Sugar gliders (Petaurus breviceps) are small, nocturnal, arboreal marsupials native to New Guinea and the Eastern coast of Australia. They are social animals, and in native conditions will live in a colony of 6–10 individuals occupying an area of up to 1 hectare (Johnson-Delaney, 2021). In captivity, it is essential that they live with other conspecifics, as a solitary sugar glider will be at high risk of developing stress induced self-mutilation. Sugar gliders are more frequently being seen in veterinary practice.
Sugar gliders require a large enclosure with branches and shelves at various levels to encourage their normal behaviour of jumping and climbing. Sugar gliders will need access to a sleeping area, such as nest boxes or fabric sleeping pouches. They are omnivorous and will need a diet to replicate what they would have in the wild, where they eat sap and gums, nectar and pollen, manna and honeydew, as well as a variety of invertebrates (Johnson-Delaney, 2014).
In captivity, sugar gliders should be provided with a nectar food source, gut-loaded insects such as Dubia roaches and crickets as well as other protein sources, and a limited portion of fruits and vegetables (Johnson-Delaney, 2014). Commercial diets are also available, but published scientific feeding trials are scarce, therefore these should not be used as the only source of food. An artificial nectar, known as Leadbeater's, can be fed to sugar gliders as part of a varied diet. This mixture can be batch made at home and frozen in ice cube trays. The frozen mixture can also be useful to keep in veterinary practices that regularly see sugar gliders, as it can be thawed on the day required and fed immediately post-surgery. To make Leadbeater's mix 150ml water, 150ml honey, one shelled hard-boiled egg, 25g high protein baby cereal and one teaspoon of vitamin and mineral powder such as Nutrobal. This can be refrigerated (or frozen and thawed) and an adult glider can be fed one tablespoon of this daily. As well as this artificial nectar, an adult glider can also be fed one tablespoon of a commercial diet, such as a zoo-based insectivore diet, half a teaspoon of chopped mixed fruits such as mango and banana, and 1–3 adult gut-loaded insects. . For enrichment, fresh food items can be offered in the form of forage, which is best given in the evening as sugar gliders are nocturnal (Johnson-Delaney, 2021).
Male sugar gliders weigh 100–160g and females 80–130g. They have soft grey dense fur with a black stripe within the dorsal midline. A gliding membrane (patagium) stretches between their front and hind limbs (Figure 1). They have five toes on their hind feet, with the first digit being opposable. Female sugar gliders have a pouch containing four teats. Sugar gliders have a cloaca, a terminal opening for the rectum, genital tract and urinary ducts. Average heart rate is 200–300 beats per minute and respiratory rate is 16–40 breaths per minute (Booth, 2000). The metabolism of marsupials is approximately two-thirds that of eutherian (placental) mammals. The average cloacal temperature is 32°C, which is cooler than actual body temperature of 36.3°C (Johnson and Hemsley, 2008).

The testes are located in a prepenile pendulous scrotum. The penis is bifurcated, with urination occurring from the base and not the forked tip (Figure 2). Males have a large prostate gland and two pairs of bulbourethral (Cowper's) glands.
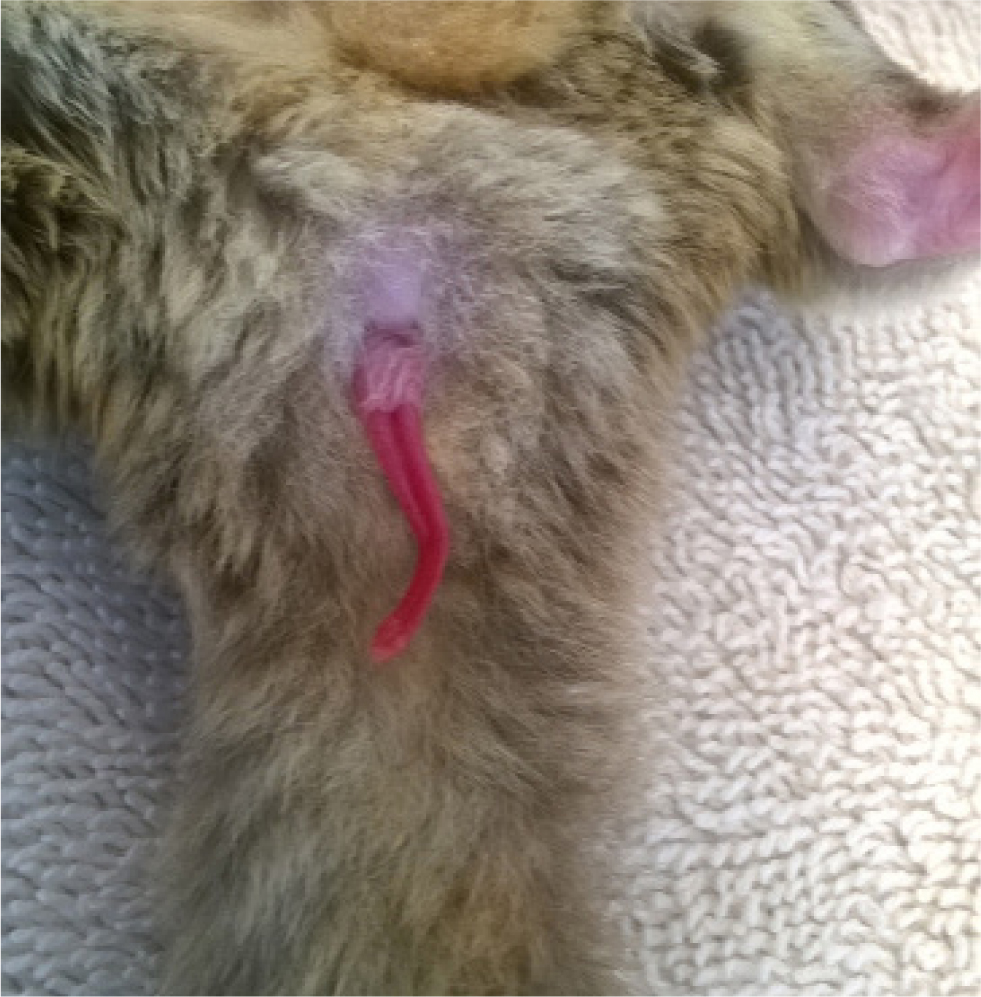
Female gliders have have two uteri running medially to two long, thin lateral vaginas. Both male and female gliders have three paired paracloacal glands.
Breeding season in native habitats occur between June and November. Sugar gliders are seasonally polyoestrous and the oestrus cycle is 29 days. They are polygamous, with the dominant male breeding with all the mature females within the colony. Litter size is often two joeys, with the young migrating to the pouch after a 15–17 day gestation period, weighing only 0.2g. At around 70–74 days, the joeys emerge from the pouch and remain in the nest until weaning at 110–120 days old.
With sugar gliders being kept as pets, normally as mixed sexed colonies, it is important that males are neutered to prevent unwanted offspring. Castration is a simple process, but a full understanding of the anatomy and anaesthesia and analgesia is needed to ensure a smooth surgical recovery and prevent incidences of self-mutilation.
Anaesthesia
It has been documented that a sugar glider should be fasted for approximately 4 hours before anaesthesia for castration (Pye and Carpenter, 1999). However, in the authors' experience, because of the concerns of hypoglycaemia affecting the anaesthesia, food has been safely withdrawn for 30–60 minutes before anaesthetic without any complications.
Sugar gliders are prone to self-mutilation after surgery. To minimise this, multimodal analgesia is recommended (Table 1). Pre-medication with buprenorphine and midazolam is beneficial to minimise fluctuations in blood pressure and heart rate during induction. Induction is via sevoflurane or isoflurane in an induction chamber with maintenance on oxygen and a volatile anaesthetic gas at 2–3% via a facemask (Figure 3). It is preferable to use sevoflurane, if possible, owing to the adverse smell associated with isoflurane and the increased risk of causing irritation to the mucous membranes (Guedes et al, 2017; Kubiak, 2021). Larger sugar gliders can be intubated with a 1mm Cook endotracheal tube aided with a stylet and a fine-bladed laryngoscope.
Table 1. Formulary.
| Drug | Dose |
|---|---|
| Midazolam | 0.25–0.5 mg/kg intramuscular or subcutaneous |
| Buprenorphine | 0.01–0.03 mg/kg subcutaneous or per os every 12 hours0.05 mg/kg intramuscular (Keller et al, 2014)0.06mg/kg intramuscular (Dr Sophie Jenkins) |
| Ketamine | 2–5mg/kg intramuscular |
| Meloxicam | 0.2 mg/kg per os or subcutaneous every12–24 hours for 3–4 days0.5 mg/kg per os or subcutaneous 12–24 hours (SJ) |
Adapted from Morrisey and Carpenter (2021)
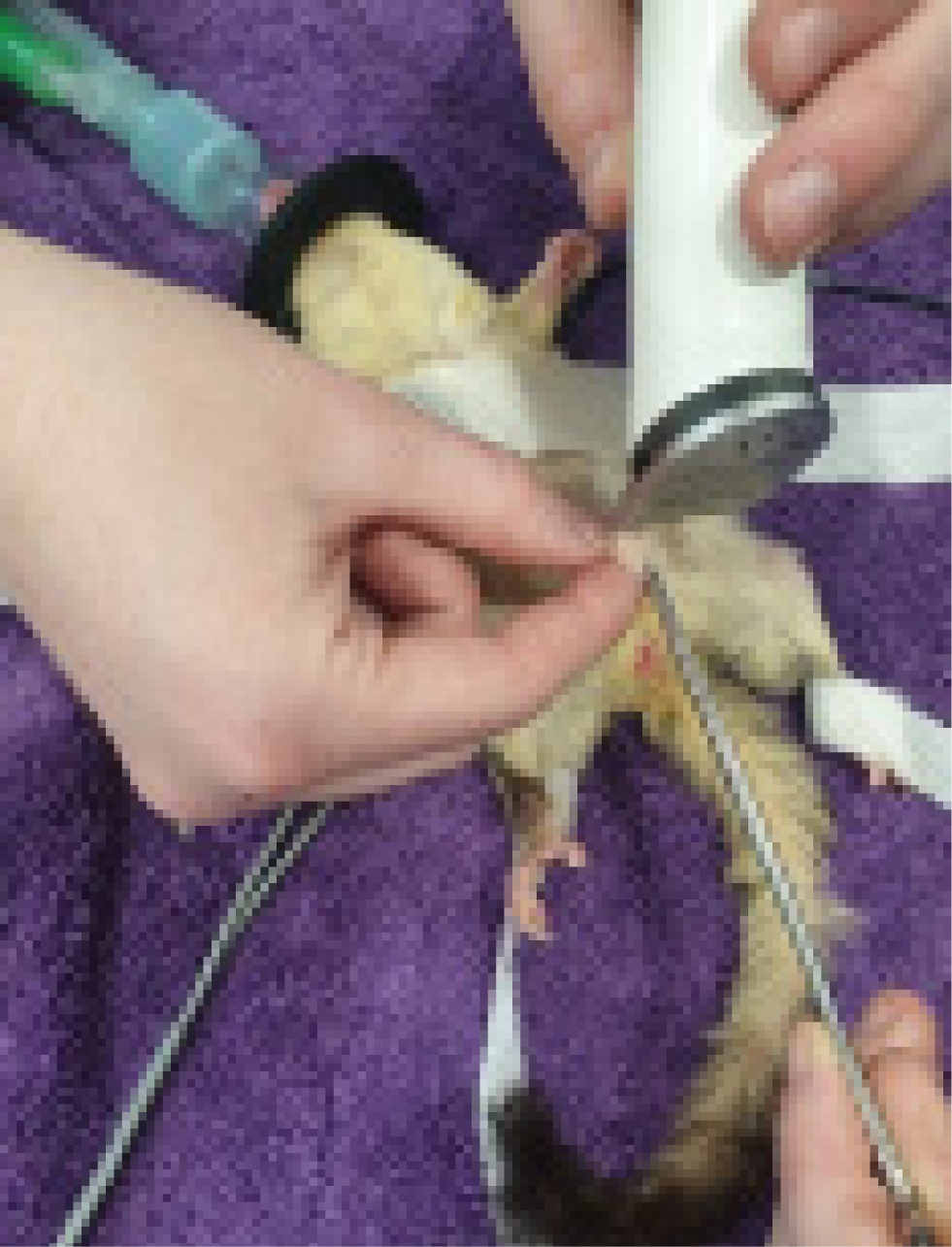
For additional analgesia, the authors recommend using local anaesthesia. Lidocaine at 1mg/kg, diluted at a ratio of 1:10 in saline solution is divided between each testicle and injected directly into the base of the stalk and the testicle. After the procedure, bupivacaine at 1mg/kg is administered via a splash block before closure. Local anaesthesia is administered to reduce the contraction of the cord during ligation and to reduce the occurrence of self-mutilation of the surgical site (Miwa and Sladky, 2016). Meloxicam is given after the procedure. One of the authors (Sophie Jenkins) will administer meloxicam at 0.5mg/kg subcutaneously and then postoperatively will dispense meloxicam orally twice daily for 5 days and if required tramadol at 5mg/kg per os for 3 days.
To aid in a smooth, rapid recovery and to minimise the risk of inducing torpor, preoperative body temperature must be maintained. Castration of sugar gliders is a relatively quick procedure, so warmed subcutaneous fluid (2–3ml Hartmann's solution) is sufficient. It is important to avoid injecting in the patagium as this would be very painful. Intravenous catheterisation for intraoperative fluids is often difficult. On recovery, the authors recommend having a sugar glider sap food ready as sugar gliders will often eat straight away. This helps distract and prevent interference of the operation site and will also help maintain glucose levels.
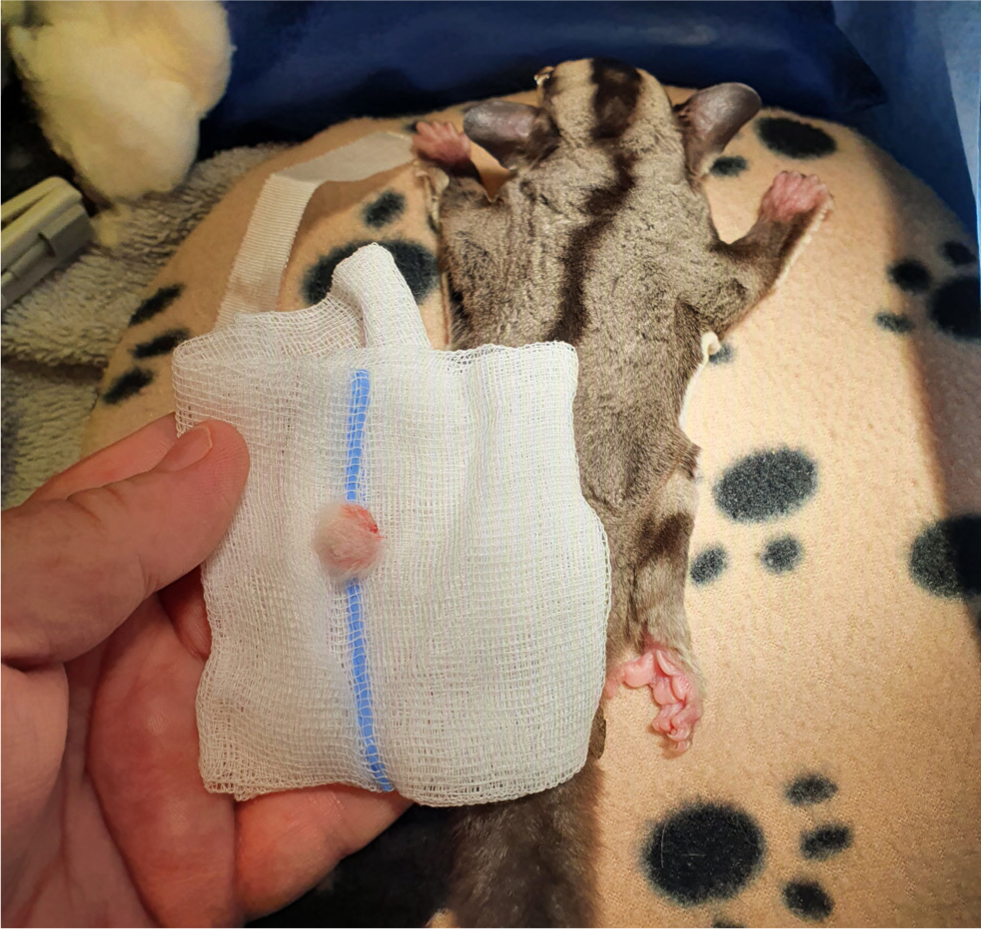
Self-mutilation is not uncommon in sugar gliders and often occurs because of stress or pain. In the authors' experience, with provision of multimodal analgesia intra- and postoperatively, by closing the skin with tissue glue instead of a skin suture, using a K-laser after surgery and by providing food on recovery, the use of an Elizabethan collar is rarely necessary. In one case, cardboard was used to make an Elizabethan collar. Alternatively, a rodent Elizabethan collar can be cut down to prevent trauma to the surgical site on recovery. Once the sugar glider had recovered and eaten, the collar was no longer necessary (Figure 4).
Orchidectomy and scrotal ablation
Castration is a fairly simple, commonly performed elective procedure for population control. Castration may also be requested as a method to try and control self-mutilation, especially mutilation of the genitalia (Raftery, 2015). Surgery can be performed either with a carbon dioxide laser, by radiosurgery, or a surgical K-laser or blade can be used. To minimise postoperative trauma, a laser can be used to incise in transverse section. The laser cuts and cauterises, so the procedure is quicker and suture ligation is not required. If using radiosurgery or laser technique, place a tongue depressor between the sugar glider's ventral abdomen and the scrotal stalk to protect the skin of the ventral abdomen.
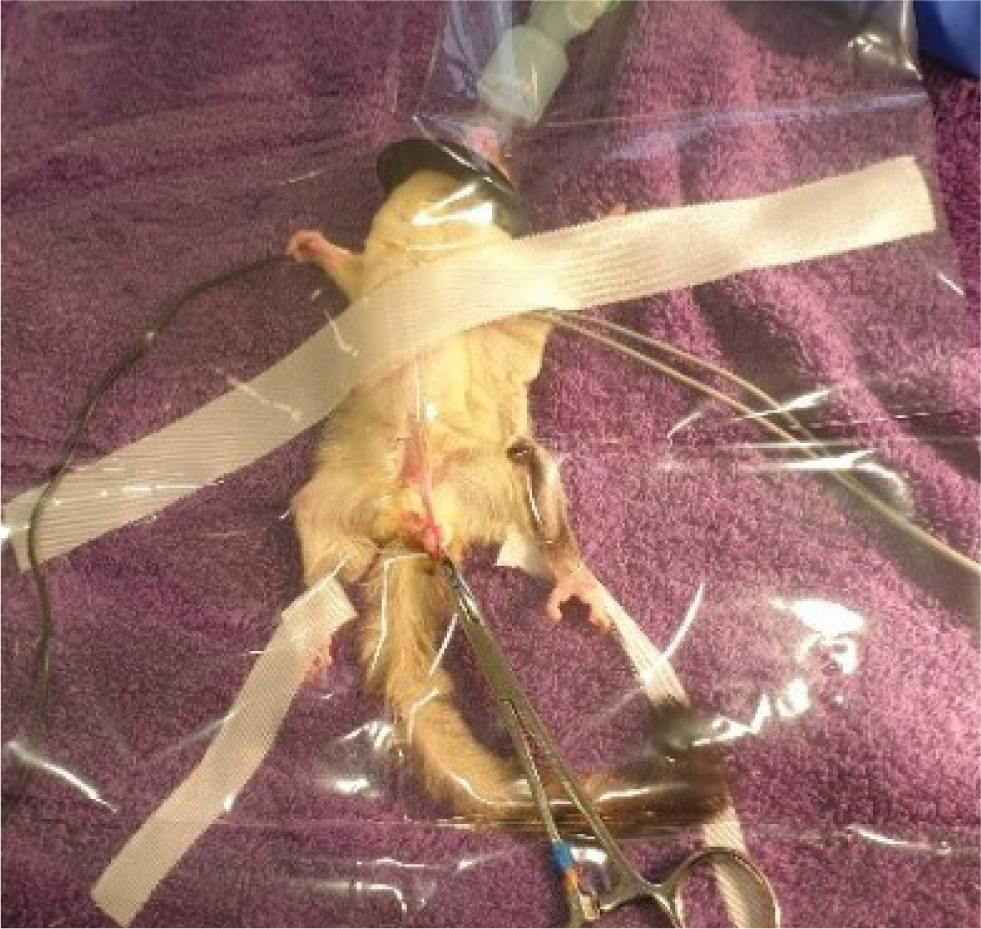
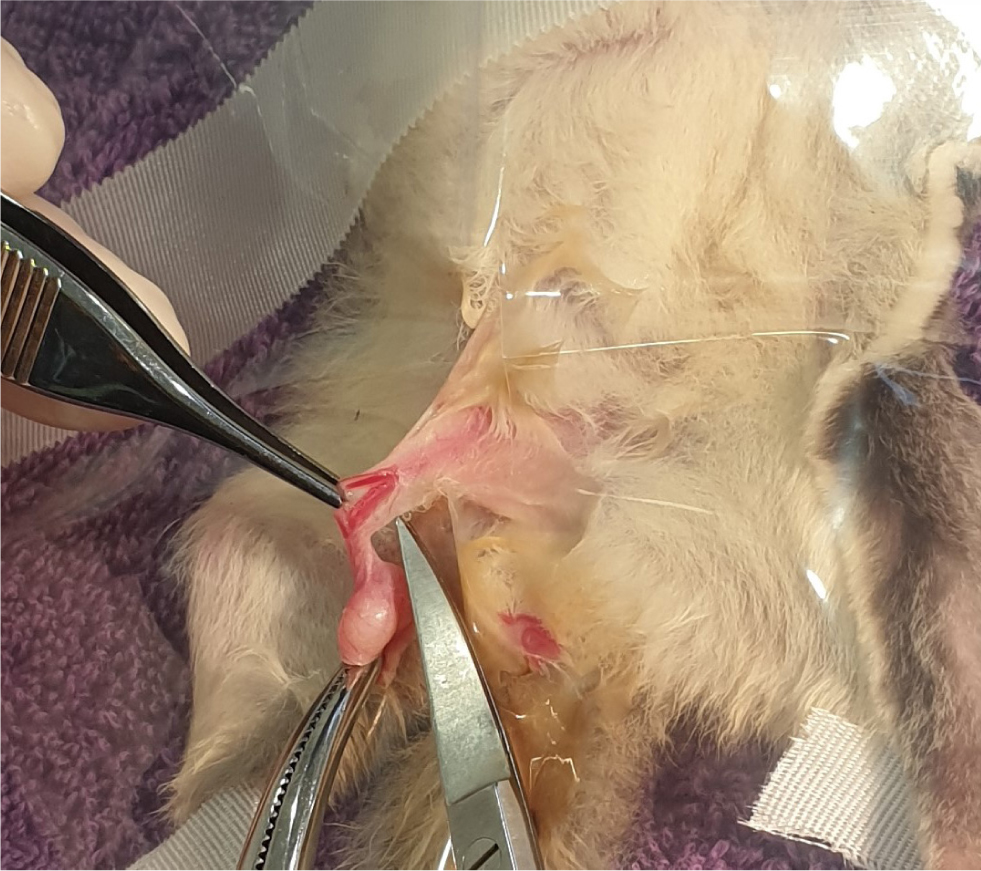
Traditional surgery can be used if laser or radiosurgery is not an option. Surgical preparation is important, and the use of mini clippers (Figure 5) is best for avoiding accidental skin damage. The use of transparent drapes aids visual monitoring of the patient (Figure 6), in addition to the use of monitoring equipment such as a cardiac doppler, rectal thermometer and electrocardiogram. A longitudinal incision is made approximately 1cm from the body wall on the stalk, to expose the spermatic cords (Figure 7). Blunt dissection is used to expose the blood vessels and vas deferens. The cords and blood vessels are clamped and ligated using a monofilament suture material to minimise tissue drag and tissue reaction, such as 1.0 metric or 5-0 imperial polydioxanone. The scrotal sac, testicles and the distal stalk are removed (Figure 8). A suture is placed incorporating the ligated stalks in the abdominal fascia to prevent herniation (Johnson-Delaney, 2002; Morges et al, 2009; Johnson-Delaney, 2010). Tissue glue can be used to close the skin incision or alternatively, the skin can be held together for a few seconds and a K-laser can be held over the area to aid in healing. Postoperative analgesia is essential, as previously described in Table 1.
Ovariohysterectomy
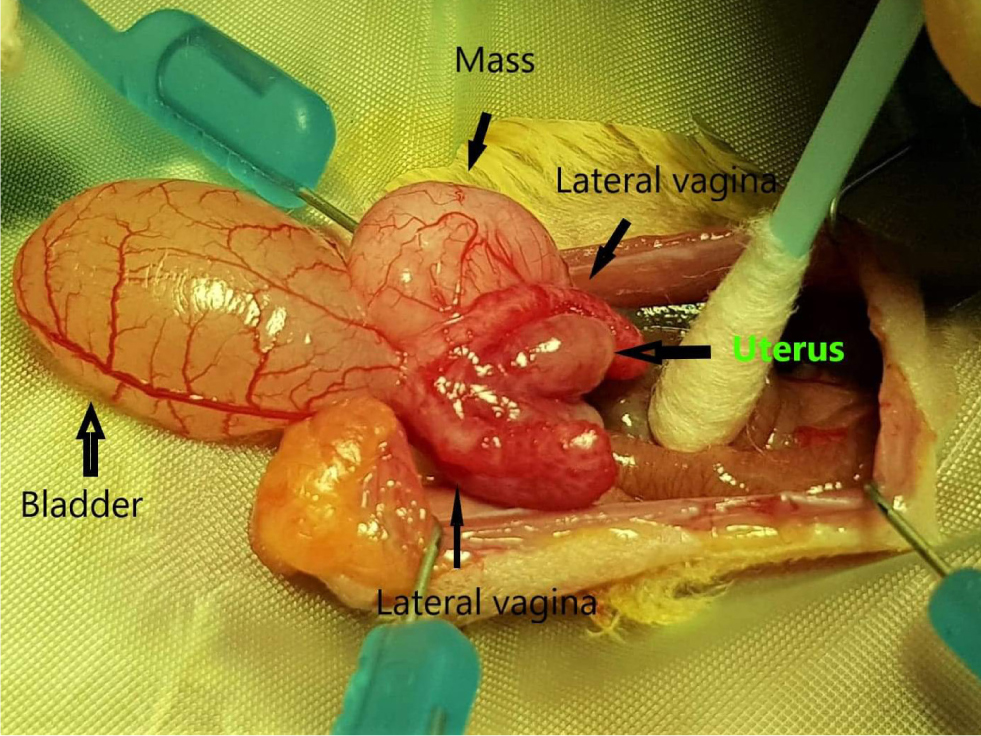
This is performed less frequently, and not usually as an elective procedure owing to the unique reproductive anatomy of female marsupials. Indications for ovariohysterectomy would include pyometra, tumours and other reproductive pathology. It is worth noting that in marsupials, there are two lateral vaginas which are separated along their length by a soft tissue median septum, referred to as the median vagina. The lateral vaginas open separately into the urogenital sinus at the level of the urethral opening (Vogelnest, 2015). The ureters pass medially along the genital ducts and are in very close proximity to the reproductive system. Therefore, the surgeon must be mindful when handling the delicate tissue as to not damage the ureters. Figure 9 illustrates the female reproductive tract.
If an ovariohysterectomy needs to be performed, a paramedial incision to the pouch is made at approximately 1–2 cm in length. It is essential to leave enough skin along the edge of the pouch for closure (Johnson-Delaney, 2021; Ness and Johnson-Delaney, 2012). The linea alba is located by blunt dissection, and the abdominal cavity is entered. The reproductive tract is visualised by carefully exteriorising the bladder. The ovaries are identified as small red granular structures. The ovarian branch of the ovarian artery is ligated. The uterus is ligated and excised at the level of the cervix above the lateral vaginal canals (Johnson-Delaney, 2021). Closure is routine and skin closure is with subcuticular sutures using 4/0–5/0 monofilament suture material. The authors recommend cold laser therapy postoperatively to aid healing and to minimise self-trauma at the operation site.
Summary
Sugar gliders are popular companion animals and as clinicians, we must be aware of their normal anatomy and be able to provide a suitable anaesthetic regime and analgesia when performing common elective procedures such as castration. Being aware of the options for suitable analgesia will prevent potential postoperative complications such as self-mutilation.


