Most dog and cat owners feed their animals commercial food, but also raw meat. According to a European survey of 5000 pet owners, an average of 19% fed raw meat to dogs and 16% to cats (McNamara et al, 2018). Reports from the United States and Australia have indicated that 97% of households with dogs provided them with mainly commercial diets, with 69% providing only commercial diets. Although owners may additionally feed their dogs table scraps, other specially prepared meals, bones, raw meat or other unprocessed products (Laflamme et al, 2008).
Owners who provide raw meat-based diets to their pets have several reasons for this. There are oft en feelings of mistrust toward commercially prepared feeds. For example, concerns about additives used or the possible presence of harmful contaminants. Furthermore, there is mistrust of the quality of the ingredients used and subsequent concerns about the influence of the production process on the availability of nutrients (Pitcairn and Pitcairn, 2005; Remillard, 2008). Allergies and other health problems are also listed as reasons for providing self-prepared food (Handl et al, 2013). Some owners may want to spoil their animal with self-prepared meals and others might think that they are giving the best to their pet. However, feeding self-prepared meals carries a risk of imbalances and deficiencies. When feeding raw meat products, there may be microbial hazards for humans and animals. It is the job of the veterinarian and technician to properly inform the owner about various feeding options and their associated benefits and risks. The optimal goal is to give sufficient amounts and correct proportions of the available nutrients, presented in a tasty and safe way.
Raw meat-based diets
Every owner is aware that fresh meat is appreciated by most dogs and cats, but so are many wet commercial foods. The smell, taste and shape of meat probably meets the instinctual need. In general, meat contains animal protein, which is easily digested, resulting in relatively little stool. However, advocates of feeding raw meat-based diets and bones are oft en very vocal about the negative side of commercially prepared feeds compared to the positive sides of raw meat-based diets (Lonsdale, 2001). Commercial foods, especially dry foods, are said to be bad for overall health, especially the teeth, coat and gastrointestinal tract. Many owners state on internet forums that after switching their pet on to a raw meat diet, they saw a miraculous improvement in the health and wellbeing of their animal. Scientific evidence to support these claims has so far failed to materialise. An explanation of this phenonema was described by Dobenecker et al (2002), who demonstrated that some dog owners, who believe in the supposed effects of a diet or drug, observed enormous improvements in dogs, even in the placebo group. Possible reasons for these observations could be the study coinciding with the onset of another treatment, a spontaneous transient improvement in clinical problems, or an optimistic perception of the effects. Another more recent publication similarly demonstrated the effect of misinformation by studying 16 500 owners from around the world, who presented their experience of the transmission of a pathogen from their pets (fed with raw meat-based diets) to the human. Such infections were reported by 0.2% of households and it was therefore concluded that this was actually a very sporadic occurrence. Furthermore, better analyses are needed before advice against raw meat feeding can be circulated (Anturaniemi et al, 2019).
Feeding rations with a high proportion of raw meat and bones was particularly publicised in the 1990s (Lonsdale, 2001). The main advocates in the discussion were the Australian vets Ian Billinghurst and Tom Lonsdale, as well as a Danish-American chemist called Mogens Eliasen. The term ‘BARF’, originally an acronym for ‘Bones And Raw Flesh’, became a widespread term for raw diets. Because the diets recommended by Billinghurst contained more than just animal products, the meaning of ‘BARF’ was later changed to ‘Biologically Appropriate Raw Food’. Lonsdale in particular seemed to be most convinced that dogs are real carnivores, who can only thrive on a diet of as many raw animal products as possible, preferably whole prey including skin and organs. Eliasen and Billinghurst recommended also including pureed fruit and vegetables in the diet. Comparable food plans nowadays are the Volhard's Natural Diet and the Schultze-Ultimate Diet. The often-heard argument from owners that we should ‘go back to nature’ and therefore feed a dog like a wolf is nonsense. The dog is not a true carnivore like the wolf, since the enzyme activity in the dog during domestication has adapted to a carbohydrate-rich diet (Axelsson et al, 2013). The wolf's diet consists of almost 100% animal prey and a single study describes their low plant intake, of up to 2-3% of total food intake (Messier and Crête, 1985). Moreover, adult wolves can survive for days and even weeks without food if they have to and the most a large grey wolf can eat at one time is about 22.5 pounds (Mech and Boitani, 2003; International Wolf Center, 2020), while dogs are served their food daily. Finally, a wolf in the wild does not have the same high life expectancy as the average domestic dog (International Wolf Center, 2020).
Health risks of raw meat nutrition
The risk of self-formulated foods is that they do not meet the same nutritional requirements as complete dog and cat foods, which are outlined in the European Pet Food Industry Federation (2020) and the Association of American Feed Control Official (2014) standards. In one survey, 90% of homemade elimination diets, where small groups of foods are eliminated to detect the cause of a food allergy, prescribed by 116 veterinarians in North America, were not nutritionally adequate for adult canine or feline maintenance. Few of the numerous homemade food recipes for dogs and cats have been tested to document performance over sustained periods (Remillard and Crane, 2010). Unlike most commercial foods, many published homemade recipes are not complete or balanced enough to fulfil animal requirements. This increases the risk of nutritional disorders. Examples include taurine deficiency, steatitis as a result of vitamin E deficiency, vitamin A intoxication in cats, and alimentary secondary hyperparathyroidism as a result of calcium deficiency in dogs and cats. Hyperthyroidism can also occur in dogs when they are fed raw laryngeal meat containing thyroid tissue (Vodégel et al, 2016). Also, owing to an unbalanced calcium-phosphorus ratio, growing pains can recur regularly, especially in fast-growing puppies of large dog breeds (Schoenmakers et al, 2000). In addition to this, feeding older animals with kidney disease a fresh meat diet can cause problems because of the high protein and phosphorus content. Feeding bones can lead to suffocation, damage to the digestive tract and constipation (German and Zentek, 2008).
Finally, feeding raw meat-based diets brings a risk of microbiological contamination. While pets are directly exposed to foodborne pathogens when they ingest food, there are several ways in which pet owners and other household members can also encounter such pathogens. This can be through direct contact with the food; through contact with a contaminated pet, such as sharing the same bed and allowing licking of the face and hands (Overgaauw et al, 2020); through contact with household surfaces; or by ingesting cross-contaminated human food. Crosscontamination may occur after preparation of raw meat or by cleaning infected food bowls on the kitchen sink (Van Bree et al, 2018). However, surveys from Europe and North America have also consistently found Salmonella spp. in fresh-frozen commercial diets. Another emerging issue concerns the risk of introducing antimicrobial-resistant bacteria. Raw pet food commonly exceeds hygiene thresholds for counts of Enterobacteriaceae. Other infectious organisms that may be of concern include Listeria, Shiga-toxigenic Escherichia coli, Brucella suis, Mycobacterium spp. (tuberculosis) and parasites such as Toxoplasma gondii and Sarcocystis spp. (Frost, 2017; Van Bree et al, 2018; Davies et al, 2019; O'Halloran et al, 2019).
This review discusses the risks of parasite infections in pets from raw meat-based diets.
Parasites and raw meat-based diets for companion animals
Parasites that may be present in raw meat-based diets are protozoa of apicomplexan genera, such as Neospora, Toxoplasma, Sarcocystis, Cystoisospora and Hammondia; nematodes such as Toxocara and Trichinella and tapeworm stages of Taeniidae (such as Taenia spp and Echinoccus spp.).
The more often raw meat is fed to pets, the greater the chance that they will be exposed to the infectious stages of parasites. The risk depends on the chance that the meat of the slaughter or prey animal is contaminated. This risk will be greater when feeding with wild caught or extensively kept production animals, than when feeding with animals from livestock farms with a higher degree of biosecurity. However, there is often not enough information available about the origin of the raw meat or the biosecurity status of livestock farms.
Protozoa
Neospora caninum
Dogs acquire Neospora caninum infection mainly by ingesting cysts containing bradyzoites located in the tissues of infected intermediate hosts, in particular cattle. Placentas from aborted cattle are the main source of infection for dogs and the feeding of raw beef has also been identified as a risk factor for canine neosporosis. Cattle, sheep, goats and other domestic and wild ungulates, as well as rodents and birds, are natural intermediate hosts of the parasite. The majority of dogs become infected after birth via transplacental transmission, so most cases of clinical (neonatal) neosporosis are reported in puppies less than 6 months old. Clinical signs which should arouse suspicion of neosporosis include hind limb paresis and ataxia, which becomes progressively more severe. The ascending paralysis can often be fatal and several litter mates can be affected (Dubey et al, 1998; ESCCAP, 2018). Neospora infections are a known problem in cattle and a prominent cause of infection-related abortion (Bartels et al, 2006; 2007).
Toxoplasma gondii
Toxoplasma gondii is a parasite similar to Neospora, except the cat is the final host. Cats usually acquire infection by the ingestion of tissue cysts, most commonly by preying on rodents and birds, or by feeding on raw or undercooked meat from infected livestock, or less commonly, on aborted material. Afterwards they can excrete large numbers of oocysts. It is likely that all mammals (including humans, cats and dogs), as well as birds, can act as intermediate hosts where tissue cysts occur in meat and tissue products. Acute toxoplasmosis is rare in cats. Kittens infected in utero can show signs of infection after birth and these prenatal infections are frequently fatal (ESCCAP, 2018). In dogs (mainly puppies), neurological complaints may be associated with Toxoplasma infections, when a pregnant bitch infects herself by eating raw meat with tissue cysts or by absorbing sporulated oocysts from the environment. Mainly lamb, but also beef is a real risk when it is consumed after insufficient cooking (Opsteegh et al, 2011).
Sarcocystis spp.
For many Sarcocystis species, dogs and other carnivores serve as the definitive host, while livestock might serve as intermediate hosts, carrying cysts in their tissues. For example, cattle are intermediate hosts of Sarcocystis cruzi, S. hirsuta and S. sinensis, but the definitive hosts of the latter are not yet known. Sheep are intermediate hosts of S. tenella. The prevalence rates in the intermediate hosts (sheep, cattle, pigs with outdoor access) are up to 100%, because of the ubiquitous distribution of the parasites. Carnivorous animals become infected by ingesting undercooked meat containing tissue cysts (ESCCAP, 2018). In the definitive host, the development of the parasite is restricted to the final stages and does not cause clinical signs. The clinical and hygienic importance of infection with Sarcocystis is restricted to the intermediate host, where outbreaks as a result of faecal contamination of feedstuff or water and can result in clinical signs. Cysts in carcasses can lead to meat condemnation. Therefore, it is advisable not to feed raw meat diets to dogs that live on livestock farms.
Cystoisospora spp.
The genus Cystoisospora is host-specific; Cystoisospora canis, C. ohioensis and C. burrowsi are the common species that infect dogs and cause intestinal coccidiosis, especially in young dogs. From a study of dogs in Belgian kennels, 26.3% were found to be infected with this parasite compared to 2% of domestic dogs (Claerebout et al, 2009). It is unknown whether these shedding animals were infected after eating raw meat, but it is a possible route of infection, after the faeco-oral route, via the ingestion of sporulated oocysts. Paratenic hosts, including rodents and ruminants, play a role in the cycle of these protozoa, after oral uptake of oocysts.
Hammondia spp.
Two species of Hammondia parasitise cats and dogs – H. hammondi and H. heydorni respectively. The life cycle resembles that of other cyst-forming coccidia, such as Sarcocystis, Neospora and Toxoplasma. Dogs and cats are the definitive hosts and acquire the infection after ingestion of infected meat (warm-blooded animals). The infection usually takes a subclinical course. Very rarely, anorexia and severe diarrhoea, which are unresponsive to antibacterial therapy, have presented in infected puppies (ESCCAP, 2018).
It is worth noting there is no risk that protozoa will be transmitted to either animals, human beings or the environment, when the raw meat is sold frozen. Freezing meat at −20°C for 1–2 days inactivates protozoa such as Sarcocystis spp., T. gondii and N. caninum (Gestrich and Heydorn, 1974; Kotula et al, 1991; Lindsay et al, 1992).
Nematodes
Toxocara spp.
Roundworms are the most common intestinal nematodes in dogs and cats. Toxocara canis is a parasite affecting the dog and T. cati affects the cat. The prevalence of patent infections in dogs varies with age (especially puppies), ancestry (high infestation in strays and shelters) and frequency of deworming. In Europe, the average prevalence was 14.6% for T. canis in all dogs. For young dogs up to 6 months old, this was 41.2%, for mature dogs it was 11.1%, and 17.6% in stray dogs. The prevalence seemingly has not decreased over the last 25 years (Overgaauw and Nijsse, 2020). Infections can result in serious gastrointestinal complaints in puppies (Nijsse et al, 2015).
Eating meat infected with larvae differs from becoming infected after ingestion of embryonated eggs from the environment. The larvae that hatch from the eggs undergo a tracheal migration in the dog and cat, in order to develop a patent infection. Egg shedding starts earlier when larvae are ingested via raw meat or prey, because these larvae no longer have to go through the entire migration process, instead they mature at the intestinal level. In the human alimentary canal, toxocarosis may occur from ingestion of larvae. This result from the consumption of raw vegetables and undercooked organ and muscle tissue of infected paratenic hosts, such as chickens, cattle and sheep (Overgaauw, 1997). Infections are reported in various paratenic hosts and in the meat of consumption animals (Davidson et al, 2012; Strube et al, 2013; Oliveira et al, 2018).
Trichinella spiralis
Trichinellosis is a parasitic disease caused by the nematode Trichinella spiralis. Wild meat-eating animals can be infected, but generally there are no signs of trichinellosis in dogs. Most infections in domestic and wild animals go undiagnosed (Miterpáková et al, 2017). People become infected when they eat undercooked infected meat, usually pork or bear and severe infections may produce serious illness and occasionally death. European Union legislation establishes special rules for the control of trichinellosis, including the requirement for systematic tests for Trichinella in all slaughtered pigs, wild boar and horses. Therefore, there is no risk of dogs contracting a Trichinella infection as a result of consuming these animals.
Tapeworms
Echinococcus granulosus and E. multilocularis
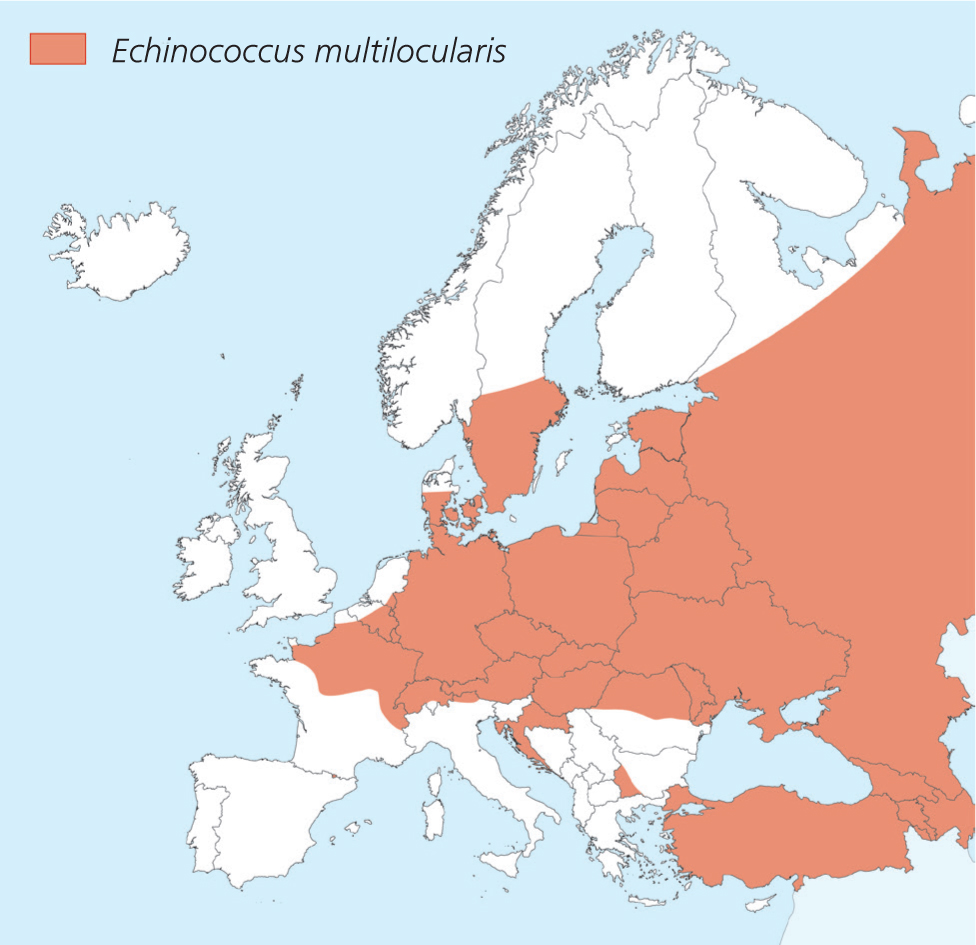
Echinococcus spp. can pose a risk to animals and humans (zoonosis). The distribution of the fox tapeworm E. multilocularis is increasing in Europe (Figure 1). This cestode inhabits the small intestine of foxes, raccoon dogs, some other canids, dogs (rarely) and very seldom, in cats. Intermediate hosts are small rodents that contain cysts (alveolar echinococcosis). If dogs eat these prey animals, adult tapeworms can develop in their intestines, releasing proglottids with eggs that are excreted in the faeces. In this way, dogs, in addition to foxes, can pose a risk to other animal species and public health (ESCCAP, 2020). In Germany, it was found that 0.2% of more than 20 000 examined dogs were shedding E. multilocularis eggs in their faeces (Dyachenko et al, 2008). Meat from endemic areas can pose a risk.
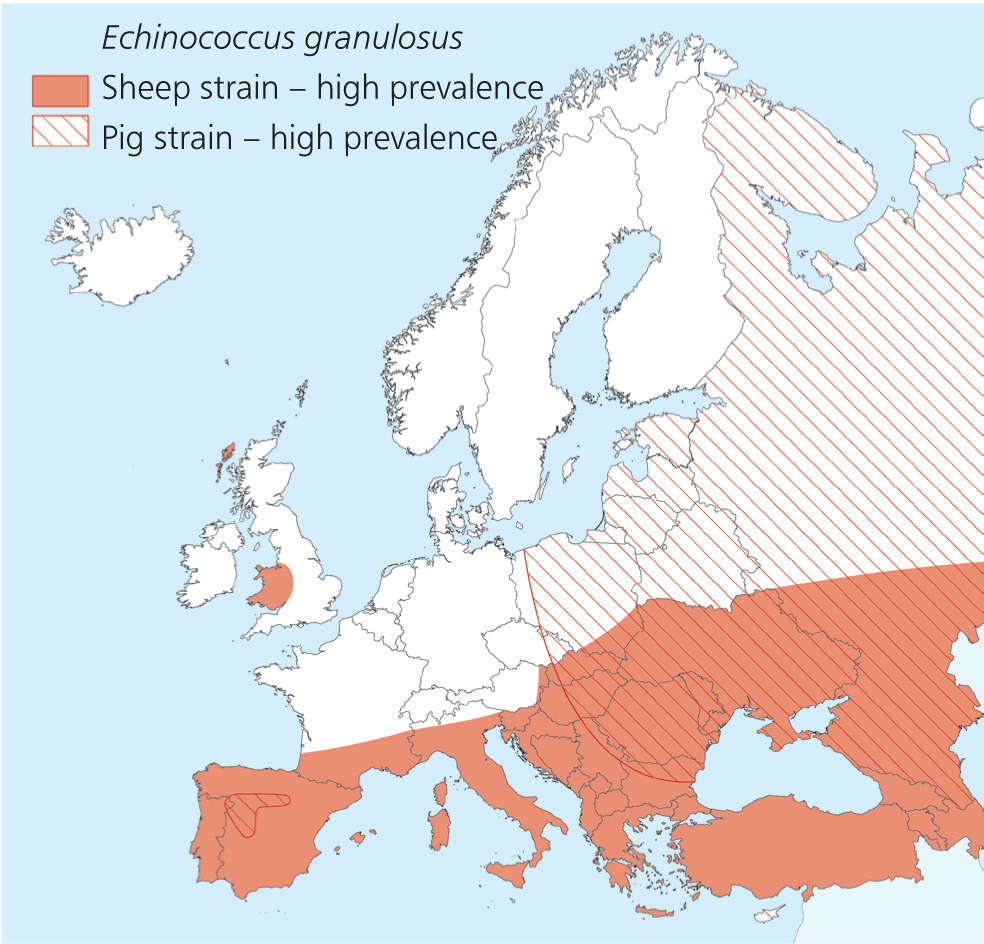
E. granulosus (dog tapeworm) is a small cestode that inhabits the small intestine of dogs and some other canids, excluding foxes. Consumption animals are the intermediate hosts (cystic echinococcosis). The parasite is endemic to southern and eastern Europe and western Great Britain (Figure 2). Raw meat and organs from animals from these areas should therefore not be fed to pets. Infection of intermediate hosts, such as the human, occur following the oral ingestion of the immediately infective eggs or proglottids, excreted in the faeces of the definitive hosts.
Taenia spp.
Dogs and cats can be final hosts of various Taenia species and are a risk to other animal species (intermediate hosts) where cysts are formed. The final host does not become ill from the infection, but intermediate hosts such as rodents, lagomorphs and ruminants can develop disease (Table 1). In the slaughtering process, the cysts, if large enough, can be detected. Therefore meat which has passed inspection, will present a smaller risk of infection with these tapeworms for the dog. Catching and eating prey animals can be more of a risk in endemic areas.
Table 1. Taenia spp. found in dogs and cats
| Final hosts | Dogs | Cats | |||||
|---|---|---|---|---|---|---|---|
| Species | Taenia multiceps | Taenia serialis | Taenia crassiceps* | Taenia pisiformis | Taenia hydatigena | Taenia ovis | Taenia taeniaeformis |
| Prepatent period (appr. in weeks) | 6 | 4–6 | 6–8 | 7–10 | 6–8 | 5–10 | |
| Intermediate host | Sheep, goats and cattle | Rabbits (and rodents) | Rodents | Rabbits/hares (and rodents) | Sheep, goats, cattle and pigs | Sheep and goats | Rodents |
| Intermediate stage and site | Coenurus larvae in brain and spinal cord | Coenurus larvae in connective tissue | Cysticercus larvae in body cavities or subcutaneous tissue | Cysticercus larvae in abdomen or liver | Cysticercus larvae in abdomen or liver | Cysticercus larvae in muscles | Strobilocercus larvae in liver and abdomen |
Conclusions
This article mainly discusses the parasitic infections of dogs and cats that can occur after they are fed raw meat-based diets or eat prey animals. The risks of feeding other animal products, such as raw eggs and fish, have not been considered. To prevent (zoonotic) parasitic infections in pets, risk analysis is of great value. Risk is the multiplication of hazard and exposure. The hazard depends on the prevalence of parasite stages in raw meat or prey animals. Unfortunately, these data are mostly lacking and it is therefore not easy to perform a good risk analysis for the aforementioned parasites. However, exposure is dependent on eating infected raw meat or prey. Infections can lead to disease in the animals themselves, but also in humans and other species of animals, while contamination of the environment can take place with eggs or oocysts. Although regularly reported anecdotally, it is unknown how often parasite infections occur as a direct result of eating raw meat. In addition, the origin of the raw meat fed to companion animals is often insufficiently known. Freezing does inactivate protozoa such as Toxoplasma and Sarcocystis, but not viruses, bacteria or Toxocara spp. The safest advice is therefore to give complete commercial food or to cook raw meat before feeding.
KEY POINTS
- Feeding companion animals raw meat-based diets carries the risk of the animal contracting parasitic infections.
- Parasites that may be present in raw meat and organs, and those for which dogs and cats are part of the life cycle, include several nematodes, tapeworms and protozoa.
- Infections can induce disease in intermediate hosts, but also in humans and other animal species, while the environment can be contaminated with oocysts or eggs.
- The best prevention method is to feed complete commercial food or to cook raw meat before feeding.

