Canine atopic dermatitis (CAD) is a genetically predisposed inflammatory and pruritic skin disease with easily recognisable clinical signs; the appearance of clinical signs is associated with immunoglobulin E (IgE) antibodies, most commonly against environmental allergens (Olivry et al, 2015a). The complex pathogenesis of CAD is not fully understood; it has been implied from human medicine that atopic dogs may have epidermal dysfunction, causing increased penetration of environmental allergens and pathogens. Consequently, this leads to a T helper 2 (Th2) cell-mediated inflammation and further perturbation of the epidermis (Elias and Schmuth, 2009; Marsella et al, 2011; Santoro et al, 2015). In addition, atopic dogs have microbial dysbiosis of their skin microbiome, making them more susceptible to developing secondary infections (Santoro et al, 2015; Pierezan et al, 2016).
The diagnosis of CAD is a diagnosis of exclusion and confirmed by a combination of history, clinical presentation, no response to a strict exclusion–provocation elimination diet and a good response to anti-inflammatory, immunomodulatory or antipruritic medication (Table 1). Very simply, there are three ways in which CAD is treated:
- Acute flare treatment
- Long-term treatment
- Proactive treatment.
Table 1. Favrot et al’s (2010) criteria for canine atopic dermatitis
| 1 | Onset of signs under 3 years of age |
| 2 | Dog living mostly indoors |
| 3 | Glucocorticoid-responsive pruritus |
| 4 | Pruritus sine materia at onset (i.e. alesional pruritus) |
| 5 | Affected front feet |
| 6 | Affected ear pinnae |
| 7 | Non-affected ear margins |
| 8 | Non-affected dorso-lumbar area |
When five criteria are met the sensitivity is 85% and specificity 79%. When six criteria are met specificity increases to 89% but sensitivity decreases to 58% (Favrot et al, 2010).
These criteria are now over a decade old and were validated before the publication of many revealing studies, such as the difference of breed phenotypes for canine atopic dermatitis (Wilhem et al, 2011), multiple studies on canine barrier function and the release of several new treatments (lokivetmab and oclacitinib).
Each time the diagnosis of CAD is confirmed the approach to the case should be individualised and treatment selected with client factors in mind, such as compliance issues and cost. Furthermore, treatment should be multifaceted; knowledge of the different medications and therapies available will increase the chance of remission and minimise the possibility of side effects (Olivry and Sousa, 2001).
This article revises the treatment of CAD — acute flares versus long-term treatment — and discusses the importance of communication for compliance, medical adherence and patient health.
Treatment of canine atopic dermatitis
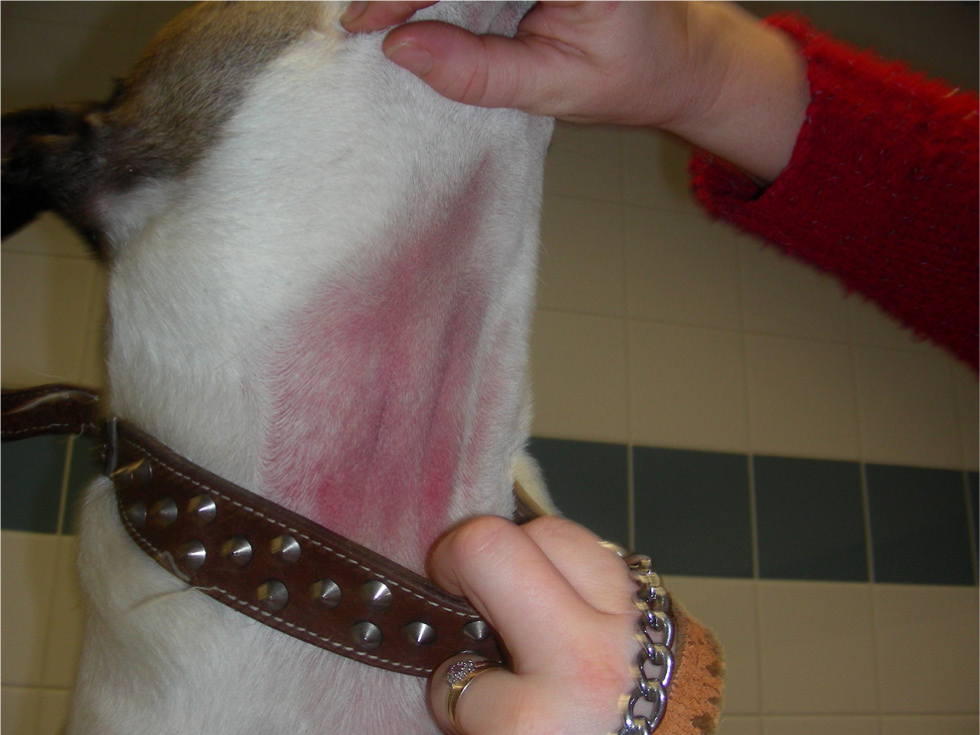
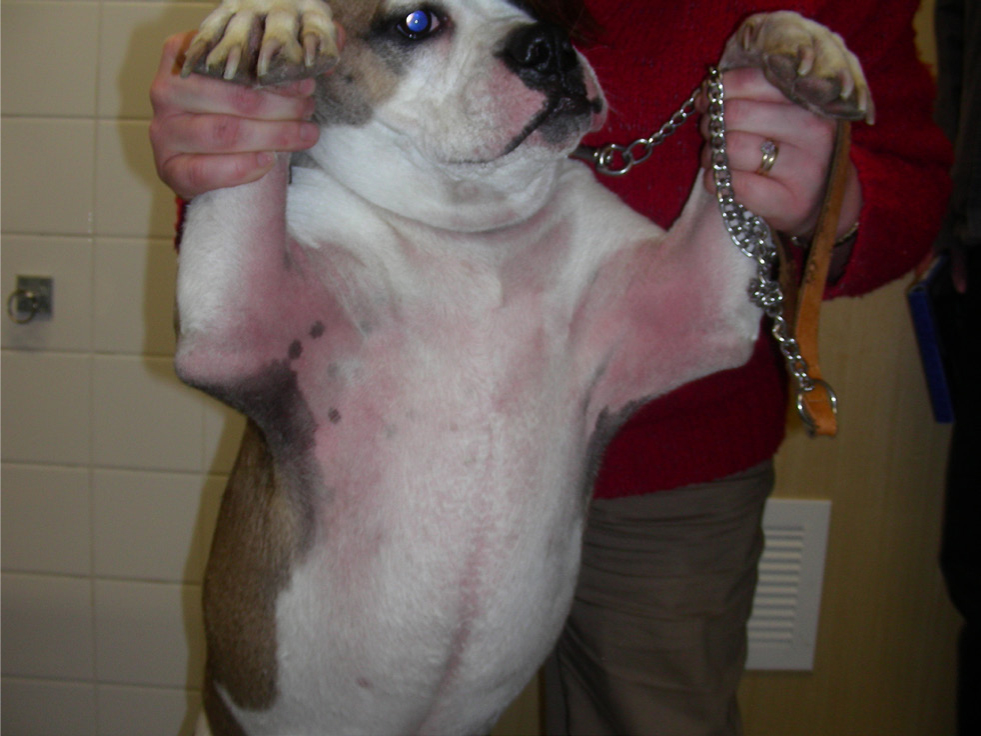
Treatment of acute flares It is a recognised phenomenon that dogs with atopic dermatitis will suffer acute flares when exposed to an allergen to which they are hypersensitive (e.g. flea saliva, dietary proteins and house dust mites). In the event of an acute flare, an attempt should be made to identify the trigger and eliminate it; human studies have identified environmental (e.g. low humidity, clothing and detergents) and psychological (e.g. stress) issues as flare factors for atopic patients (Morren et al, 1994).
In addition to environmental allergens, bacterial and/or yeast infections of the skin and/or ears can also cause acute flares and should be treated appropriately (Olivry et al, 2015a; Santoro et al, 2015).
To help control acute flares, treatment with topical and/or systemic anti-inflammatory or antipruritic medication is required (Olivry et al, 2015a). Topical glucocorticoid treatment is helpful for localised lesions that are erythematous and/or pruritic (Nuttall et al, 2009; Nam et al, 2012). The study performed by Nuttall et al (2009) monitored the topical use of hydrocortisone aceponate for up to 70 days in atopic dogs and no adverse effects were reported. However, topical corticosteroids remain unlicensed for long-term use because of the reported risk of developing cutaneous atrophy (Kimura and Doi, 1999). Innovations in topical treatments have also suggested that some shampoo and mousse combinations may be useful in settling flares, but independent studies are still to be published (Gatellet et al, 2020).
When topical therapy is insufficient to control acute flares, systemic treatment can be initiated with a short course of oral glucocorticoids (prednisolone, prednisone or methylprednisolone) or oclacitinib (Olivry et al, 2015a). The use of oral glucocorticoids should always be evaluated on a case-by-case basis and avoided in patients with widespread pyoderma. Glucocorticoid side effects are dependent on each individual and directly proportional to potency, dosage and duration of administration (Olivry et al, 2015a); when given orally they can be managed more easily by the administrator. The safe, short-term alternative to oral glucocorticoids is oclacitinib (Cosgrove et al, 2013; Gadeyne et al, 2014); co-administration of oral glucocorticoids and oclacitinib should be avoided because of the potential of a combined immunosuppressive effect.
Furthermore, when a rapid response to either oral glucocorticoids or oclacitinib is not observed, differential diagnoses and/or secondary problems (e.g. infection, ectoparasitism and non-atopic food reactions) should be reconsidered (Olivry et al, 2015a).
The second-generation consensus guidelines for the treatment of CAD published information on interventions that are of little or no benefit when managing acute flares (Olivry et al, 2015a):
- Type 1 antihistamines — there is no conclusive evidence for their efficacy
- Oral essential fatty acids — the length of time required for a beneficial effect makes them unhelpful in the event of acute flares
- Calcineurin inhibitors (e.g. ciclosporin) — their slow onset of action makes them an unsuitable antipruritic for acute flares.
Long-term treatment
Long-term treatment of CAD differs to the treatment of acute flares as flare factors, pruritus treatment and prevention strategies are all addressed.
Flare factors
As part of the identification of flare factors, dogs with perennial atopic dermatitis should undergo a dietary exclusion–provocation trial to determine the involvement of food; dogs should be fed a hydrolysed or novel protein diet for at least 8 weeks (Olivry et al, 2015b). In addition, atopic dogs should be treated throughout the year with effective ectoparasite control as they are predisposed to developing hypersensitivity to flea salivary antigens and possibly more prone to contracting sarcoptic mange (Carlotti and Jacobs, 2000; Taszkun, 2011). The study performed by Dryden et al (2013) revealed that oral spinosad provided more reliable flea control than topical spot-on fipronil/(S)-methoprene, highlighting the importance of appropriate flea control for atopic dogs.
Identification of hypersensitivity to environmental allergens in atopic dogs can be demonstrated by intradermal allergy testing and/or IgE serology; dogs without clinical signs of atopic dermatitis can have positive reactions thus the tests are not used to differentiate atopic from non-atopic dogs (Lian and Halliwell, 1998). The main purpose of intradermal allergy testing and/or IgE serology is to provide more information about allergen hypersensitivities before the formulation of allergen-specific immunotherapy (Willemse, 1986). Moreover, allergen avoidance strategies are sensible to consider but often impractical and generally unproven. There is still no evidence to support the use of IgE serology for the diagnosis of food hypersensitivity in dogs with food-induced atopic dermatitis (Mueller and Olivry, 2017); food hypersensitivity can only be diagnosed following a dietary exclusion–provocation trial.
Atopic dogs are generally hypersensitive to house dust mites regardless of geography (Swinnen and Vroom, 2004). Therefore, it is reasonable to implement house dust mite control measures for atopic dogs; however, the most effective control measures still need to be determined. The study completed by Swinnen and Vroom (2004) showed that treating the home environment of mite-hypersensitive atopic dogs with benzyl benzoate led to a decrease of atopic clinical signs. Following on from this, anecdotally, atopic dogs may benefit from being moved to outdoor kennels, sleeping on impermeable mattress covers, regular cleaning of their home environment and possibly the use of allergen-neutralising sprays.
Dogs with atopic dermatitis are more predisposed to acquiring skin and/or ear infections because they have a defective skin barrier, allowing penetration of commensal microbes and overstimulation of the immune system (Elias and Schmuth, 2009; Marsella et al, 2011; Santoro et al, 2015). Clinical signs and infection develop as a result of a combination of both microbial virulence and the host response (Casadevall and Pirofski, 1999). When infection does occur, and is diagnosed with or without cytology, appropriate treatment should be initiated: topical and/or systemic. The use of antimicrobial therapy should comply with official and up-to-date guidelines, for example Responsible use of Antimicrobials by the British Small Animal Veterinary Association (2020), and Advice on Responsible use of Antimicrobials by the Federation of Companion Animal Veterinary Associations (2018).
To relieve pruritus and physically remove allergens and microbes from the surface of the skin, atopic dogs should be bathed at least once weekly with an appropriate shampoo: emollient, anti-seborrhoeic and/or antiseptic. If the skin starts to become dry post bathing, a moisturiser can be added to the protocol or the shampoo changed. In addition, oral essential fatty acids improve both skin barrier function and hair coat appearance thus it may be beneficial to include essential fatty acids into the long-term treatment plan; they can be administered as an oral supplement or enriched diet (Olivry et al, 2015a).
Pruritus treatment
The hallmark criteria of CAD is pruritus; treatment should reduce pruritus thereby controlling self-trauma to help resolve skin lesions. The study by Nuttall et al (2011) showed topical treatment with hydrocortisone aceponate versus oral treatment with cyclosporin equally reduced pruritus in dogs with atopic dermatitis. However, CAD is typically treated with systemic medication and multiple randomised controlled trials have been published to confirm the efficacy of several different medications available (Olivry et al, 2010; Olivry and Bizikova, 2013). To date the four anti-inflammatory, immunomodulatory and antipruritic medications include:
- Oral glucocorticoids (prednisolone, prednisone or methylprednisolone). For rapid remission of clinical signs oral glucocorticoids are an effective anti-inflammatory and after a short induction period, they should be tapered to the lowest dosage and frequency to maintain stability and minimise side effects (Olivry et al, 2010; Table 2). For this reason, long-acting injectable glucocorticoids should be avoided. One of the side effects of chronic glucocorticoid therapy is an increased risk of developing urinary tract infections, thus patients receiving oral glucocorticoids long term should routinely have their urine analysed with cytology and/or cultured.
- Oral ciclosporin. Oral ciclosporin also has anti-inflammatory effects and works by inhibiting different cell types involved in allergic reactions: Langerhans cells, lymphocytes, eosinophils and mast cells (Marsella, 2005). Oral cyclosporin takes 4–6 weeks to have a clinical effect, so initially it can be administered with oral glucocorticoids or oclacitinib (Dip et al, 2013; Panteri et al, 2016). Once remission is achieved, the dose should be tapered by either increasing the dosing interval or decreasing the daily dose (Table 2). Side-effects from initial use are most commonly gastrointestinal, and some uncommon, well documented, and usually reversible side effects are seen with chronic use (Olivry and Mueller, 2003; Olivry et al, 2010; Olivry and Bizikova, 2013). Repeat urinalyses have been recommended, as per glucocorticoids, to screen for occult urinary tract dysbiosis or infection (Radowicz and Power, 2005).
- Oclacitinib. Oclacitinib is a janus kinase (JAK) inhibitor; it has been identified that atopic dogs have increased levels of circulating interleukin-31 (IL-31) and this cytokine binds to JAK receptors on neurons, causing the release of pruritogenic signals and the manifestation of pruritus (Gonzales et al, 2013). Oclacitinib has a fast onset of action and is administered, in dogs over 12 months’ old, twice daily for 2 weeks, then once daily (Cosgrove et al, 2013; Gadeyne et al, 2014; Table 2). If remission is achieved with once daily administration, the dose should be tapered further if remission is still maintained; nevertheless, long-term, once daily administration appears to be safe (Cosgrove et al, 2013). Some dogs will require off-label twice daily administration to remain controlled long-term. Pre-treatment screening for neoplasia and monitoring periodically with complete blood counts and serum biochemistry when on long-term treatment is recommended.
- Lokivetmab. The newest antipruritic available is lokivetmab. Compared to the other medications, it has an extremely specific mode of action: it is a monoclonal, anti-canine IL-31 antibody and binds to and neutralises circulating IL-31 (Michels et al, 2016). Randomised controlled trials performed so far have revealed lokivetmab to have a rapid onset of action, suppress pruritus for up to 4 weeks, and to have a favourable safety profile (Michels et al, 2016; Moyaert et al, 2017; Table 2). It is licensed for use in dogs of all ages and is the ideal choice in the face of concurrent infection.
Table 2. Treatment for chronic canine atopic dermatitis
| Oral glucocorticoids | 0.5–1 mg/kg SID/BID PO for 7–14 days then taper to the lowest possible alternate day dosage |
| Oral ciclosporin | 5 mg/kg SID PO for 4–6 weeks, 1–2 hours before food, then decrease the frequency of dosing/total daily dose |
| Oclacitinib | 0.4–0.6 mg/kg BID PO for 14 days then SID +/– decrease the frequency of dosing/total daily dose |
| Lokivetmab | 1.0 mg/kg Q 4 weeks SC +/− increase the dosing interval |
Alongside these medications, there is some evidence to suggest that ultra-micronised palitoylethanolamide, a naturally occurring, endocannabinoid-like, bioactive lipid compound, may prove to be useful adjunctive medication (Noli et al, 2015).
Prevention strategies
As part of the prevention strategy for CAD, allergen-specific immunotherapy is a very safe and potentially efficacious treatment option; it is reported to be effective in 60–70% of atopic dogs. Allergen-specific immunotherapy is the only treatment option that works to reverse the pathogenesis of atopic dermatitis and it does this by influencing a reduction in effector cell activity (eosinophils, basophils and mast cells), followed by an immunological shift (from Th2 cell to Th1 cell) and immunological tolerance (DeBoer, 2017).
It is reported that allergen-specific immunotherapy will take months to have a clinical effect, thus anti-inflammatory/immunomodulatory/antipruritic medication will be required concomitantly during the initiation phase (Dell et al, 2012). Allergen-specific immunotherapy should be administered for at least 1 year to completely evaluate its efficacy and if considered effective, injection frequency and dose should be individually adjusted (Olivry et al, 2015a). In the authors’ experience, although off-licence, pulse dosing of topical hydrocortisone aceponate can be used as an effective proactive measure for atopic dogs with skin and/or ear issues.
For example, allergic otitis externa can be controlled with a small, measured volume of topical hydrocortisone aceponate applied on 2 consecutive days a week. This treatment helps prevent the initiation of pruritus and secondary infection and in mild cases may be used as monotherapy (Nuttall et al, 2009; Lourenço et al, 2016; Bergvall et al, 2017).
Communication, compliance and medical adherence
In veterinary medicine compliance is defined as ‘the degree of client adherence to doctor recommendations and prescribed treatment protocols’ (Wayner and Heinke, 2006). To help veterinary professionals appreciate and understand what is involved in achieving compliance, the American Animal Hospital Association devised the formula:
Compliance (C) = Recommendation (R) + Acceptance (A) + Follow-Through (FT).
Wayner and Heinke (2006) simplified the CRAFT formula further to:
Compliance = Communication.
Communication is key to optimal patient care and is therefore dependent on the skill of veterinary professionals (Shaw et al, 2004; Wayner and Heinke, 2006). Not only does effective communication lead to improved patient health through acceptance and understanding, it also nurtures client relationships and promotes a healthy working environment (Stobbs, 1999; Shaw et al, 2004). When good communication is compromised, it causes a breakdown of client relationships, misunderstanding and loss of confidence (Stobbs, 1999). Human medicine studies have also shown that communication impacts patient morbidity (Stewart et al, 1999).
In human medicine the complexity of non-compliance has been studied and divided into two categories: unintentional versus intentional. Patients that unintentionally do not comply may do so because of misunderstanding, drug administration issues and language barriers; patients that intentionally do not comply make that conscious decision for reasons such as medication concern(s) and personal belief(s).
On top of this categorisation, patients may additionally fail to comply due to forgetfulness, stress and a busy lifestyle (Cochrane et al, 1999). This model of non-compliance can be extrapolated and applied to veterinary clients owning pets that require regular, long-term medication. Furthermore, regardless of the type of non-compliance, good communication from the outset will prevent the issue of non-compliance, or resolve it, while treatment is in place.
Medical adherence is an important topic in human medicine and in the review by Stewart et al (1999) four factors were identified to affect medical adherence:
- Patient education. A positive correlation exists between patient education and compliance; the more concise the medical instructions are the higher the compliance (Garrity, 1981). In the literature there are many suggestions to improve medical adherence, for example simple directions, repeating instructions, checking for understanding and using different forms of communication (Stewart et al, 1999)
- Mutual expectations. The paper by Golin et al (1996) reviewed the literature on patient–doctor relationships and how this affected medical adherence in patients with diabetes; they reported that disagreement or mismatch between patient and doctor expectations can lead to a decrease in medical adherence
- Shared decision making. It has been shown that patients that acquire an active role in their treatment decisions are more likely to be adherent (Garrity, 1981; Stewart et al, 1999)
- Positivity. Emotional support, friendliness, empathy and a good patient–doctor relationship all appear to encourage and increase medical adherence (Squier, 1990; Stewart et al, 1999).
Conclusions
Acute flares of CAD can be treated topically with hydrocortisone aceponate or systemically with oral glucocorticoids or oclacitinib. The mainstay of treatment for CAD is anti-inflammatory/immunomodulatory/antipruritic treatment and the options available are oral glucocorticoids, oral cyclosporin, oral oclacitinib and injectable lokivetmab. Allergen-specific immunotherapy is used as safe individualised therapy and is reported to be effective in 60–70% of atopic dogs. As CAD is a chronic skin disease requiring treatment for life, it is important to establish a healthy relationship with clients to achieve compliance and medical adherence; essentially, effective communication with clients leads to improved patient health.
KEY POINTS
- Treatment of canine atopic dermatitis should depend on whether presentation is acute or chronic, and clinical signs are localised or generalised.
- Acute flares should be treated by identifying the trigger and eliminating it, if possible, and with proven fast-acting topical and/or systemic treatment.
- Long-term treatment for canine atopic dermatitis involves identifying flare factors, providing anti-inflammatory/immunomodulatory/antipruritic treatment and prevention strategies.
- The essence of achieving compliance, medical adherence and improved patient health is effective communication.


