A 5-year-old, female, Springer Spaniel presented with a widely dilated pupil in the left eye. A history of blue cornea was noted 2 days before. The owner felt that she seemed to have lost her vision in that eye, as she had bumped into things a couple of times on that side. The dog seemed depressed, had stopped eating the previous night and was now reluctant to walk. The owner had noticed these changes and wondered whether they had any relationship to her recent spay.
On arrival, the dog's left eye had a brisk dazzle reflex but did not respond to a menace and had no pupillary light reflexes (direct or consensual). The neuro-ophthalmic examination of the fellow eye was within normal limits.
Question 1 — Describe the ocular abnormalities (Figure 1a, 1b).
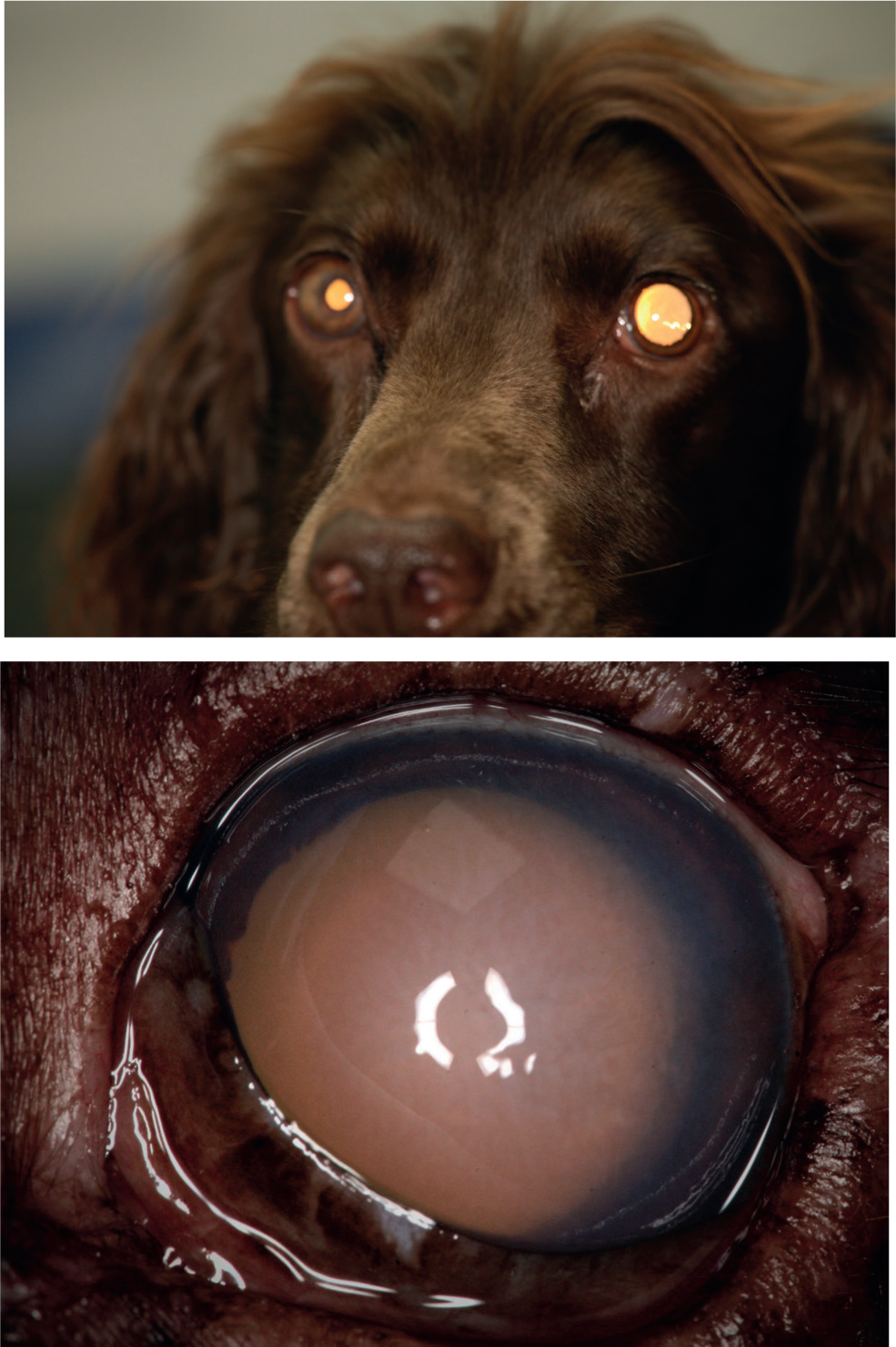
Answer — The left eye had mild protrusion of the third eyelid, episcleral congestion (not easy to see on the photographs), diffuse corneal oedema and fixed mydriasis (aniscoria).
Question 2 — The intraocular pressure (IOP) was measured with a TonoVet as 67 mmHg. What is the most likely diagnosis?
Answer — The high IOP (normal range 15–18 mmHg in the dog) along with the clinical signs lead to a diagnosis of glaucoma.
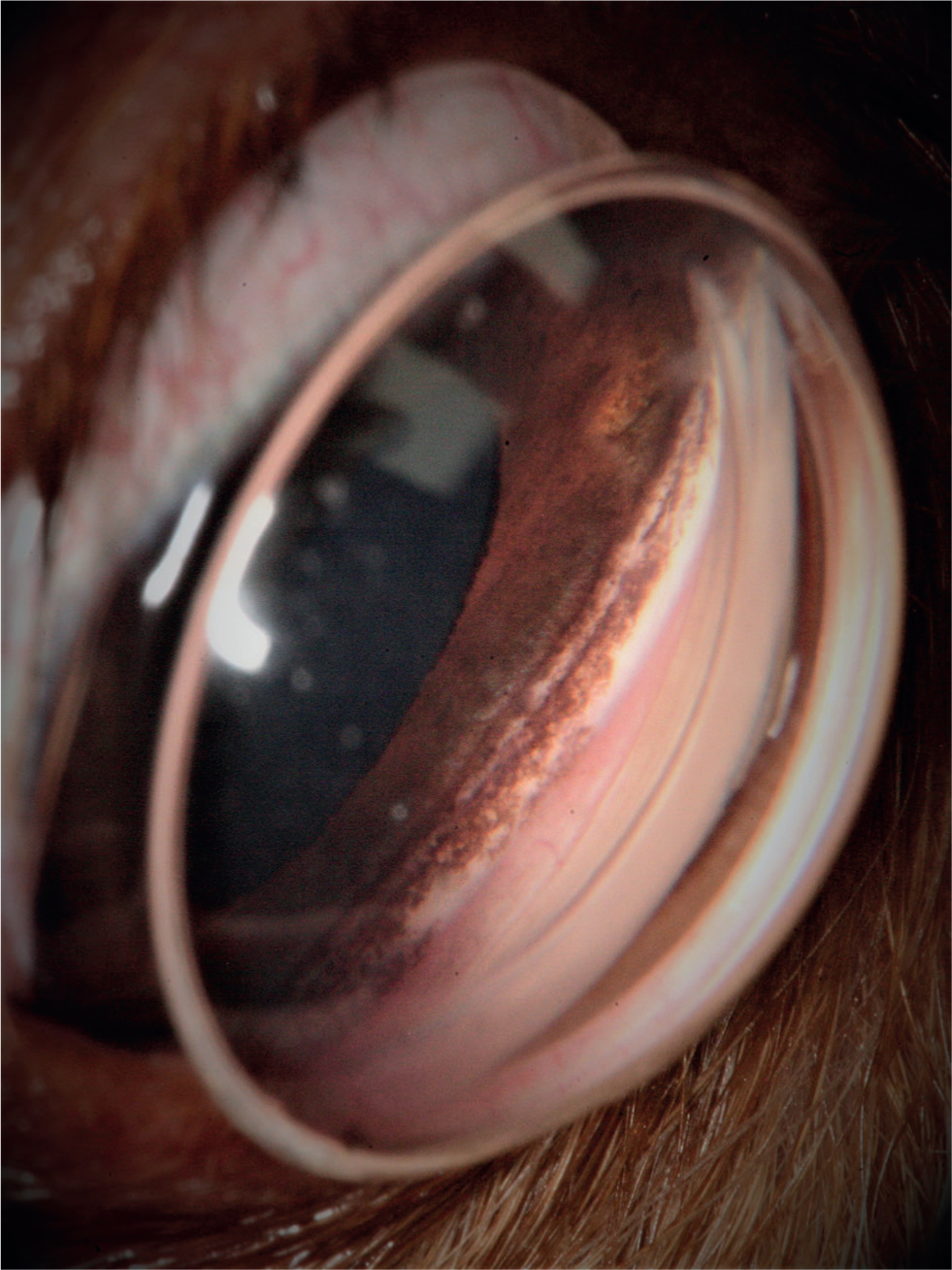
Question 3 — What test should be performed and what additional information is this adding to the diagnosis?
Answer — Gonioscopy is performed on the fellow normotensive eye (right eye). This is a diagnostic technique used to study the iridocorneal angle (ICA), between the iris and the cornea. The ICA of the dog cannot be directly visualised. This is because light rays returning from the ICA exceed the critical angle of the cornea to air interface and are totally internally reflected. To overcome this problem, gonio lenses (Figure 2) are used to assess the ICA. Accurate gonioscopy is important in the classification of glaucoma (primary or secondary and open or closed angle) and therefore its appropriate management.
Question 4 — The image of the left (Figure 3a) and right (Figure 3b) fundus are pictured. Describe the clinical findings.
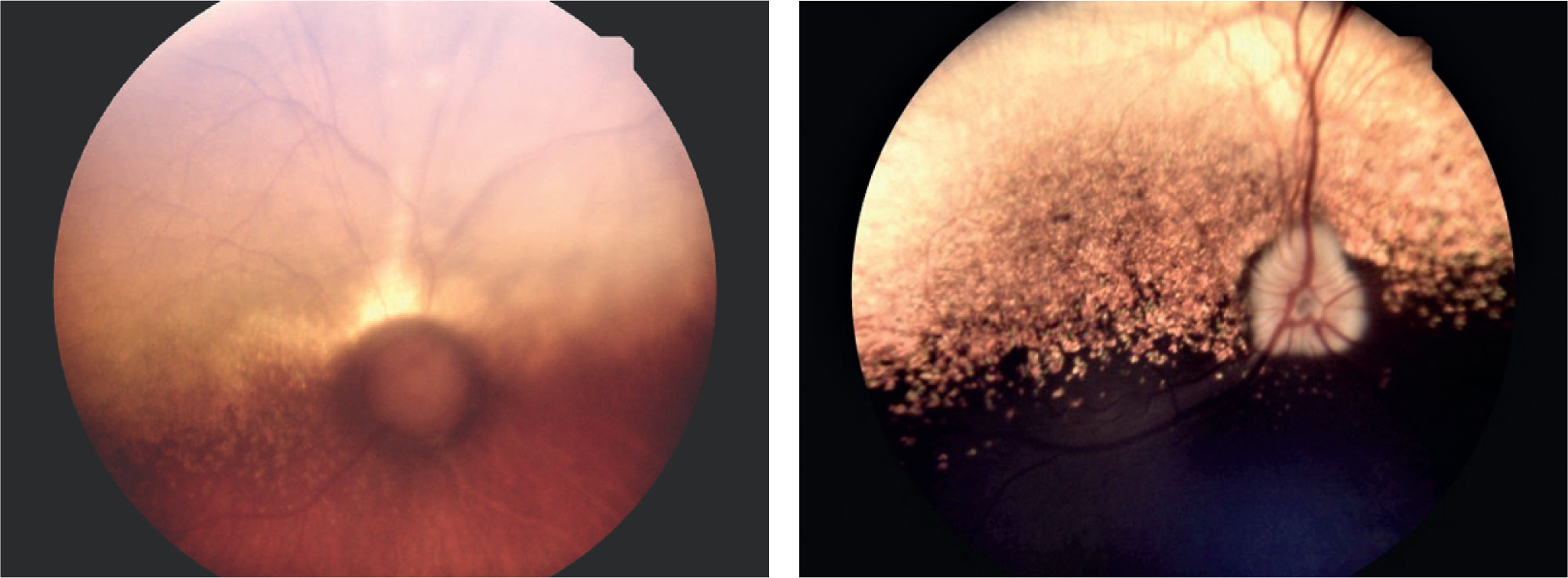
Answer — In the left glaucomatous eye, the fundus and optic nerve changes are consistent with chronic elevation of the IOP. The optic nerve head is round in shape and appears demyelinated and dark. The retinal vessels dip sharply over the edge of the optic cup. A peripapillary region of tapetal hyperreflectivity is present along with moderate retinal vessel attenuation. In the right eye, a normal retina and a healthy, well myelinated optic nerve is noted.
Question 5 — What is the characteristic presentation in the optic disk?
Answer — The centre of the optic nerve head is not in focus at the level of the retina, it has moved posteriorly. This phenomenon is called optic cupping and is consistent with optic nerve axonal and ganglion cell death. When the IOP rises, the scleral lamina cribrosa, the perforated region of sclera where the optic nerve fibres and blood vessels exit the eye, is stretched and distorted posteriorly. This distortion compresses the optic nerve fibres, reducing their axoplasmic flow (mechanical theory), as well as potentially disturbing the optic nerve head blood supply (vascular theory).
Question 6 — What is the clinical significance of the optic nerve head pathology on vision?
Answer — At the time of glaucoma diagnosis, the apoptotic cascade (programmed cell death of the retinal ganglion cells) has already started and will continue even in the presence of controlled IOP. The ultimate aim of glaucoma treatment is to delay this process and maintain functional vision for as long as possible. If the eye is left untreated and the IOP is not lowered, this damage will eventually lead to blindness in a shorter time frame.
Question 7 — What are the treatment options for this condition?
Answer — Both medical and surgical treatment options are available for the treatment of glaucoma. It is important to understand that there is no cure for glaucoma, we can only manage the disease aiming for the retention of vision and maintenance of a normal IOP. Treatments can either aim to lower the production of aqueous humour and/or increase the outflow. Which treatment is selected depends on whether there is visual potential.
Medical treatments aimed at reducing the production of aqueous humour include: topical carbonic anhydrase inhibitors (e.g. dorzolamide, brinzolamide); and beta-adrenergic antagonists (e.g timolol). Oral carbonic anhydrase inhibitors (e.g. acetazolamide) have also been used to reduce aqueous humour production but with marked systemic side effects. Topical prostaglandins (e.g. latanoprost, travoprost) and parasympathomimetic drugs (e.g. pilocarpine) act increasing the aqueous humour outflow. Hyperosmotic agents (mannitol, glycerol) can also be used temporarily to lower the IOP by dehydrating the vitreous.
In eyes with the potential for vision, surgical therapies can similarly be classified into those decreasing aqueous humour production by ablation of the ciliary body (for example trans-scleral or endoscopic laser cyclophotocoagulation and cyclocryotherapy), and those increasing the outflow of aqueous humour (for example gonioimplants). In irreversibly blind eyes and those with intractably high IOPs, enucleation is more appropriate. In some cases, evisceration with an intrascleral implant or a cyclo-destructive procedure (e.g. intravitreal gentamicin injection) may be other options.
Question 8 — This dog eventually had surgery for glaucoma. What is the function of the device seen in the anterior chamber of this dog's eye (Figure 4)? When is this surgical procedure indicated?
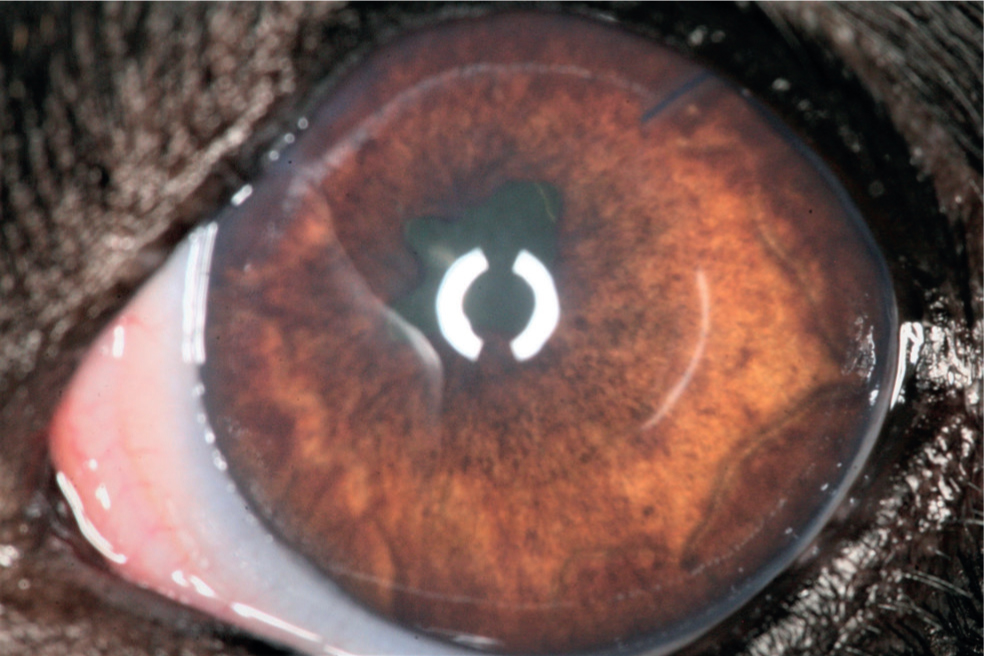
Answer — The tube of a non-valved (Molteno implant) is in the anterior chamber (dorsolateral quadrant), this is a shunting device designed to bypass the abnormal ICA and drain aqueous into the subconjunctival space. This procedure is indicated when the eye has visual potential and is unresponsive to medical treatment.
Definition of glaucoma
Historically, the definition of glaucoma in veterinary medicine was an elevation of IOP (Gelatt et al, 2013). However, glaucoma is not that simple. Glaucoma is currently defined as the final common pathway of a group of different diseases most of which are associated with increased IOP, which does not permit the optic nerve and retina to function normally, leading to neural degeneration and blindness (Shaarawy et al, 2009; Miller and Bentley, 2015; Pizzirani, 2015)
Pathophysiology
In order to understand glaucoma, one must be aware of the way the aqueous humour is produced and how it exits the eye. Aqueous humour is produced by the non-pigmented epithelium of the ciliary body by active secretion and passive processes (Pizzirani and Gong, 2015). In the conventional outflow route, aqueous humour passes through the pupil into the anterior chamber and drains the ICA. At the ICA, aqueous humour passes between the strands of the pectinate ligament and flows through the sponge-like trabecular meshwork before draining into the scleral veins and general circulation via the avascular aqueous plexus. A small portion of aqueous outflow occurs via the supraciliary and suprachoroidal space, the unconventional or uveoscleral pathway.
Clinical symptoms
The clinical signs have been described earlier, it is important to emphasise the effect this disease can have on a dog's quality of life. An acute increase in IOP is typically associated with headache and nausea in humans (Yanoff and Duker, 2018). It is therefore likely that animals experience a similar degree of pain. Blepharospasm or rubbing of the eye can be present. Glaucoma should be considered in dogs with a wide range of symptoms such as altered behaviour (depression, reduced activity or aggression), reduced appetite or vomiting.
Outcome
Glaucoma commonly masquerade as non-ocular illness, often leading to delayed diagnosis and treatment, increasing the chance of a poor outcome (Alario et al, 2015). The longer the IOP remains increased, the lower the chance of vision retention. Irreversible vision loss can occur very rapidly. At diagnosis, vision has very often been in lost in the affected eye due to owners' failure to recognise symptoms early, such cases usually end up requiring enucleation. Histopathology of the globe is strongly advised (Komáromy et al, 2019).
Primary glaucoma in dogs is a bilateral disease, retention of sight is therefore dependent on early recognition of the problem in the fellow remaining eye. Prophylactic treatment and regular ophthalmic examinations including IOP checks are recommended.


