Euthanasia literally means ‘easy death’ and is defined by the Oxford English Dictionary as ‘the painless killing of a patient suffering from an incurable and painful disease or in an irreversible coma’. The Royal College of Veterinary Surgeons (2012) code of conduct describes it as ‘painless killing to relieve suffering’ and recommends that all staff are fully trained and that a ‘planned, rehearsed and coordinated approach is taken’, given the often emotionally charged nature of these situations.
Good euthanasia practice includes:
- Client communication and education
- Appropriate animal handling and technique choice
- Pre-euthanasia sedation or anaesthesia, to reduce anxiety and allow safe restraint
- Thorough confirmation of death.
These should be followed for all species that are kept as pets, just as for cats and dogs (American Veterinary Medical Association (AVMA), 2020). Indeed, a conservative and humane approach to the care of any creature is warranted, justifiable, and expected by society (AVMA, 2020).
Many exotic species mask signs of disease or pain, or do not show these signs in easily recognisable ways. As a result, these pets are sometimes not presented to vets until disease is advanced and quality of life is significantly impaired. Sadly, this often means that euthanasia is the only humane welfare outcome for these animals. However, it is important to note that where treatment is possible (within client constraints such as finances and husbandry issues) this, or referral to an appropriate centre, should always be offered.
There is often no one ‘ideal’ method of euthanasia, and the choice of method will depend on many variables. The regularly updated AVMA (2020) Guidelines for the Euthanasia of Animals provide a comprehensive overview of methods of euthanasia in different species and environments, which should be consulted if there is any doubt over the suitability of a particular method in an unfamiliar species. The recommendations for humane euthanasia may change and develop, so it is prudent to consult the most up-to-date guidelines.
Many methods described will not be available in general practice, such as carbon dioxide inhalation, so this article will address practical options available to veterinary general practitioners.
The aim, at all times, should be to make sure that euthanasia is as pain- and stress-free as possible with animal welfare at the forefront of any decision-making. Good client communication as to what is happening and why, is paramount.
Decision-making
The euthanasia of pet animals always poses ethical, emotional and practical challenges. These may be compounded in the case of exotic pet species because of:
- Difficulties in assessing quality of life (for example, assessing this in a pet lizard can be more difficult than in a pet dog)
- Hiding of clinical signs until pathology is severe (particularly with prey species, such as rabbits or psittacine birds)
- Unfamiliarity of clinician with the species
- Potential distress at being in an unfamiliar place (such as prey species being able to smell predators, or handling a species that is not used to being restrained)
- Safety concerns for the veterinary staff and clients (such as the handling of potentially aggressive species, zoonoses, or inhalant gas exposure, particularly in vulnerable or at-risk staff)
- The owner's emotional bond with the pet
- Other client factors (such as husbandry provision or welfare concerns)
- Financial restrictions (precluding treatment)
- Potential difficulties with intravenous access
- Variable access to drugs or particular techniques.
The British Veterinary Association (BVA, 2016) guide to euthanasia has a useful decision tree with guidelines for when vets are faced with euthanasia as a management decision for an animal under their care. The AVMA (2020) guidelines also give useful decision trees for making a euthanasia decision, and as well as evaluating the morality of that decision.
Clear and calm communication with the client is vital when making a decision regarding euthanasia, and obtaining written consent from the client is advised.
Should the client be present?
For all pet species, this can be an emotional subject and there is no definitive ‘right’ answer. For many exotic species, anaesthesia is advised before injectable administration of a barbiturate overdose. Anaesthesia, whether by inhalant gas or injection, may appear distressing to an owner and this should be kept in mind when discussing whether a client should be present. The pithing of reptiles in particular will not be palatable to the layperson's eye. If anaesthesia is provided by inhalant gas via a facemask, members of the public should not be present for health and safety reasons.
Careful communication as to what exactly will be happening and why, is vital (including warning of potentially unpalatable events such as vocalisation or movement after euthanasia).
Emotional support and grief counselling should be considered for clients and for other companion animals. The AVMA (2020) guidelines state that ‘the depth of the emotional attachment between animals and their owners or caretakers requires an additional layer of professional respect and care, beyond the ethical obligation to provide a good death for the animal’. Indeed, many exotic pets such as psittacine birds, tortoises and chinchillas are relatively long-lived and owners can form deep bonds with them, so their grief when they die may be profound (Harris et al, 1984). There are organisations that can help support individuals with pet bereavement, such as the Blue Cross, and clients may be signposted to these. It is also important to consider the impact of euthanasia practice on veterinary staff (Hamilton, 2019).
How to euthanise
This will be discussed in more detail for each species group. The method used should result in rapid loss of consciousness followed by cardiac or respiratory arrest and, ultimately, a loss of brain function (AVMA, 2020). The method should be practicable, safe for vet, staff and owner, allow good welfare, be aesthetic where required and take into account any needs once the animal is dead, such as the minimal destruction of tissue if a postmortem is to be performed (Cooper, 2019). Care should be taken to consider species-specific handling techniques and stress reduction before loss of consciousness (AVMA, 2020). Even in species or taxa where pain perception is poorly understood (such as in reptiles, amphibians, invertebrates and fish) euthanasia methods that give ‘rapid loss of consciousness’ and ‘minimize pain and distress’ should be strived for, even where it is difficult to determine that these criteria have been met (AVMA, 2020).
Hypothermia is not considered to be an appropriate method of euthanasia (AVMA, 2020). The formation of ice crystals during freezing is painful, and it must be remembered that reduced movement is not an indicator of anaesthesia or analgesia. Rapid freezing, for example using liquid nitrogen, is also not considered to be a humane sole method of euthanasia other than for small (<4 g) reptiles, amphibians and <5-day-old rodent neonates where immediate death occurs (AVMA, 2020). Physical methods such as decapitation are often unpalatable to owners and require training to be humane, and so are rarely considered in general practice.
Sedation or, more commonly, anaesthesia before administration of a barbiturate overdose is generally recommended for most exotic pet species. Anaesthesia is generally preferred to sedation given the lower chance of arousal or consciousness during the procedure. Anaesthesia may be via inhalant gas delivered via a mask or induction chamber, by parenteral injection, or by immersion (for fish or amphibians). Once the animal is completely insensible, then pentobarbitone is usually administered intravenously if possible, or via intra-cardiac, intra-peritoneal, intra-coelomic or intra-osseous injection. Non-intravenous injection of pentobarbitone is likely to be painful so anaesthesia is mandatory. Intramuscular injection of pentobarbitone is also not advised by the British Small Animal Veterinary Association (BSAVA).
Suggested pentobarbitone doses are as follows (BSAVA, 2020):
- Mammals: 150 mg/kg intravenous (IV), intra-peritoneal, as rapidly as possible or to effect
- Birds: 150 mg/kg IV
- Reptiles: 60–100 mg/kg IV, intracoelomic or intracardiac
- Amphibians: 60 mg/kg IV, intracoelomic, or via lymph sacs such as with frogs and toads
- Fish: 60–100 mg/kg IV, intracoelomic, intracardiac.
Confirmation of death
This may be simple and easily transferrable from experiences with a cat or dog, particularly in small mammals, but can be surprisingly challenging with other species such as reptiles, amphibia and invertebrates. This will be discussed in more detail when considering each species group. A combination of criteria is most reliable in confirming death. Recommended criteria include:
- Lack of pulse
- Apnoea
- Absent corneal reflex
- Absent toe-pinch/pedal reflex
- Absent heartbeat
- Rigor mortis.
No single test alone, other than rigor mortis, confirms death (AVMA, 2020). If there is doubt about death confirmation, it may be prudent to keep the body at the surgery at high ambient temperatures for 24 hours to allow definite confirmation of death before release for burial or cremation.
Carcass disposal
Following euthanasia, a decision will need to be made as to the disposal of the carcass. This must be carried out in accordance with local law. This is usually cremation or home burial. Individual cremation can be performed, as for cats and dogs, and indeed many clients may choose this (it is important not to assume client's decisions based on perceptions). Home burial may be appropriate in many cases, but as with cats and dogs, there is a risk of exhumation by other pets or wildlife, which is both unpalatable and a risk to potential scavengers regarding drug residues. Potential zoonotic risks should also be considered such as Chlamydophila (psittacosis) from birds.
Home burial is not permitted by the Department for Environment, Food and Rural Affairs (2015) in the UK for chickens or other farm animals, as they are classed as ‘fallen stock’. In addition, care should be taken when disposing of water containing sedatives from the euthanasia of fish and amphibians. The water should go into clinical waste rather than directly into a drainage system.
Species-specific information
Mammals
Rabbits
Rabbits may be given intravenous pentobarbitone via an intravenous cannula. These can be placed in the marginal ear, cephalic or saphenous veins. Local anaesthetic cream can be applied before cannula placement, although this may take some time to take effect. Anaesthesia or sedation may be performed before pentobarbitone administration, depending on the rabbit's temperament and vet and client preferences. There are many anaesthetic options available such as alfaxalone, combinations of alpha-2 agonists with ketamine and butorphanol or buprenorphine; midazolam with buprenorphine; or midazolam with fentanyl/fluanisone (Carpenter, 2017; BSAVA, 2020). Inhalant gas induction alone is not recommended as this can be aversive and stressful. If intravenous access is not possible, pentobarbitone may be given via the intra-cardiac route, or potentially intra-peritoneally once the rabbit is completely anaesthetised.
Rodents and pygmy hedgehogs
For rodents, such as mice, rats, hamsters, degus, chinchillas, guinea pigs, and pygmy hedgehogs euthanasia is generally performed by intravenous, or often intra-cardiac administration of pentobarbitone after induction of anaesthesia. Anaesthesia may be induced via inhaled gas such as isoflurane or sevoflurane via an induction chamber or facemask, or by subcutaneous or intramuscular injection (such as medetomidine, ketamine and butorphanol combinations) (BSAVA, 2020). Sevoflurane is likely to be less irritant and aversive than isoflurane when used for induction of anaesthesia. The author advocates the use of zero dead space induction masks for rapid anaesthetic induction. Both gas induction and injections may be aversive to rodents so there will need to a consideration of the best option to use for a particular rodent and circumstance (Figure 1).

Ferrets and skunks
Intravenous access and handling issues may be challenging in these species, so anaesthesia is usually performed before intravenous or intra-cardiac pentobarbitone administration. Anaesthesia can be induced by inhalant gas or intramuscular injection of a medetomidine, ketamine and butorphanol combination.
Small pet primates
Anaesthesia should be performed before intravenous or intra-cardiac pentobarbitone. Anaesthesia is usually induced via inhalant gas via facemask, or more usually an induction chamber, or by intramuscular injection of ketamine, combined with either diazepam or medetomidine (Masters, 2010). Sevoflurane is the preferred choice of inhalant gas because of its minimal odour, lower pungency and airway irritation, compared with isoflurane (Popilskis et al, 2008).
Death can be confirmed for most mammals by checking for a heartbeat with a stethoscope and checking corneal and toe pinch reflexes.
Birds
In most cases, birds will be euthanised by induction of anaesthesia using inhalant gas (isoflurane or sevoflurane) via a chamber, or more usually a facemask. The author advocates the use of zero dead space masks for rapid anaesthesia induction. Inhalant gas alone may lead to death. This is acceptable in birds and may be preferable if postmortem examination is required as there will be minimal tissue damage (Rae, 2006; AVMA, 2020). Although, considerations should be made regarding the potential for increased exposure of veterinary staff to inhalant gases. However, euthanasia is usually completed by intravenous (medial metatarsal, basilic/ulnar or right jugular vein), intracardiac or intraosseous administration of pentobarbitone. If the owner wishes to be present, use of inhalant gas is problematic. Intramuscular ketamine (Chitty, 2005) or midazolam with butorphanol have been suggested, or intranasal midazolam (2 mg/kg) plus butorphanol (2 mg/kg) (Montesinos, 2018). After waiting 15 minutes, an intravenous cannula can be placed or an intravenous injection given (Montesinos, 2018). Consideration of the level of sedation or anaesthesia is important because of the potential for arousal.
It must be remembered that birds lack a diaphragm, so intra-coelomic injections should be avoided as material may be injected into the air sacs, causing drowning or marked pain and irritation. Some bones such as the humerus and femur are pneumatised and communicate directly with the respiratory system. Drugs should not be administered by the intra-osseous route in these bones because of the risk of drowning. Intraosseous catheter placement can be performed safely in the proximal tibiotarsus or distal ulna (AVMA, 2020). Care should be taken with intracardiac injection to make sure there is no lung or air sac injection. Intrahepatic injection just under the keel may also be appropriate in anaesthetised birds. Physical methods of euthanasia such as cervical dislocation are feasible but are likely to be unpalatable in general practice and require appropriate training.
Death can be confirmed for most birds by checking for a heartbeat with a stethoscope and checking corneal and toe pinch reflexes.
It must be remembered that in the UK, food-producing birds such as chickens are considered ‘fallen stock’ and must not be allowed to be buried at home.
Reptiles
Reptiles are poikilotherms, meaning their body temperature and metabolic rate are dependent on the temperature of their environment. Reptiles' slow metabolic rate and resistance to hypoxia can make euthanasia and even death confirmation challenging (Baines and Davies, 2004a; Cooper, 2019; Nevarez, 2019; AVMA, 2020).
Hypothermia or rapid freezing are not considered acceptable methods of euthanasia for reptiles (Baines and Davies, 2004a; b), apart from rapid freezing of reptiles weighing less than 4g (AVMA, 2020). There is no evidence that freezing is clinically effective for euthanasia (Martin, 1995). It is also believed that freezing can result in the formation of ice crystals in tissues that could cause pain (Close et al, 1997; Cooper et al, 1989).
Euthanasia is generally performed by intravenous injection of pentobarbitone. Initial anaesthesia, such as intramuscular ketamine, intravenous or intramuscular alfaxalone, or intravenous propofol, is advised, so that the reptile becomes insensible. Gas anaesthesia may be used in very small reptiles such as geckos, but is usually ineffective in larger reptiles, particularly chelonians. The subcutaneous route may also be used for anaesthesia in certain species such as leopard geckos, avoiding the use of volatile anaesthetic gas. The reptile should be kept warm during this period to increase the metabolic rate. Once anaesthetised, pentobarbitone may be given intravenously usually via the dorsal tail vein, jugular vein, subcarapacial venous sinus or brachial vein in larger individuals (such as chelonians) or via the ventral tail vein in snakes and lizards (Figure 2), or the ventral abdominal vein (in lizards). The jugular vein may be accessible in small lizards such as leopard geckos. Where intravenous access is not possible, intracardiac injection may be used, particularly in snakes. In small juvenile tortoises, intracardiac injection through the plastron where the scutes intersect in the mid-line, after sedation, may be used, or alternatively can be performed using a longer needle introduced parallel to the neck and directed towards the midline. Intracoelomic injection is not recommended, as uptake can be unpredictable and may be painful (McArthur et al, 2004; Chitty and Raftery, 2013). However, if there is no alternative then it may be used under anaesthesia as an acceptable method (AVMA, 2020).
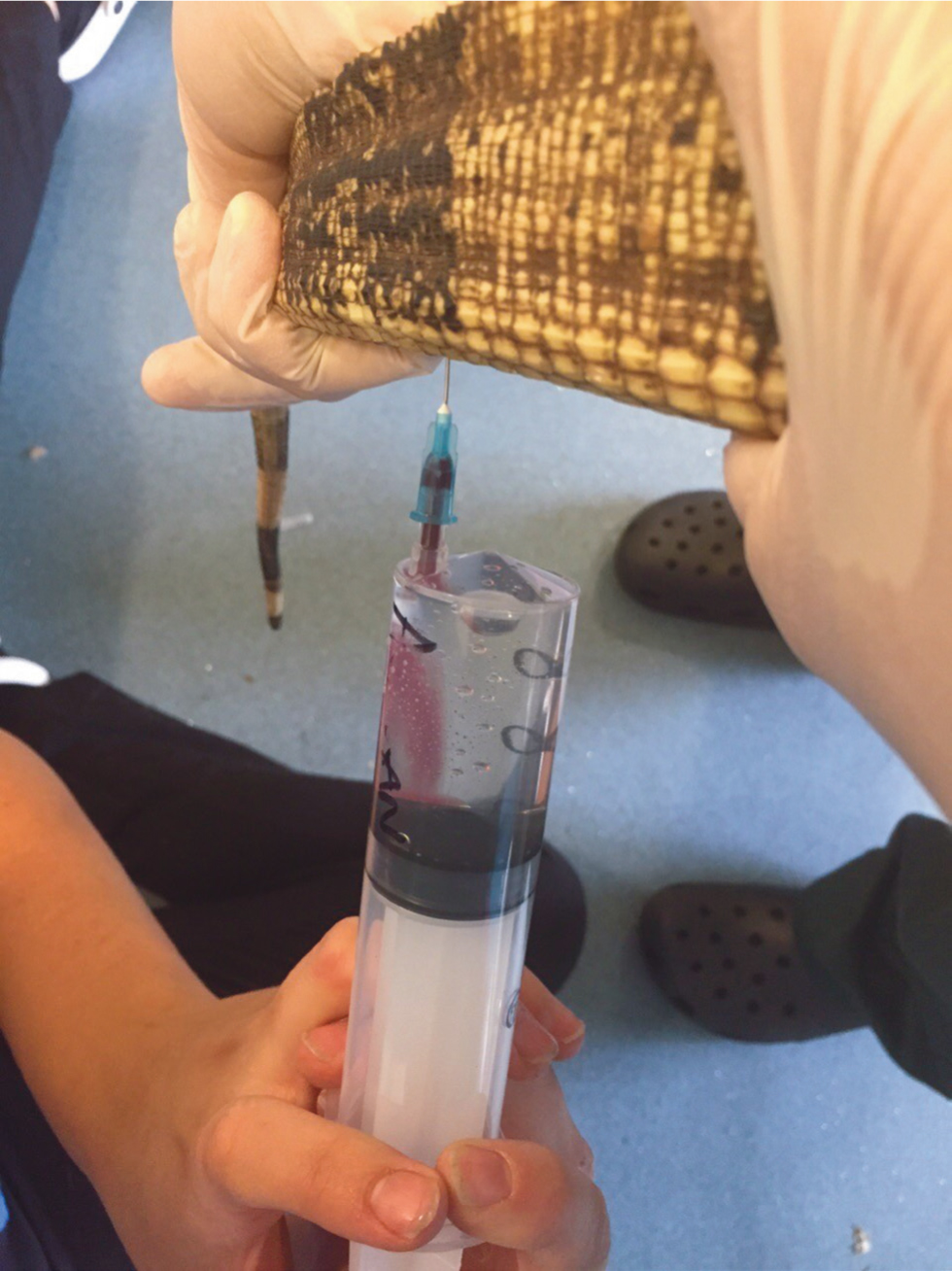
The reptile should be pithed before disposal of the body. This is done by mechanically disrupting the brain using an appropriate tool (such as a needle or dental elevator) to pierce through the roof of the mouth (more cosmetically palatable if the body is to be taken home by the owner) or through the foramen magnum (Nevarez, 2019). Placing cotton wool inside the reptile's mouth after pithing may help reduce blood leakage and aid cosmetic appearance if the owner is taking the body home for burial. Movement is still possible even after pithing and owners should be warned of this if they are taking the body home for burial soon after euthanasia.
Death confirmation in reptiles can be challenging. Dead reptiles will show total immobility, an absence of spontaneous breathing and an open larynx, but the heart may continue to beat after brain death, making confirmation of death difficult. Pedal withdrawal, palpebral, corneal and cloacal reflexes may be tested, although negative findings do not necessarily indicate death. Performing an electrocardiogram (ECG) can be useful, although weak signals may not be picked up by standard veterinary equipment. Also, ECG signals may be detected for up to 24 hours after apparent death as a result of ventricular pacing. An 8 MHz Doppler probe, such as those used to measure feline blood pressure, can be used to check for a carotid pulse (Figure 3). The probe can be placed on the lateral neck in chelonia and over the heart in snakes and lizards and moved until a pulse is found. It should be remembered that reptilian heart rates will be much slower than those of mammals, and will also be temperature dependent. An ultrasound probe can also be used in the fossa between the lateral neck and the forelimb in chelonia, or over the heart in lizards and snakes, to try and visualise whether the heart is beating. If the fossa is too small to allow probe contact, an acoustic standoff may be used (for example an inflated glove filled with water). Death may be confirmed by the lack of all withdrawal and ocular reflexes, no ECG trace and the use of 8 MHz Doppler or ultrasonography to show no heartbeat (Baines and Davies, 2004b; McArthur et al, 2004; Mader, 2006; Chitty and Raftery, 2013; Hyndman, 2018). If there is any doubt as to death, the reptile should be kept somewhere warm (24–30°C) for 24 hours before considering cremation or burial.

Amphibians
The same basic considerations for the euthanasia of reptiles apply to amphibians, although their skin permeability allows some unique methods of anaesthesia before euthanasia (Nevarez, 2019). Hypothermia or rapid freezing are not considered acceptable methods of euthanasia, other than rapid freezing of amphibians weighing less than 4g (AVMA, 2020).
Euthanasia may be achieved by an overdose of an immersion anaesthetic such as tricaine mesylate (MS222), benzocaine or 2-phenoxyethenol. The easiest agent to obtain in the UK is likely to be 2-phenoxyethanol and the author recommends that a veterinary practice keep this in stock in case amphibian or fish anaesthesia or euthanasia is required. MS222 should be buffered with bicarbonate at a 1:1 weight ratio, or until the solution reaches a pH of 7–7.5 (Nevarez, 2019). Alfaxalone baths (10 mg/l) or intramuscular injection of alfaxalone may be an alternative option. After the amphibian becomes insensible, pentobarbitone may be administered by an intravenous (ventral tail vein in axolotls or the lingual plexus, ventral abdominal vein or medial femoral vein in larger anurans), intracardiac or intra-coelomic route. Injection may cause tissue disruption which may affect postmortem examination.
Death confirmation can be challenging for amphibians, as it is for reptiles, and the methods listed for reptiles may be used. If there is any doubt as to death, the amphibian should be kept somewhere warm (24–30°C) for 24 hours before considering cremation or burial. Safe disposal of the water must be considered – water with anaesthetic agents should not be flushed down a sink.
Fish
Euthanasia is generally performed via immersion in an overdose of anaesthetic solution (usually 5–10 times that used for anaesthesia). Water from the fish's own housing should be used to reduce stress. Anaesthetic agents include MS222, benzocaine and 2-phenoxyethanol. MS222 should be buffered with bicarbonate. The amount of MS222 should be calculated in grams and then double the amount of sodium bicarbonate weighed out (Francis-Floyd and Petty, 2010). Alfaxalone baths (10 mg/l) may be an alternative option. Isoflurane or sevoflurane can be added to water, but they are generally not very water soluble. Injecting through a syringe and small gauge needle underwater can aid dispersal. Doses of 5–20 ml/l can be used, although, owing to agent volatility, there are human safety concerns and staff must be used in a well-ventilated area (AVMA, 2020). Intramuscular injection of alfaxalone, ketamine or medetomidine and ketamine can be considered in large fish.
Immersion in anaesthetic agent alone may be sufficient for euthanasia, particularly for small fish (and may be necessary for postmortem examination to avoid tissue damage), but intracardiac, intravenous (caudal peduncle vein or veins on the dorsomedial aspect of the opercula in large fish (Ross, 2001) or intra-coelomic injection of pentobarbitone after anaesthesia may be used. Pithing after anaesthesia may be required in larger fish.
Physical methods such as blunt trauma, pithing or cervical transection are possible but unlikely to be palatable in a general practice situation with a companion ornamental fish. Chilling or freezing, death by anoxia after removal from water, exposure to caustic chemicals or after prolonged traumatic injury before loss of consciousness, are not acceptable (AVMA, 2020).
Death confirmation indicators include respiratory arrest (no opercular activity for a minimum of 30 minutes) and a loss of eyeroll (the movement of the eye when the fish is rocked from side to side). The heart can continue to beat after brain death, so a heartbeat is not an indicator of life, but prolonged absence of a heartbeat is a good indicator of death (AVMA, 2020). As with amphibia, care should be taken not to dispose of anaesthetic solution into a drainage or water course.
Invertebrates
There is limited guidance available on appropriate methods for the euthanasia of invertebrates (AVMA, 2020), although the British and Irish Association of Zoos and Aquariums (2020) recommendations for ethical euthanasia of invertebrates have been recently updated. The sheer diversity of species and taxa limits generalisations and the reader is advised to seek invertebrate-specific literature, for example Murray (2011), Braun et al (2006) and Cooper (2011). The innervation and circulatory systems, which are not easily comparable to vertebrate systems, make developing humane methods of invertebrate euthanasia challenging (AVMA, 2020) (Figure 4). While there is debate about pain perception and welfare in invertebrates, a humane approach should be developed using methods that aim to minimise pain and distress. In practical terms for pet invertebrates, this usually involves using anaesthetic agents, then pithing and immersion in a fixative such as formalin or freezing (Pizzi, 2010). For example, immersion in 5% laboratory grade ethanol or an undiluted, uncarbonated beer (5% ethanol content) induces anaesthesia in land snails (Succinea putris) with no overt signs of distress. This can be followed by immersion in solutions of 70–95% ethanol or neutral-buffered 10% formalin which acts to euthanise the snails (Gilbertson and Wyatt, 2016). Further work is necessary to establish whether it is valid to apply this two step method and other methods to terrestrial invertebrate species (AVMA, 2020). For euthanasia of African land snails, a 100% solution of phenoxyethanol may be used to cover the muscular foot, avoiding the pneumostome. Pentobarbitone may be injected into the body cavity once the snail is unresponsive and no heartbeat is found on using 8-MHz Doppler probe (Pellett et al, 2020). Injection of potassium chloride has been suggested as an effective method for terrestrial arthropod euthanasia (Bennie et al, 2012).

Conclusions
Euthanasia is a challenging but important aspect of veterinary practice. Often clinicians may be unfamiliar with performing this in exotic pet species, but it is important that a humane, compassionate and safe approach is used, keeping animal welfare in mind while respecting the strong bond between the owner and pet.
KEY POINTS
- Euthanasia is an important aspect of veterinary practice and may be especially challenging when dealing with exotic pet species.
- Whatever the species, euthanasia should be performed humanely and compassionately whilst respecting the bond between pet and owner.
- Good client communication and education; appropriate animal handling and technique choice and pre-euthanasia sedation or anaesthesia, where appropriate, to reduce anxiety and allow safe restraint and thorough confirmation of death, are essential.
- In most exotic pet species, administration of a barbiturate overdose after induction of general anaesthesia is generally recommended.
- Hypothermia and rapid freezing are not acceptable methods of euthanasia.


