Autoimmune skin diseases are characterised by reactions of the immune system against ‘self’ antigens. The reaction can be mediated by antibodies or by cells belonging to the immune system (https://pathology.jhu.edu/autoimmune/definitions). In cats, autoimmune skin diseases are considered uncommon to extremely rare, with the exceptions of pemphigus foliaceus (pemphigus foliaceus) and vitiligo. Currently, the existence of some autoimmune skin disease, such as cutaneous lupus erythematosus, is questioned in the feline species. The autoimmune skin diseases reported in cats are summarised in Table 1. Pemphigus foliaceus is the most common autoimmune skin disease, although it represents only 1% of cases seen by veterinary dermatologists (Scott et al, 2013a).
Table 1. Autoimmune diseases in cats
| Antibody-mediated autoimmune skin disease | Prevalence | References |
|---|---|---|
| Pemphigus foliaceus | Uncommon | Bizikova, 2020; Bizikova and Burrows, 2019; Irwin et al, 2012; Preziosi, 2019; Preziosi et al, 2003 |
| Pemphigus vulgaris | Extremely rare | Manning et al, 1982; Scott et al, 2013b |
| Mucous membrane pemphigoid | Two cases | Olivry et al, 2002 |
| Bullous pemphigoid | One case | Olivry et al, 1999 |
| Cell-mediated autoimmune skin disease | ||
| Systemic lupus erythematosus | One case reported | Vitale et al, 1997 |
| Cutaneous lupus erythematosus (nasal, discoid) | Five cases | Kalaher and Scott, 1991; Willemse and Koeman, 1989 |
| Vitiligo | Uncommon | Tham et al, 2019 |
| Antibody- and cell-mediated autoimmune skin disease | ||
| Paraneoplastic pemphigus | One case | Hill et al, 2013 |
Pathogenesis
Pemphigus foliaceus is an antibody-mediated autoimmune skin disease. Autoantibodies belonging to the IgG subclass attack and destroy the desmosomes, the special structures connecting one keratinocyte to another. The clinical result of the detachment of keratinocytes in the upper layers of the epidermis is a pustule, which is the primary pemphigus foliaceus lesion in all affected species (Bizikova, 2020). The target autoantigen within the desmosome has been identified in humans as desmoglein-1 and, more recently, in dogs as desmocollin-1, while it has not yet been identified in cats. A recent study showed that circulating anti-keratinocyte IgG antibodies were present in 77% of cats affected by pemphigus foliaceus. The authors postulated that the target autoantigen is likely to be different from the one identified in dogs and humans (Levy et al, 2020).
Pemphigus foliaceus in cats is spontaneous in the majority of cases, and may be uncommonly associated with drug administration, particularly with antibiotics and methimazole (Bizikova and Burrows, 2019; Jordan et al, 2019). Two cases associated with thymoma (Biaggi et al, 2009; Coyner, 2011) and one case associated with feline leishmaniosis (Rufenacht et al, 2005) have also been reported. Two studies described development of pemphigus foliaceus in cats with a history of chronic allergic skin disease (Preziosi et al, 2003; Jordan et al, 2019). In the author's experience, it is not uncommon to diagnose pemphigus foliaceus in cases referred for the work-up of allergic pruritus.
Clinical features
There is no apparent breed predisposition, with over 60% of affected cats being domestic short- or long-haired cats. The median age of onset is 6 years and it is more common in females (female to male ratio: 1:5). The average time between onset of disease and diagnosis is 3 months (Preziosi et al, 2003; Bizikova and Burrows, 2019; Jordan et al, 2019).
The primary lesion of feline pemphigus foliaceus is a large subcorneal pustule, spanning multiple hair follicles. However, the lesion is easily ruptured and often cannot be appreciated. The most common lesions identified in felines are superficial erosions and honey-coloured crusts that are often coalescing, together with alopecia and scaling (Figure 1). The parts of the body most commonly involved are the pinnae (over 80% of the reported cases), the nasal planum, muzzle, eyelids and the footpads, although the disease may be generalised (Figures 2 and 3). The areas surrounding the nipples represent a peculiar location for pemphigus folliaceus in the cat, while the most distinctive localisation of pemphigus foliaceus is the claw fold. A thick, yellowish, caseous exudate can be expressed from the claw folds and, when present, this is a useful hint at the diagnosis (Figure 4). In the majority of cats, the disease involves two or more body sites, but localised lesions have been reported in 19% of cases. Lesions are usually symmetrical and pruritus is apparent in over 60% of cases (Preziosi et al, 2003; Bizikova and Burrows, 2019; Jordan et al, 2019).
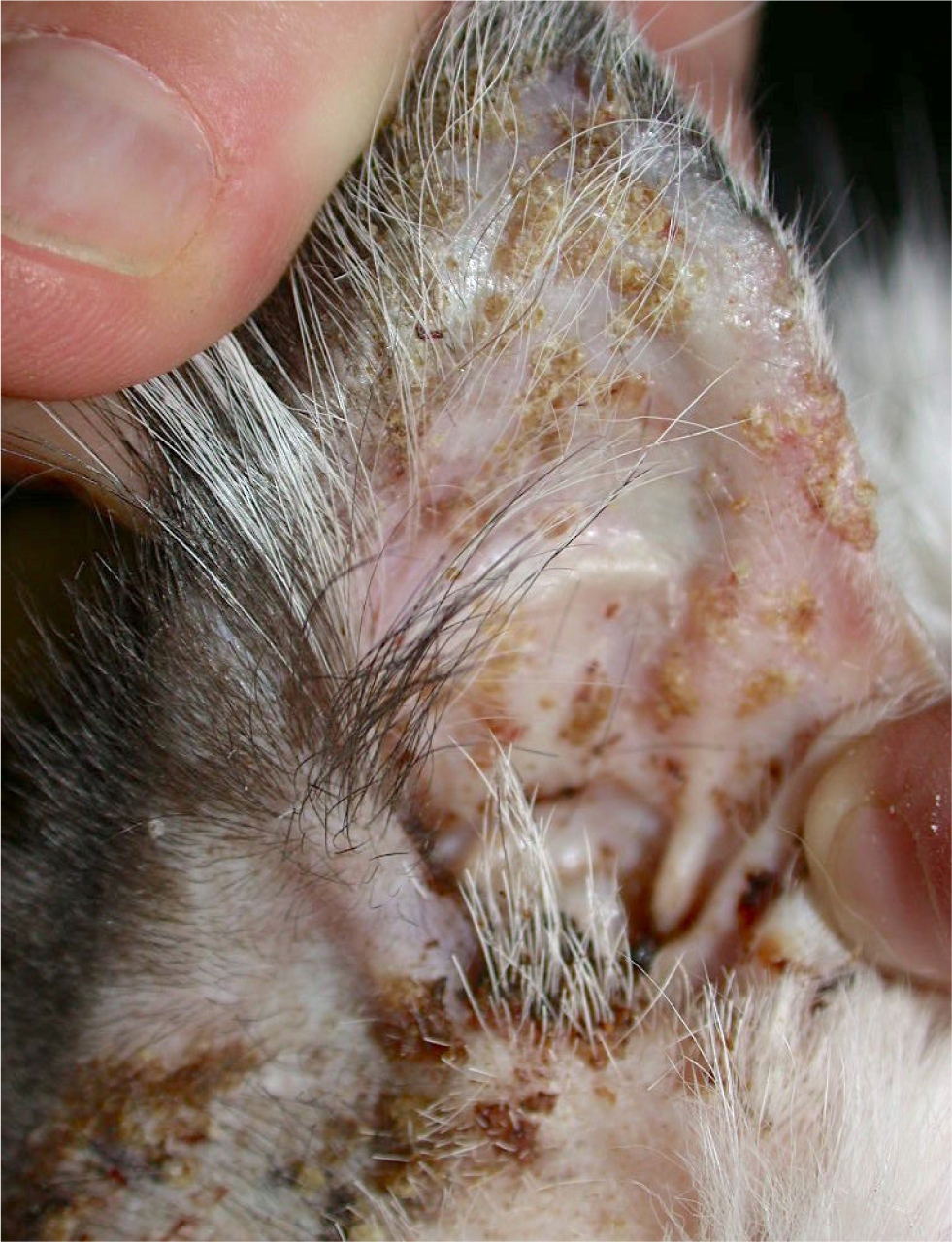
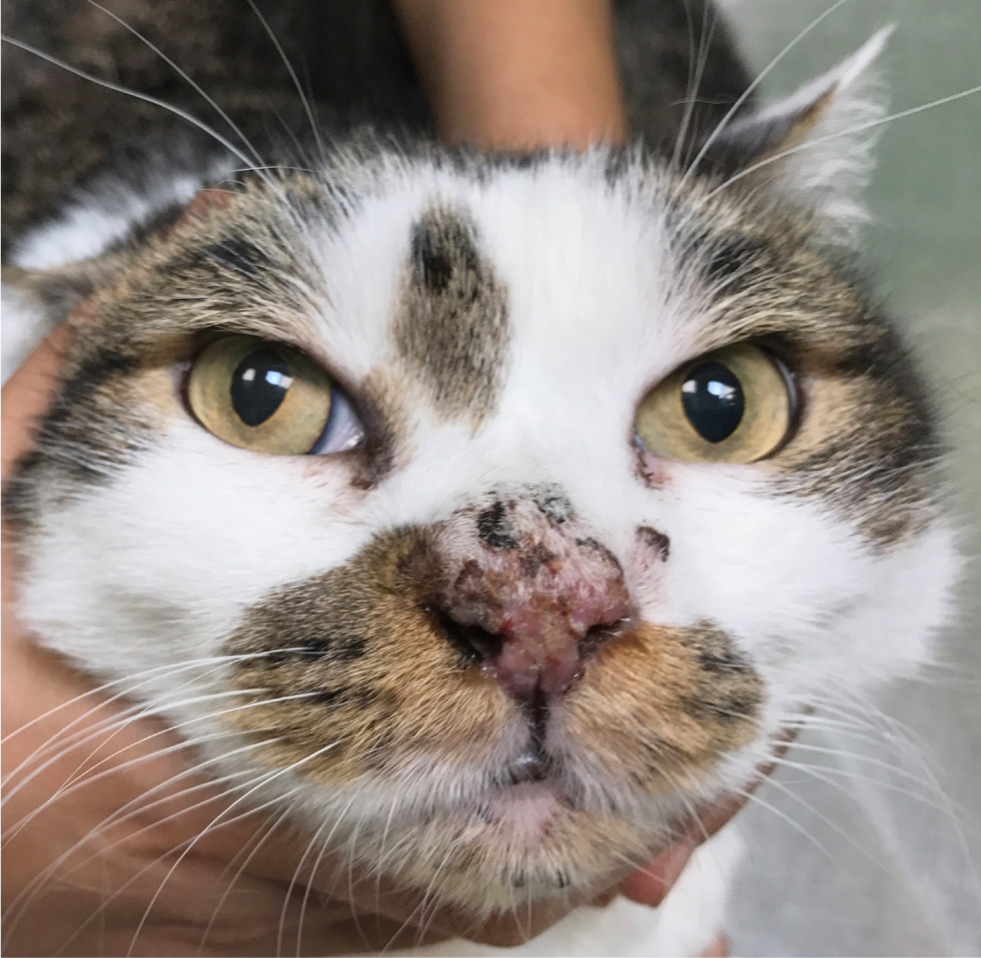
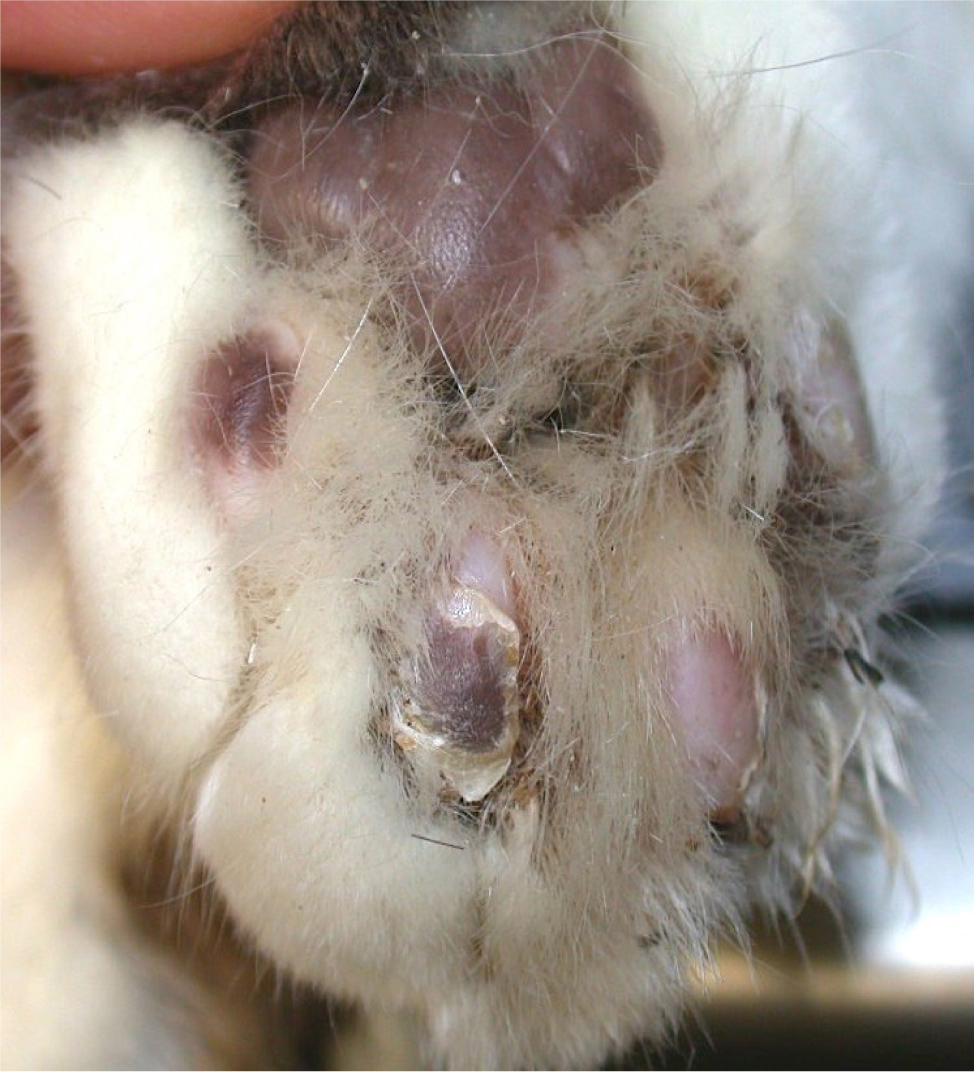
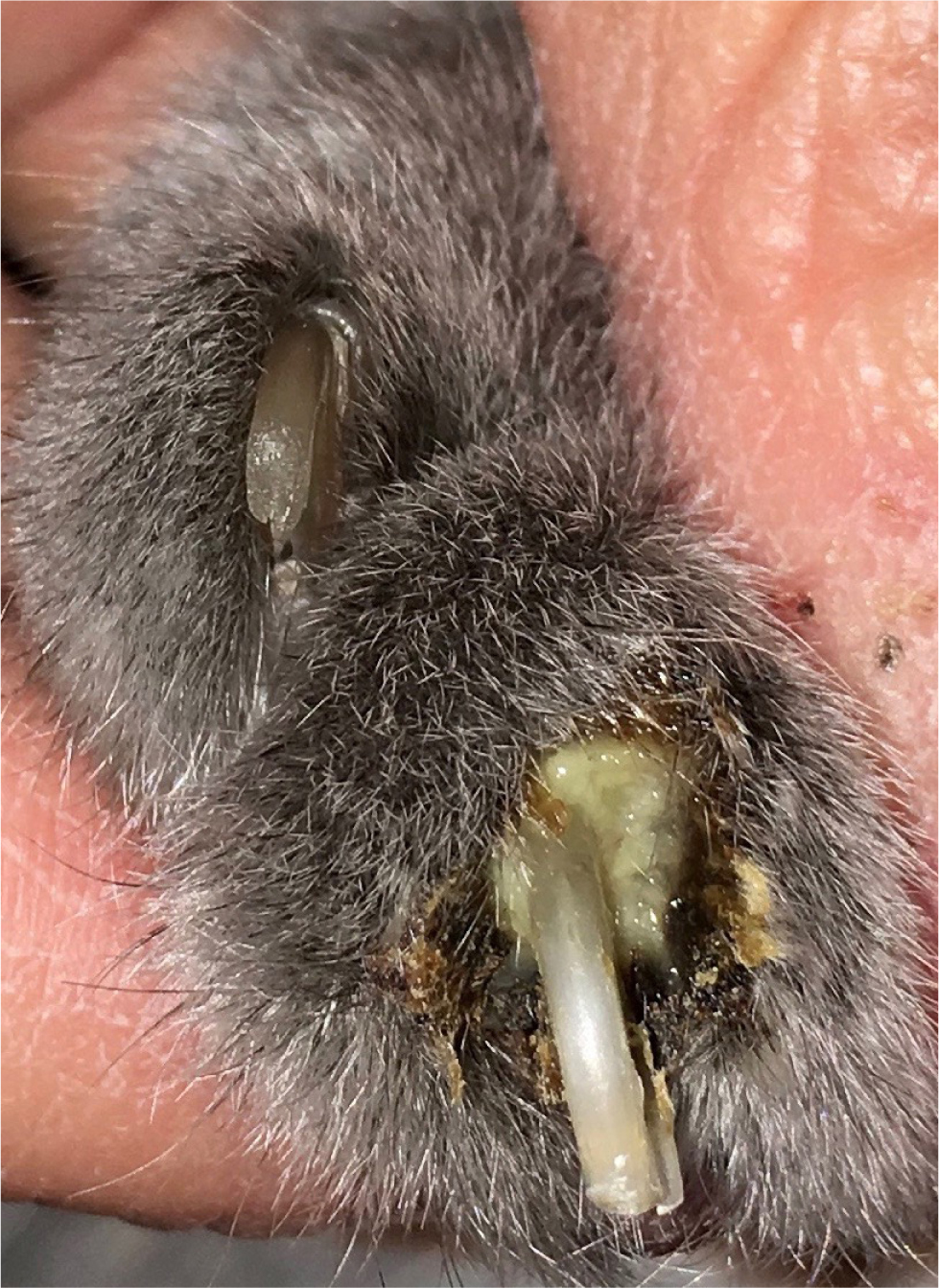
Concurrent otitis externa, more often purulent and with secondary bacterial infection, may be diagnosed in approximately 30% of affected cats (Jordan et al, 2019). When pedal lesions are severe, cats are often systemically ill, having anorexia, lethargy, hyperthermia, enlarged lymph nodes and lameness. The most common clinicopathological abnormalities include leukocytosis and neutrophilia. Differential diagnoses include dermatophytosis, adverse drug reaction, (drugs are recognised triggers for pemphigus foliaceus), leishmaniosis (so far, only once reported with a clinical presentation similar to pemphigus foliaceus) and superficial pyoderma, which is uncommon in cats (Preziosi et al, 2003; Rufenacht et al, 2005; Bizikova and Burrows, 2019; Jordan et al, 2019).
Diagnosis
Despite the difficulty of identifying intact pustules in cats, useful samples for cytological examination can be obtained from the underneath of a crust or from the claw folds. Cytology shows large numbers of non-degenerate neutrophils and acantholytic cells, which may be in rafts. Acantholytic cells are round, nucleated and usually basophilic, as they come from the spinous layer of the epidermis (Preziosi et al, 2003; Bizikova and Burrows, 2019; Jordan et al, 2019). Bacterial infection as a complicating factor has been reported in 63% of cases, and Malassezia overgrowth in 19% of cases after cytological examination (Jordan et al, 2019).
However, superficial pyoderma in cats does not usually present as a pustular dermatitis, and the role of the bacterial infection should be further investigated with culture and sensitivity testing. Secondary infections should be treated before taking biopsy samples for histopathological examination. Dermatophytosis should be ruled out by performing a fungal culture.
The confirmative diagnostic test is histopathology from skin biopsy samples and crusts. Microscopic examination of skin biopsies may show sub-corneal pustules containing neutrophils and acantholytic cells, sometimes admixed with eosinophils (Gross et al, 2005). Crusts are often multilayered with degenerating inflammatory cells, corneocytes and serum alternating. Including crusts in the samples can be extremely useful to the pathologist, because ghost acantholytic cells may be observed within the crust and intact pustules are very difficult to find in the feline patient. In the dermis, there is perivascular to interstitial inflammation, with neutrophils being the most common cell type, followed by mast cells, plasma cells, lymphocytes and eosinophils (Gross et al, 2005). In a series of cases, vascular changes with hyaline thrombi, hyalinised vascular walls, microhaemorrage and leucocytoclasia were described in 17% of the cases (Jordan et al, 2019). Direct/indirect immunofluorescence testing kits are available only for use in research establishments.
Treatment
In general, cats affected by pemphigus foliaceus respond to treatment better than dogs. The vast majority of cats achieves remission in 4 to 6 weeks on average. Spontaneous remission has been reported for drug-related cases (Bizikova and Burrows, 2019). Death as a result of the disease itself, adverse effects of treatment or concurrent diseases directly related to the drugs administered (for example, diabetes mellitus) is reported in 20% or less of cases (Preziosi et al, 2003; Bizikova and Burrows, 2019; Jordan et al, 2019).
Glucocorticoid monotherapy is the first treatment and the majority of cats do not require association with other immunosuppressive drugs to keep the disease in remission. Recommended induction doses are 2–3mg/kg/day when using prednisolone or methylprednisolone, 0.1–0.2mg/kg/day when using dexamethasone and 0.3–0.5mg/kg when using triamcinolone. In one review, a pulse therapy protocol with high-dose glucocorticoids was used in 39% of the cases, as an attempt to gain quicker control of the disease. However, no advantages over traditional glucocorticoid therapy were observed (Bizikova and Burrows, 2019). Details of glucocorticoid pulse therapy are summarised in Table 2.
Table 2. Details of high-dose glucocorticoid pulse therapy in cats
| Induction phase | Maintenance phase | |
|---|---|---|
| Triamcinolone 1 mg/kg orally once daily for 3 days | Prednisolone 10 mg/kg orally once daily for 3 days | Dexamethasone 1 mg/kg orally once daily for 3 days |
| Prednisolone 2–3 mg/kg orally once daily | Taper and repeat induction phase if needed | |
The dosage is gradually reduced when the disease is in remission and a slow reduction of 20–25% every 2–4 weeks is recommended (Preziosi et al, 2003; Bizikova and Burrows, 2019; Jordan et al, 2019; Preziosi, 2019). The ideal maintenance dose should be 0.5mg/kg orally once daily or on alternate days, when using prednisolone or methylprednisolone. A study of 37 cats explored the results obtained with glucocorticoid monotherapy. The authors reported complete remission within 8 weeks in 97% of cats, with a median induction dose of 2mg/kg of prednisolone administered once daily. The median maintenance dose in the study was 1.2mg/kg/week. Even with very slow dose reduction, relapses occur in over 50% of the cats on glucocorticoid monotherapy (Simpson and Burton, 2013). Another study evaluated the management of feline pemphigus foliaceus in general first-opinion practice, and some interesting observations were made. Firstly, remission was easily achieved in 90% of cats with glucocorticoid monotherapy, however more than 70% of cases relapsed when the dose was reduced. Second, severe adverse effects resulting from glucocorticoids were observed in 16% of cats, including two cats treated with long-acting injectable glucocorticoids, which should be avoided whenever possible (Coyner et al, 2018).
If glucocorticoids are not effective in maintaining remission when the dose is decreased, or severe adverse effects occur, discontinuation or a significant dose reduction may be required. Other immunosuppressive drugs should be added at this point, in order to decrease the dose and, if possible, ultimately discontinue glucocorticoids (Preziosi et al, 2003; Bizikova and Burrows, 2019; Jordan et al, 2019). The most commonly used immunomodulatory drugs in combination with glucocorticoids are ciclosporin (5–10mg/kg) and chlorambucil (0.1–0.2mg/kg/day). To achieve disease control, glucocorticoid dosage should be initially increased back to a higher dose or the initial dose when the second drug is added, and then gradually tapered again. Results with chlorambucil or ciclosporin as steroid-sparing drugs have been compared in a recent study, although the number of cats included was low (six cats per group of treatment). According to this study, the two drugs were equivalent in terms of response to treatment and time to obtain disease remission. However, all cats treated with ciclosporin were eventually weaned off glucocorticoids, compared to one cat only in the chlorambucil group (Irwin et al, 2012). Further studies with more cases are necessary to confirm these findings.
Adverse effects occur in approximately 30% of cases (Bizikova and Burrows, 2019; Jordan et al, 2019) and are usually result from using glucocorticoids (Table 3). Chlorambucil may induce leucopoenia, thrombocytopenia, anaemia and increased liver enzymes. Monitoring for adverse effects with haematology, biochemistry and urinalysis is recommended every 6 months if the cat is treated with glucocorticoid monotherapy or ciclosporin. When chlorambucil is administered, haematology should also be performed every 2–3 weeks over the first 3 months of therapy. Diarrhoea, hypertrichosis and disseminated mycobacteriosis have been observed in cats treated with ciclosporin.
Table 3. Adverse effects reported in cats treated for pemphigus foliaceus with systemic glucocorticoids
| (Transient) diabetes mellitus | Polyuria | Lethargy |
|---|---|---|
| Upper respiratory tract infections | Polydipsia | Anorexia |
| Hepatopathy | Urinary tract infections | Skin atrophy/fragility |
| Polyphagia | Diarrhoea | Pancreatitis |
| Weight gain | Melena | Demodicosis |
Topical therapy with glucocorticoids or tacrolimus may be an option when the disease is localised (Bizikova and Burrows, 2019). Good results with oclacitinib have been described recently, although in a single case report (Carrasco et al, 2021). When secondary bacterial infection is identified on cytological examination, systemic antibiotics are recommended. Ideally, the antibiotic should be selected based on culture and sensitivity testing (Jordan et al, 2019). Other immunosuppressants suggested in older papers include doxycycline, gold salts, megestrol acetate and azathioprine, which is not recommended in cats because of the high risk of bone marrow suppression.
Prognosis
The prognosis for pemphigus foliaceus in cats is good, providing that patients are followed up with frequent checks and treatment modifications are introduced as soon as required. For example, in cats requiring high doses of glucocorticoids to maintain remission, a second immunomodulatory drug should be introduced in a timely fashion. Long-term remission with discontinuation of all drugs is not uncommon in cats (approximately 15% of cases). However, the majority of cats require long-term treatment with glucocorticoids, ciclosporin or chlorambucil either alone or in combinations.
KEY POINTS
- Pemphigus foliaceus is the most common feline autoimmune skin disease, yet it only represents 1% of cases seen by veterinary dermatologists because other autoimmune skin diseases are even more rare.
- The clinical result of the detachment of keratinocytes in the upper layers of the epidermis is a pustule, which is the primary pemphigus foliaceus lesion in all affected species.
- The primary lesion is a pustule, although it is not often seen because it can be easily ruptured.
- The best diagnostic hint is a thick, yellowish, caseous exudate that can be expressed from the claw folds.
- When taking biopsy samples, always include crusts in the vial, because ‘ghost’ acantholytic cells may be present.
- Glucocorticoid monotherapy is the mainstay of treatment, but iclosporin or chlorambucil may be added to discontinue or reduce the glucocorticoid dose.


