Snakes and lizards are commonly seen in practice now. Large numbers of certain species, such as Royal or ‘Ball’ pythons (Python regius); Inland Bearded dragon (Pogona vitticeps) and Veiled chameleon (Chamaeleo calyptratus), are bred readily in captivity, so are relatively inexpensive to purchase and are thus widely kept. Therefore, there is a growing expectation that all veterinary practitioners should be able to examine and treat these species - and also the lower financial investment in the animal may result in decreased incentive to seek out more specialised vets, to pay for more involved investigations, or to drive further to these centres
The special features of snakes' and lizards' highly adapted skin include the formation of scales and the necessity to regularly shed the skin at intervals to allow their growth. This inevitably presents a different range of diseases to the mammals more normally seen in small animal clinics and requires a different approach to their investigation (Figure 1). Most importantly, when dealing with reptiles, their poikilothermic status means that environment and husbandry issues are particularly important to consider and a full husbandry evaluation must be carried out in all cases.
While a wide range of species may be encountered in practice, the differential diagnoses discussed in this article are seen commonly across many of these species groups. This article looks at the most common differential diagnoses based on clinical signs and discusses a practical approach to investigating these cases, as well as how to treat or manage them.
In this article drug doses are not given as they may vary between the many species, and because reptile therapeutics is a rapidly evolving field, meaning dose rates are often changing. Readers are encouraged to research drug doses regularly in referenced formularies (Carpenter and Harms, 2022; Hedley, 2023) and in the wider peer-reviewed literature.
Role of environment and stress
Environment and stress play major roles in development of reptile disease, including skin disease. These stressors will also affect prognosis, as effects on the reptile's immune system will influence the degrees of pathogenicity and invasiveness of potential pathogens. A variety of factors contribute to the environmental stress on reptiles.
Husbandry factors
Husbandry factors are paramount in the development of reptile disease. As such, a full husbandry review must be carried out in all cases. Before embarking on the treatment of reptiles, clinicians need to be aware of:
- How to take a history and conduct a husbandry review
- The accepted needs and standards of keeping for the species they are seeing.
A full review of conducting a husbandry review is beyond the scope of this article and the reader is directed to the further reading section at the end.
Table 1 summarises the basics of what needs to be known.
Table 1. Essential information for a reptile husbandry review
| Parameter | How to gather data | Text |
|---|---|---|
| Vivarium set up |
|
|
| Social groups |
|
|
| Temperature |
|
|
| Humidity |
|
|
| Ultraviolet (For detailed information on this subject, see http://www.uvguide.co.uk) |
|
|
| Diet |
|
|
Social factors
As well as permitting spread of organisms that may be commensal in one species, yet pathogenic in another, mixing of species in co-habitation may be stressful to one or more of the species. This may result in immunosuppression, encouraging the invasiveness and subsequent spread of potential pathogens.
Even in single species groups, inappropriate social groupings may result in stress and immunosuppression. Most reptiles are solitary and generally should be housed alone. However, some will permit housing in small groups. For example, Bearded dragons may be kept in small groups of one male and 2–3 females, as long as there is sufficient space and resource. However, as Bearded dragons and other reptiles can be hard to definitively sex, it is easy to get the grouping wrong. Groups with multiple males may experience direct male–male aggression. This may be physical, but can also be much more subtle with displays, such as head bobbing and driving subordinate males away from food and heat sources. As such, some keepers suggest that if in doubt, Bearded dragons should always be kept on their own.
Parasites
In the UK the vast majority of reptilian pets are captive bred, so ectoparasites are extremely rare. However, two may be seen in pratcice.
Snake mite (Ophionyssus natricis)
These are commonly found on both snakes and lizards and may accumulate under scales or around the eyes (Figure 2), nostrils and other apertures. They may cause direct irritation and lead to hyperkeratosis and ulceration. Infected animals may spend more time in the water bowl in an effort to remove mites. The mites may also be an important vector of disease, such as arenavirus in snakes. Treatment (all reptiles should be treated each time) may involve ivermectin (topically on affected areas, or systemically), but this should not be used in indigo snakes, skinks or chelonia, or fipronil spray carefully applied to affected areas. Care must always be taken when using fipronil spray as alcohol fumes may be toxic to the reptile. For this reason, the author avoids this agent in very small or young animals.

After application of fipronil, reptiles must be placed in a warm well-ventilated place for 30–60 minutes to allow drying. Treatment also involves environmental control, as a majority of the mite's lifecycle is off the animal. All organic materials in the animal's vivarium should be destroyed and plastic materials washed in warm soapy water. The rest of the vivarium may be sprayed with ivermectin-or fipronil-based sprays and allowed to air dry. Both animal and environmental treatments should be repeated monthly until no more mites are found.
Prevention involves effective quarantine of new animals before entry to a collection and systemic treatment with ivermectin at least twice during quarantine. In large, complex, or naturalistic enclosures, or where chemical control is not effective, biological control using Cheyletus eruditus mites should be considered.
Myiasis
Where animals are kept outdoors (or in conservatories or green-houses), fly strike is occasionally seen. It is generally seen infecting untreated wounds and certainly represents issues with husbandry and observation. The outlook is usually guarded to poor depending on the amount of tissue necrosis and depth of maggot invasion. Treatment involves physically removing the maggots and fly eggs; topical treatment with either ivermectin or F10 insecticide spray (cypermethrin plus piperonyl; Health & Hygiene Pty, SA); and systemic ivermectin. Wounds should be thoroughly cleaned and repaired, or allowed to granulate, and all underlying factors must be corrected.
Clinical presentations
The following section gives a basic guide to approaching different presentations of dermatopathies in snakes and lizards.
Wounds
Wounds are common, but are rarely suitable for suturing and first intention healing (although this should be the route of choice where possible). Most wounds need to be managed and allowed to heal by second intention.
Initial management will include cleaning and removal of contaminated material, which may require sedation or anaesthesia. If the animal is debilitated or severely damaged, it is advisable to first stabilise for a period (possibly of several days) using fluids, assist-feeding and keeping in suitable conditions in terms of heat and ultraviolet. Additional sampling (including blood sampling) may be required to assess for underlying disease.
Use systemic antibiotics and analgesia as required. Ideally, antibiotic choice should be based on culture and sensitivity (either swabbing of underlying tissue after removal of contaminated tissue, or culture of underlying tissue taken by biopsy). However, it is likely that empirical dosing will be needed initially. If anaerobic involvement is not suspected, then licensed fluoroquinolones may be sufficient. However, if anaerobes are suspected (bite wounds) then broader spectrum antibiosis is required, for example trimethoprim-sulphonamide; fluoroquinolone plus metronidazole (with care in herbivorous reptiles); or ceftazidime.
The effect of analgesia is poorly understood in reptiles, with few trials demonstrating efficacy, and marked differences are seen in different species. For short-term use, meloxicam may be used via subcutaneous or intramuscular injection in the cranial half of the body, or tramadol may be given intramuscularly in the caudal half.
For the application of topical hydrophilic dressings, the author will typically use either wet-to-dry dressing, or a 50:50 mix of a hydrophilic gel and silver sulfadiazine cream applied daily after cleaning.
Once the tissue has properly demarcated, the wound may be debrided and then allowed to granulate. Systemic antibiosis may be discontinued once a clean granulation bed has formed. Generally a 50:50 mix of a hydrophilic gel and silver sulfadiazine cream is applied daily after cleaning. Alternatively, medical grade Manuka honey may be used topically.
A special case is tail autotomy, where a natural defence mechanism means the tail will almost always heal (and partially regrow) with no clinical intervention. Indeed, clinical intervention may well reduce the ability to regrow, therefore the remaining stump of tail should be left alone and not bathed. Systemic antibiosis and anti-inflammatories are also unnecessary.
Dysecdysis
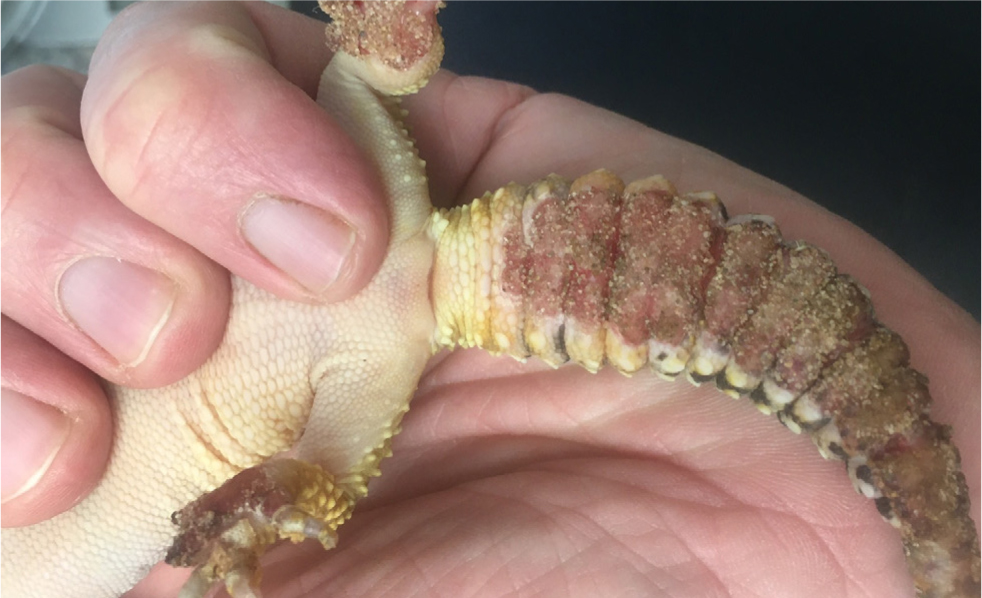
Reptiles shed skin periodically as they grow; generally, the more rapidly they are growing, the more frequently they shed. Snakes and lizards shed differently, with snakes usually shedding the whole skin in one piece and lizards shedding in a more piecemeal fashion. This process may not complete, resulting in retained skin over particular parts of the body, such as inside the eyes (in snakes and leopard geckos) (Figure 3) or feet (Figure 4). Leopard geckos appear commonly affected, partly because of the complexity of their eyes, with skin shedding from inside the eyelids. These shedding issues are generally linked to inappropriate humidity, temperature and UV provision


In terms of treatment, underlying factors need to be corrected. The retained skin should not be pulled off as this may result in damage to underlying tissues, especially eyes and feet (Figures 3 and 4). Instead, the animal should be bathed daily in warm water and the retained skin gently bathed and allowed to come away bit-by-bit. In some cases patience is required, as the retained skin will not come away until the next shed.
Several factors may affect the ability to shed properly:
- Systemic illness, especially dehydration. Any systemic illness will affect the ability to adequately shed. Shedding involves the production of an entire new skin and the old skin is cast off by means of secretion of fluid between the new skin and old. Dehydration will therefore affect formation of the ‘shedding fluid’.
- Malnutrition and parasitism – failure to adequately intake and absorb sufficient dietary protein will impact the ability to form new skin.
- Husbandry
- Temperatures and method of heating: inadequate temperature ranges (especially where too low) will affect the metabolic rate and the ability to form the new skin and cast off the old. In the author's experience, retained shed on the feet of Leopard geckos is common and particularly associated with the use of heat mats on the floor of the vivarium. This appears to overheat and dry the skin of the feet, resulting in dysecdysis. Instead, heat mats should be positioned on the wall of the vivarium so warming is achieved via heat radiation rather than direct contact.
- Humidity: low humidity is particularly associated with dysecdysis, owing to the interference with production of shedding fluid. This is a particular factor in dysecdysis of Leopard gecko eyes; while this species is a desert species living in a generally low humidity environment, it spends a lot of time under rocks in high humidity. As a result, a clean high humidity chamber filled with damp moss should be provided. This should be placed in the mid-part of the temperature range with the animal able to enter and spend increased time in high humidity when shedding. For snakes, vivarium humidity may be slightly increased (or additional bathing provided) when shedding is imminent (noted by the skin becoming blue-grey and dull as it separates).
- Ultraviolet light: research in Leopard geckos has shown that over-exposure to ultraviolet light will result in increased shedding frequency and, likely, an increased probability of dysecdysis. This species is crepuscular and it has been shown that prolonged use of ultraviolet does not result in higher plasma calcium or vitamin D3 levels, so it is advised that Leopard geckos receive 2 hours of ultraviolet light both morning and evening. This should reduce shedding frequency (Gould et al, 2018).
As stated above, these factors need addressing in order to prevent problems that will worsen with each shed.
Blistered skin
Skin blisters (formation of small fluid-filled cavities in the skin) are common in snakes (Figure 5), but relatively rare in lizards.

While not a true blister (but often presented as such), the most common presentation in lizards is the formation of subcutaneous fluid-filled sacs in the axillae of Leopard geckos. These are usually bilateral and rarely appear linked with any other clinical signs. Cytology usually shows these to be acellular and underlying causes are unknown. They can usually be left and will resolve in time.
In snakes, skin blistering is much more common and there are a number of differential diagnoses (Appendix 1). Aside from a full clinical examination and history and husbandry review, the most useful diagnostic test initially is aspiration of fluid from blisters for cytology and bacteriology (both aerobic and anaerobic) plus mycological culture (if indicated by cytology). If this is unrewarding, full thickness skin biopsies are indicated for histopathology and culture.
Ulcerated/crusted skin
Crusting of the skin will often occur secondary to skin ulceration or physical trauma (Figure 6). This category should also be considered together with the previous section on skin blistering, as ruptured skin blisters will present as ulceration and crusted lesions. Clinical history is useful in finding out if skin blisters have been noted earlier in disease.

When investigating these lesions it is important to not sample superficial crusts as these will often yield environmental organisms or secondary/tertiary invaders. Instead crusts should be removed and underlying tissues sampled for cytology/histopathology and bacterial and/or fungal sampling.
Colour changes
Skin colour changes may be seen as generalised or localised changes, and have multiple differential diagnoses.
Generalised changes
- Brighter, more vivid colours may be seen after a shed in snakes, and especially in chameleons showing heightened emotional state. This is normally males entering a breeding condition and therefore a sign of health, but can be a sign of stress in males kept within sight of other male chameleons. In Bearded dragons, dominant males will show beard (the skin under the neck) blackening (often accompanied by aggressive behaviours and head bobbing). In all-female colonies, dominant females may show this too. Similarly, subordinate animals will lose this black beard and may become a brighter yellow/orange colour. However, a black beard can also be a colour change in an unwell or stressed Bearded dragon, which emphasises the need to combine skin signs with results from the clinical examination and history.
- Dull skin colour in snakes usually shows that they are entering shed, especially if the skin (including over the eyes) shows a grey-blue tinge. May be seen in some lizards, such as Leopard geckos.
- Generalised skin blackening carries a very poor prognosis as it shows peripheral circulatory shutdown (reptile skin colour, caused by blood flow through different ‘colour cells’). If accompanied by collapse and lack of responsiveness, the prognosis is usually hopeless.
- Reddening/erythema/petechiation in snakes may indicate septicaemia or inflammation (such as in generalised skin burns, Figures 7 and 8). Care should be taken in Boa constrictors where there may be a normal rosy hue to ventral and lateral scales.

Localised
Focal lesions may be single or multiple and of varying significance and activity. If the animal is well, it may be worth observing the lesion for a period in order to assess progression before deciding to investigate. The most useful primary test is skin biopsy – further biopsy for bacterial/fungal culture may be needed depending on initial results.
Localised skin blackening is often seen following irritant sub-cutaneous injections or venepuncture, especially in chameleons. It is always worth advising owners of this before performing such techniques.
Masses
Dermal masses are common in snakes and lizards. Differential diagnoses (Appendix 2) are similar to those seen in more ‘routine’ veterinary species and approaches are similar too.
Neoplasia
Generally seen in older animals though some may be seen in younger animal (such as carcinomas, Figure 9 and 10) and it must also be remembered that some reptiles, eg chameleons, have relatively short lifespans. Tumours may be single or multiple. In general, malignancy is more common in reptile tumours than would be expected in pet mammal species. These masses tend to spread locally rather than metastasise (Figure 9), though this should be assessed in each individual case. One syndrome seen more commonly in recent years is that of carcinoma in chameleons and Bearded dragons (Figure 10). These are usually multiple and will often recur locally or at different dermal sites after excision. Prognosis is guarded but the masses are slow-growing. In cases where neoplasias occur in different sites simultaneously, there is suspicion these may be viral-linked, although this is unproven. In appearance they resemble granulomas or papillomas, emphasising the need to biopsy in these cases. Subcutaneous lipomas are common in corn snakes and may be diagnosed on appearance and cytology. These normally respond well to dietary management. However, a range of other tumours are also found, such as sarcomas in snakes and melanomas in Bearded dragons, so biopsy of masses is always recommended. Biopsy techniques are similar to those used in small animal medicine, although reptile tumours appear to be poorly exfoliative in the author's experience, making fine-needle aspirates less useful than wedge or excision biopsy.


Granulomas and abscesses
Bacterial, fungal and (rarely) parasitic granulomas are all seen in reptiles. These are usually single but there may be multiple depending on the underlying causes (injury, skin penetration, parasitic larval spread). Diagnosis is by means of biopsy and therapy generally involves excision. However, in some more abscessated cases, the lesion may be opened and pus removed by curettage (reptile pus is solid); the abscess may then be allowed to heal by second intention. One important differential diagnosis for granuloma formation is mycobacteriosis. As this carries a poor prognosis and is also zoonotic, diagnostic sampling is recommended in all granuloma cases and acid fast staining requested in cytological examination.
True cysts are extremely unusual but may be diagnosed by appearance and analysis of aspirated fluid.
Conclusions
Skin disease is common in snakes and lizards, and can be approached successfully in most cases. However, a holistic approach to the animal and its husbandry and environment is essential. Focussing solely on the skin lesions will mean that vital underlying causes are not identified and corrected.
KEY POINTS
- To understand the role of dermal signs as markers of underlying disease or husbandry issues in these species.
- To have a basic approach to investigating dermal lesions in these species.
- To have an understanding of diagnostic tests relevant to different clinical signs.

