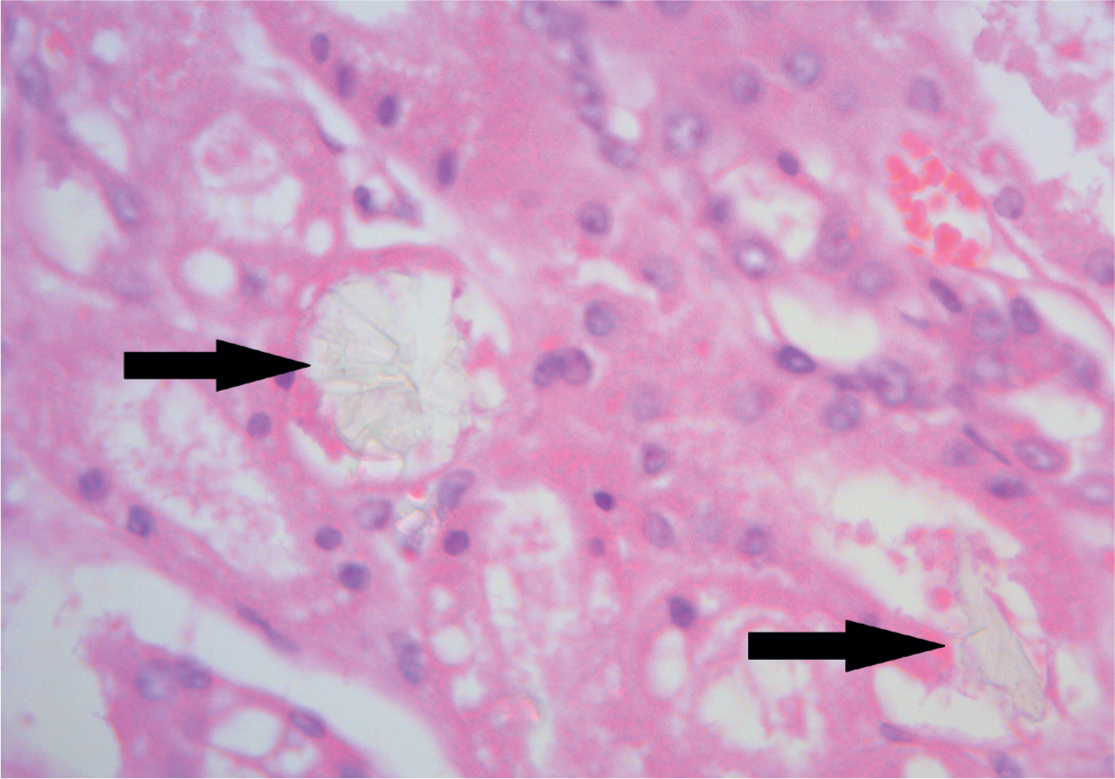
The major toxic agent in ethylene glycol poisoning is not the parent compound but the metabolites produced by the action of alcohol dehydrogenase. This enzyme oxidises ethylene glycol to glycoaldehyde. This is ultimately metabolised to oxalate, causing renal damage and hypocalcaemia by binding to calcium to form calcium oxalate crystals, which may be present in urine. Calcium oxalate crystal formation within the renal tubules (Figure 4) has been shown to be the cause of the renal toxicity in ethylene glycol poisoning (de Water et al, 1999).
Clinical effects
Although the progression of clinical signs can be divided into stages, these may overlap and not be clearly defined in many cases, particularly in cats.
In the early stages of ethylene glycol poisoning (from 30 minutes–12 hours), there are central nervous system signs owing to unmetabolised ethylene glycol including ataxia and weakness. Vomiting and tachycardia may occur. These early signs may be easily missed, particularly in an outdoor cat. Polyuria, dehydration, tachypnoea, acidosis and hypothermia may occur. Polydipsia, although common in dogs, is generally not seen in cats. Seizures can occur at this stage in severe cases.
From 12–24 hours in cats and 36–72 hours in dogs, cardiopulmonary signs develop with tachypnoea, tachycardia, acidosis, hyper- or hypotension, pulmonary oedema, arrhythmias, congestive heart failure and circulatory shock. Cats remain depressed. Cerebral oedema may be present and death can occur at this stage in some cases.
In the third stage of poisoning (which occurs from around 24 hours onwards), renal system signs including oliguria, azotaemia and/or uraemia develop and the kidney injury exacerbates acid/base and electrolyte disturbances. Kidneys may be swollen and painful and there may be vomiting, anorexia, oral ulcers and severe depression, lethargy, coma and convulsions due to uraemia (Thrall et al, 1984).
Increased urea and creatinine are generally seen from about 12 hours in cats and 24–48 hours in dogs (Grauer et al, 1984). Urine-specific gravity is low (due to osmotic diuresis induced by ethylene glycol); proteinuria, glucosuria, haematuria and albuminuria may occur. Calcium oxalate crystals can appear in the urine within 3–6 hours of ingestion (Sanyer et al, 1973; Grauer et al, 1984; Thrall et al, 1985; Connally et al, 2010), but the absence of oxalate crystals does not rule out ethylene glycol poisoning. The crystals are light yellow, arranged in rosettes, sheaves or prisms, and are birefringent in polarised light.
There may also be acidosis, hyperglycaemia, hypocalcaemia (due to binding of calcium to oxalate), hyperphosphataemia and hyperkalaemia (due to reduced excretion and acidosis). Clinical signs of hypocalcaemia generally do not occur in ethylene glycol poisoning because of the shift to the active, ionised form of calcium when metabolic acidosis occurs (Winek et al, 1978; Thrall et al, 1984).
Treatment
Diagnosis of ethylene glycol poisoning is generally based on history, clinical signs and laboratory findings. Ethylene glycol poisoning should be suspected in any animal with acute onset of signs, raised urea, creatinine, hypocalcaemia, hyperglycaemia, and other nitrogenous compounds in the blood (azotaemia or uraemia), and low or fixed urine specific gravity (Grauer and Thrall, 1982), central nervous system (CNS) depression, metabolic acidosis with or without a high anion gap or calcium oxalate crystalluria (Stuckey et al, 2012).
Many antifreeze products contain fluorescein (a green or red dye depending on the pH of the medium), which fluoresces under ultraviolet light. Sometimes the dye may be detected in urine or vomitus using a Wood's lamp, and examination of the paws, mouth and face may be useful (Winter et al, 1990). However, this is not a reliable test (Parsa et al, 2005), as it is difficult to detect fluorescence in a test sample without a positive and negative control for comparison (Wallace et al, 2001).
The aim of therapy in ethylene glycol poisoning is to prevent metabolism and the production of toxic metabolites, reverse electrolyte and acid/base disturbances, and maintain the glomerular filtration rate.
Decontamination is recommended for any quantity but gut decontamination is probably only worthwhile within 1 hour of ingestion. Adsorbents such as activated charcoal are not useful and, in many cases animals, particularly cats, do not present until the onset of signs, hours after ingestion.
In symptomatic animals, the blood pH, electrolytes and renal function should be monitored. Intravenous fluids 2–3 times maintenance are essential to ensure adequate hydration and promote diuresis. If possible, the central venous pressure and renal output should be monitored in animals with renal dysfunction because of the risk of fluid overload and subsequent pulmonary oedema. If there is oliguria or anuria, the fluid rate can be increased and diuretics such as mannitol or furosemide can be given if there is no response. Haemodialysis is very effective in ethylene glycol toxicosis if started before onset of azotaemia and can be used in animals with uraemia while waiting for possible return of renal function (Rollings et al, 2004); however, it is rarely available.
Ionised calcium concentration should be monitored in animals with renal failure or severe acid/base disturbances (Connally et al, 2010). Calcium gluconate can be given if there are clinical signs of severe hypocalcaemia. Sodium bicarbonate should be given to correct severe acidosis (Jandrey, 2016). Animals should be offered food and water if their condition allows.
The aim of antidotal therapy in the management of ethylene glycol toxicosis is to prevent formation of the toxic metabolites. This is achieved through administration of ethanol or fomepizole. Ethanol has a higher affinity for alcohol dehydrogenase than ethylene glycol, whereas fomepizole is a potent selective competitive inhibitor of alcohol dehydrogenase. With the enzyme either busy with ethanol or inhibited by fomepizole, there is time for renal excretion of the unchanged parent compound. Ethanol is cheap and readily available whereas fomepizole is generally unavailable and is very expensive, particularly in cats which require six times the dose used in dogs and humans.
The sooner antidotal therapy is started, preferably within the first few hours of ingestion, the better the outcome. A potentially lethal dose of ethylene glycol can be survived if treatment is prompt, but it should be noted that administration of an antidote is usually necessary for a prolonged period, and animals with ethylene glycol poisoning require intensive management for several days. There is no point giving ethanol or fomepizole to block metabolism if the ethylene glycol has already been metabolised. Unfortunately, cats often present late, by which time such antidotal therapy is no longer of use. Management in these cases is supportive/palliative. In animals with renal failure, antidotes increase the half-life of ethylene glycol and if renal damage has already occurred, the kidneys may not be able to effectively eliminate it.
Conclusions
Poisoning can result in adverse effects on the kidney through a variety of mechanisms, including formation of toxic metabolites, interference with normal physiological process and, in some cases, unknown mechanisms. Since haemodialysis and other extracorporeal techniques are rarely available, early intervention to prevent onset of kidney injury is essential. This can involve intravenous fluids or, in the case of ethylene glycol, use of antidotes to prevent formation of toxic metabolites. In most cases, prognosis is poor in animals with advanced kidney injury.
KEY POINTS
- A number of drugs and chemicals can affect the normal functioning of the kidney.
- Early treatment is essential to prevent kidney injury since treatment options are limited once injury has occurred.
- Aggressive intravenous fluid therapy is recommended for lily or grape toxicosis.
- Non-steroidal anti-inflammatory drug (NSAID)-induced renal injury can be managed with supportive care.
- Early administration of antidote to prevent formation of toxic metabolites in ethylene glycol toxicosis is essential, but often not practical due to late presentation of cases.
- Prognosis is generally poor in animals with advanced kidney injury.


