There are five common causes of mange in domestic dogs and these can be subdivided into surface-dwelling mites (Otodectes cynotis, Cheyletiella spp. and Neotrombicula autumnalis) and subsurface mites (Demodex spp. and Sarcoptes scabiei var canis). The historical and clinical features of the diseases that each of these mites cause, plus the best methods for their detection and diagnosis, will be considered in turn.
Otodectes cynotis
Overview and signalment
Otodectes cynotis (known colloquially as the ‘ear mite’) is the cause of otoacariosis or otodectic mange. This mite belongs to the family Psoroptidae and is an obligate parasite that inhabits the vertical and horizontal ear canals of dogs and cats, although other species such as ferrets can become infested. It is non-burrowing and lives on the surface of the ear canal lining. Dogs infested with Otodectes most commonly develop bilateral otitis externa, characterised by vertical and horizontal canal erythema and a dark brown, ceruminous otic exudate.
In addition to otitis externa, ectopic infestations of the head, neck, tail head and, in rare cases, the trunk can occur when mites escape the ear canals. Juvenile animals appear to be most susceptible to otoacariosis, and it is thought that older animals may acquire immunity to the mite (Powell et al, 1980).
Zoonotic infections have also been reported, in which mites typically produce pruritic papular lesions on areas of the body that are in contact with the infested pet. These occur much less commonly than cases of sarcoptic or cheyletiellic mange.
Diagnosis
Direct observation of the mites moving around within the ear canal can often be made using a standard auroscope. The mites are pearly white in colour and contrast well with the dark, waxy discharge that frequently develops as a result of their presence. Alternatively, a sample of cerumen can be gathered using a cotton bud, smeared onto a glass slide and examined microscopically for mites and ova. Adding a drop of liquid paraffin and a cover slip enables the sample to be viewed under a microscope (Figure 1). If ectopic infestations are suspected, adhesive tape can be used around the head and neck to collect mites and this can be placed directly onto a slide for microscopic examination.
Cheyletiella species
Overview and signalment
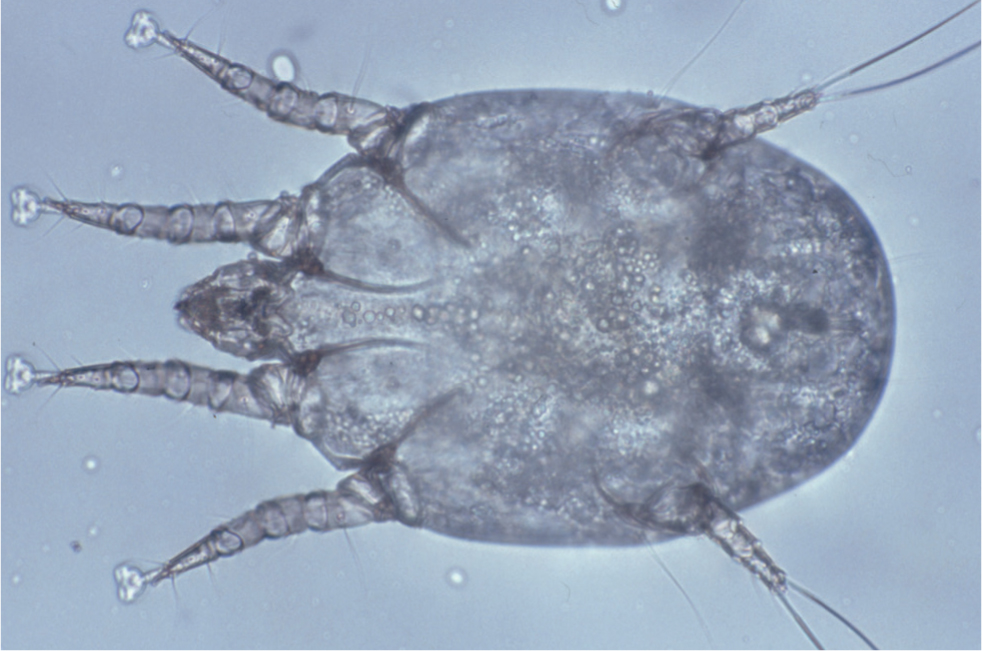
Cheyletiella spp. mites are the cause of cheyletiellosis, which is typically a mild but very contagious dermatosis, caused by mites living on the skin surface. Cheyletiella spp. are relatively large (500 × 350 µm), white, fiddle-shaped acari with legs that protrude beyond their body margin. Their most distinctive feature is a pair of crescent-shaped hooks on the accessory mouthparts (Figure 2). They feed on tissue fluid and lymph obtained by piercing the epidermis with a style-like chelicerae, and form pseudo-tunnels in the scale that is generated in response to their activity. Cheyletiella yasguri is the species most frequently isolated from dogs, but as a family Cheyletidae are not believed to be host-specific and may readily transfer between dogs, cats and rabbits (Miller et al, 2013). Humans in contact with pets carrying Cheyletiella spp. are at risk of becoming transiently infested, producing an uncomfortable, pruritic dermatosis characterised by papular lesions which appear on the arms, legs, trunk, buttocks and rarely the face.
Cheyletiellosis is primarily a dorsally distributed disease that is characterised by mild erythema and excessive scaling (Figure 3). Occasionally, infestation may lead to pyotraumatic dermatitis (acute moist dermatitis). These ‘hot-spot’ cases with concurrent dorsal or truncal scaling should be screened for the mite.
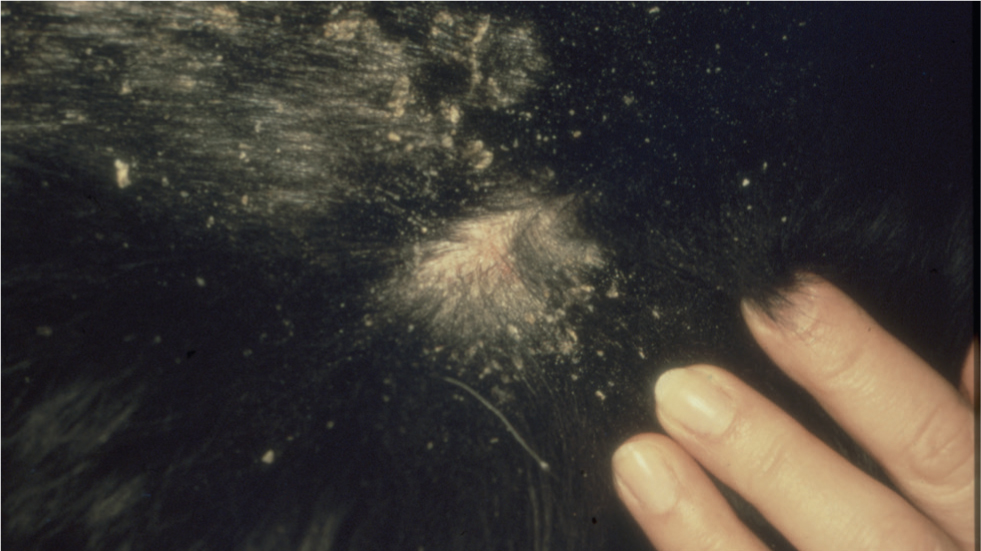
Pruritus is variable, ranging from absent to moderately severe, and young animals usually exhibit more obvious clinical signs than adults. An asymptomatic carrier status also exists and this should be kept in mind when tackling problem cases in which reinfestation and zoonotic transmission repeatedly occur.
Diagnosis
In cases where the history and clinical signs suggest cheyletiellosis, the simplest diagnostic test is to sit or stand the animal on a dark surface and using the hand, a brush or a flea comb, to dislodge some of the scale from the skin surface. On closer inspection, the scale may appear motile as a result of mite activity. This is the origin of the disease's colloquial name: ‘walking dandruff’.
More accurate methods of diagnosis involve gathering mites and eggs by adhesive acetate tape and skin scrapings. The former involves applying the sticky surface of a piece of acetate tape repeatedly along the dorsum of a scurfy animal, applying it directly to the surface of a glass slide and then examining it microscopically for evidence of mature and immature mites and their eggs. Cheyletiella spp. mites bind their ova to hairs with a thread, but this often breaks so the eggs may be free in the sample as well as bound to hairs. This distinguishes them from louse eggs, which generally remain bound to hairs because of a strong cement-like substance produced by the females. Superficial skin scrapings offer an alternative method of diagnosis. Scale should be removed using a size 10 scalpel blade and then suspended in liquid paraffin on a microscope slide beneath a cover slip. The slide should then be scanned at low power (x40) and any areas of interest examined at medium power (x100). In the majority of cases Cheyletiella spp. are present in large numbers, but the mites can sometimes be difficult to detect. In two separate studies, investigators failed to recover mites in 15% of infested dogs (Paradis and Villeneuve, 1988).
Neotrombicula autumnalis
Overview and signalment
Of the several hundred different species of Trombiculidae (chigger) mites that have been recognised, only a handful cause disease in humans (trombiculiasis) and animals (trombiculosis). Neotrombicula autumnalis, the harvest mite or berry bug, is the most familiar pathogenic trombiculid mite in the UK and Europe. Only the six-legged larval stages are parasites of mammals. The remainder of the lifecycle is completed in the environment, while the nymphal and adult stages feed on vegetable matter. Canine and feline (and occasionally human) infestations are seasonal; the majority of cases occurring between June/July and November, although one atypical infestation was reported in January (White, 2001).
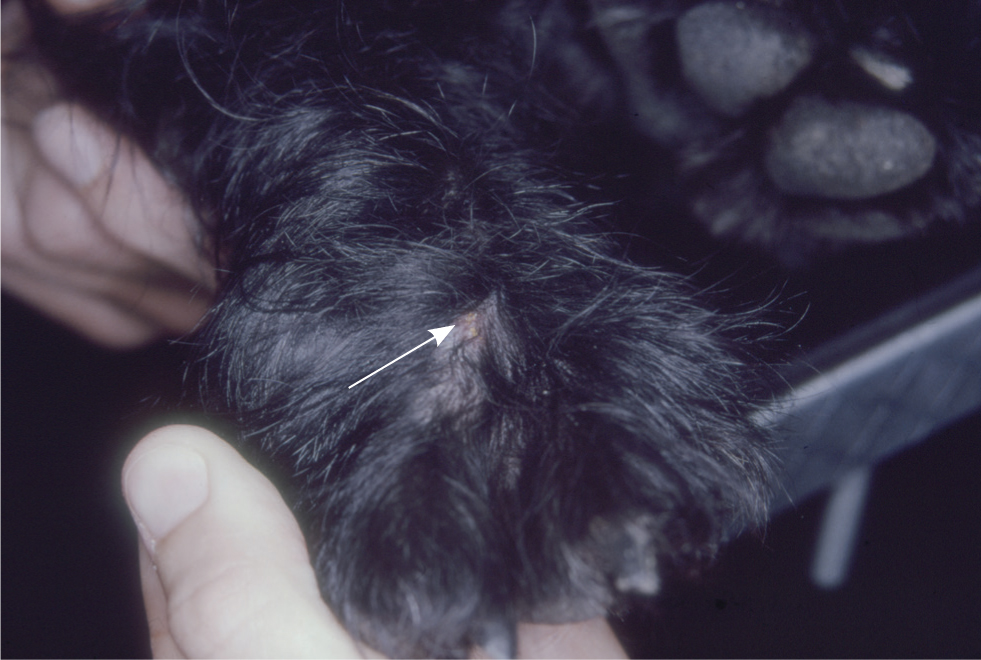
All Trombiculidae cause papular to papulocrustous lesions at their feeding sites, which tend to be located interdigitally (Figure 4), within the pinnal alar folds (Henry's pockets) or along the ventral abdomen. Neotrombicula larvae appear individually (Figure 5) or in groups as bright red-orange specks. Most infestations are asymptomatic, although some infested animals become pruritic and in extreme cases wheals can develop at the sites of mite attachment.

Diagnosis
Whenever trombiculosis is suspected, directly examine the coat, particularly the known predilection sites, as the mites are readily visible to the naked eye. If mites remain undetected, suspicious lesions should be scraped and the scrapings examined microscopically at low-to-medium power. The fact that only the six-legged larval forms are parasitic, plus the orange colour of the mites, helps with identification and diagnosis. Mites have even been detected histopathologically and it is suspected that the host's immune reaction to them may wall-off or ‘encyst’ them (Bourdeau et al, 2000), making them detectable only by deep skin scrapings or biopsy.
Demodex species
Overview and signalment
The majority of cases of canine demodicosis (demodectic mange, red mange and follicular mange) is caused by the mite Demodex canis (Figure 6), which was first described in the mid-1800s. In the 1980s and 1990s, two additional species of demodectic mite were isolated from dogs; Demodex cornei (Scarff, 1988) and Demodex injai (Hillier and Desch, 1997). All three mite species inhabit the hair follicles and, occasionally, the sebaceous glands, although Demodex cornei is thought to occupy a more superficial position within the skin. Transmission of the mite is believed to occur in the first few days of the nursing period. During early lactation, mites transfer from the bitch's skin to the facial and pedal skin of the pups as they feed, and these sites are those most frequently affected in a juvenile dog. The mite is host specific and, with the exception of its spread from bitch-to-pup, it is not generally considered to be contagious between dogs. All pups are believed to contract a complement of mites from their mother, but in the majority, mite populations remain low and clinical signs of infestation never develop. Individuals within the same litter can vary with respect to their tendency to develop signs of disease and certain breeds, for example the West Highland White terrier, English Bulldog, Shar Pei and Staffordshire Bull Terrier are more susceptible to demodicosis than others. These observations have resulted in the hypothesis that affected individuals may have a Demodex-specific immunodeficiency of differing severity, depending on whether they develop mild localised disease (capable of spontaneous resolution) or chronic generalised disease. Immunological studies suggest that this defect is most likely to be T-cell mediated, in contrast to a B-cell, innate or humoral immunity problem (Miller et al, 2013).
Infestation with demodectic mites typically results in one of two major forms of disease; localised and generalised. Most cases of localised demodicosis occur in young dogs, with an age of onset of 3–8 months. Mite overgrowth and the resulting follicular inflammation initially produce patchy hypotrichosis and alopecia, particularly of the face, forelimbs and feet (Figures 7 and 8). Pruritus is variable, but is generally absent to mild. The underlying skin may be erythematous (red mange), mildly scaly and comedones may be observed. Truly localised demodicosis at a young age of onset will typically resolve spontaneously, with or without treatment. However, when a case is first presented, it is difficult to know whether it is destined to remain localised or will generalise, so the author recommends that all cases of demodicosis are treated.


Generalised demodicosis also occurs in young dogs, but can develop at any age. Certain breeds are predisposed, but any breed of dog with a concurrent illness, or animals receiving immunomodulatory therapy could potentially develop demodicosis. Correction of the underlying disease or cessation of therapy may result in spontaneous resolution of the demodicosis. Clinically, in generalised cases, the skin lesions are more widespread, severe and chronic and the alopecic patches described above can, with time, become mildly to moderately hyperpigmented. Some dogs with generalised demodicosis have pedal involvement, whereas in others the feet alone are affected, or may remain affected when other cutaneous lesions resolve. ‘Pododemodicosis’ can be very painful and dogs frequently lick at their feet and may exhibit a shifting lameness; this latter sign may help to distinguish between this disease and, say, pododermatitis caused by a hypersensitivity disorder. It is worth noting that infestation with Demodex injai specifically may result in a different clinical picture; affected dogs do not lose so much hair and the major clinical sign may be seborrhoea and pruritus of the face and dorsum.
Secondary pyoderma, which may be superficial and/or deep, is a common complication particularly of generalised cases of demodicosis, so epidermal collarettes, pustules and furunculotic lesions may be observed. In time, the disease can progress to affect large areas of the skin and the patient may become depressed, pyrexic and may have superficial lymphadenomegaly. Before the advent of modern, effective acaricides, some cases of generalised demodicosis were severe enough to warrant euthanasia.
Diagnosis
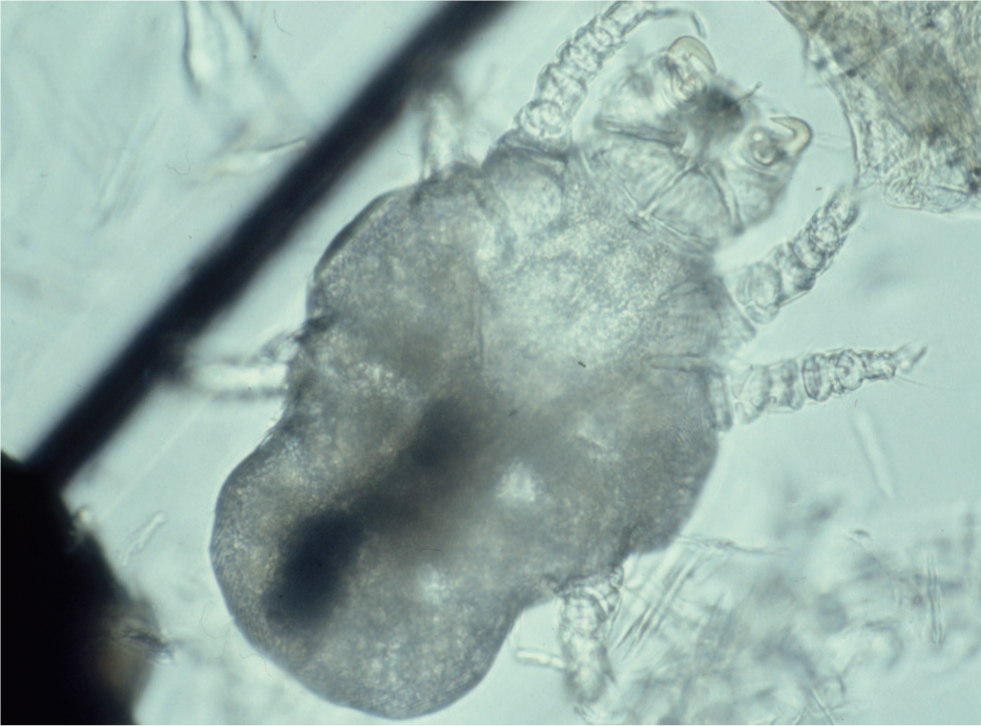
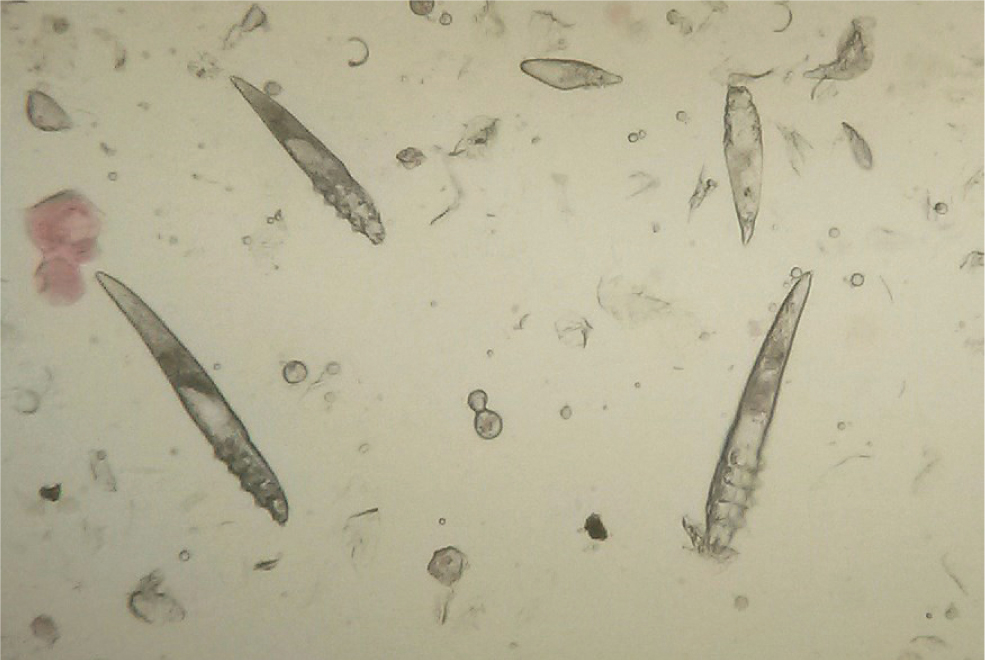
Skin scrapings are a reasonably reliable diagnostic technique for Demodex spp. mites. A size 10 scalpel blade, dipped in liquid paraffin and scraped repeatedly over a small area of skin which has been gently squeezed beforehand (to extrude the mites from the hair follicles) will often yield many mites and ova (Figure 6). Hair pluckings (trichograms) may also contain mites, and are sometimes easier and quicker to obtain from a dog with painful feet than a skin scraping. Specimens should be suspended in a drop of liquid paraffin on a microscope slide, covered with a cover slip and examined with a light microscope at ×40 and ×100 magnification.
Demodex canis and Demodex injai are long slim mites, with the latter being the longer of the two. Demodex cornei is a shorter, stubbier mite. Live, adult, eight-legged mites can be seen to be moving but the legs of the 8-legged nymphs and six-legged larvae are too small to allow any movement to be seen. The eggs are fusiform in shape and are not attached to the hairs. Mites may also be observed within hair follicles in skin biopsy specimens.
Sarcoptes scabiei var. canis
Overview and signalment
Sarcoptic mange (canine scabies, sarcoptic acariosis and fox mange) is a non-seasonal, common, highly contagious, pruritic skin condition caused by infestation with the globose mite Sarcoptes scabiei. The disease affects many mammalian species and the variant S. scabiei var. canis occurs commonly in domesticated dogs. In contrast, sarcoptic acariosis is rare in the cat and relatively few reports of feline sarcoptic mange appear in the veterinary literature. Sarcoptic mange can affect dogs of any age or breed and there is no known sex predilection. The mites are obligate parasites but can survive for limited periods away from the host. Feeding, oviposition and deposition of faeces within the skin exposes the host to mite allergens, which in the majority of infested dogs induces both humoral and cell-mediated immunological responses (Bornstein and Zakrisson, 1993; Arlian et al, 1996). These defence mechanisms can lead to spontaneous resolution of the disease after a few months, but most dogs develop a moderate to intensely pruritic dermatosis that can develop anywhere on the body, but which typically affects the elbows, hocks, pinnal margins and ventrum (Figures 9 and 10). Left untreated the lesions can extend and spread to involve almost the entire body surface.
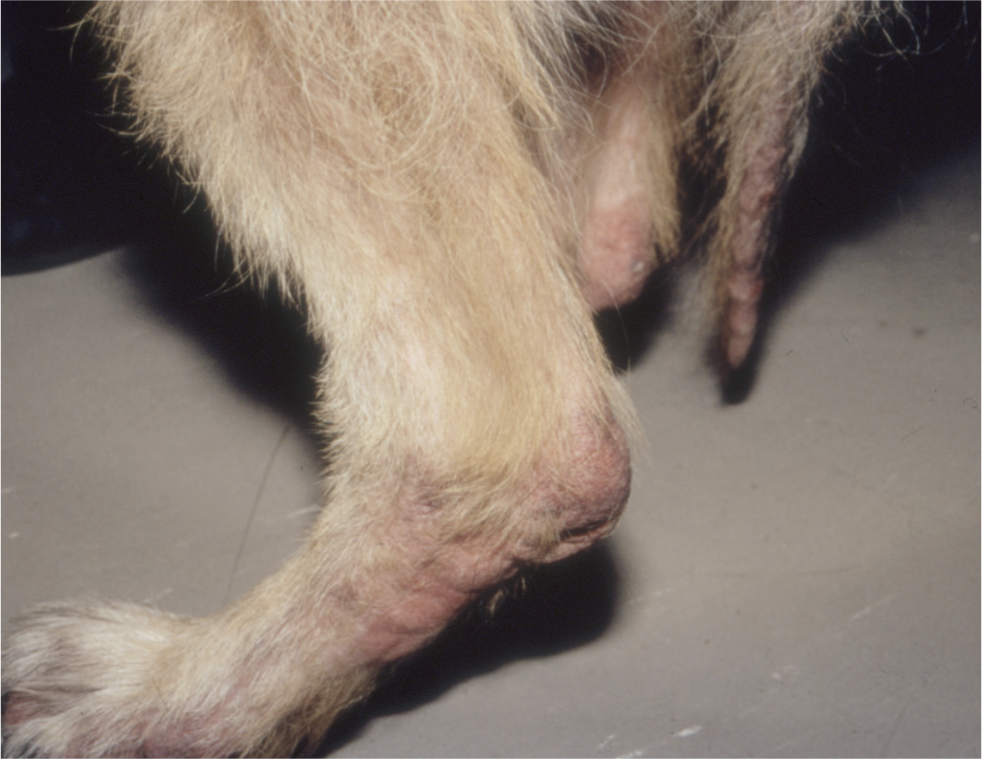
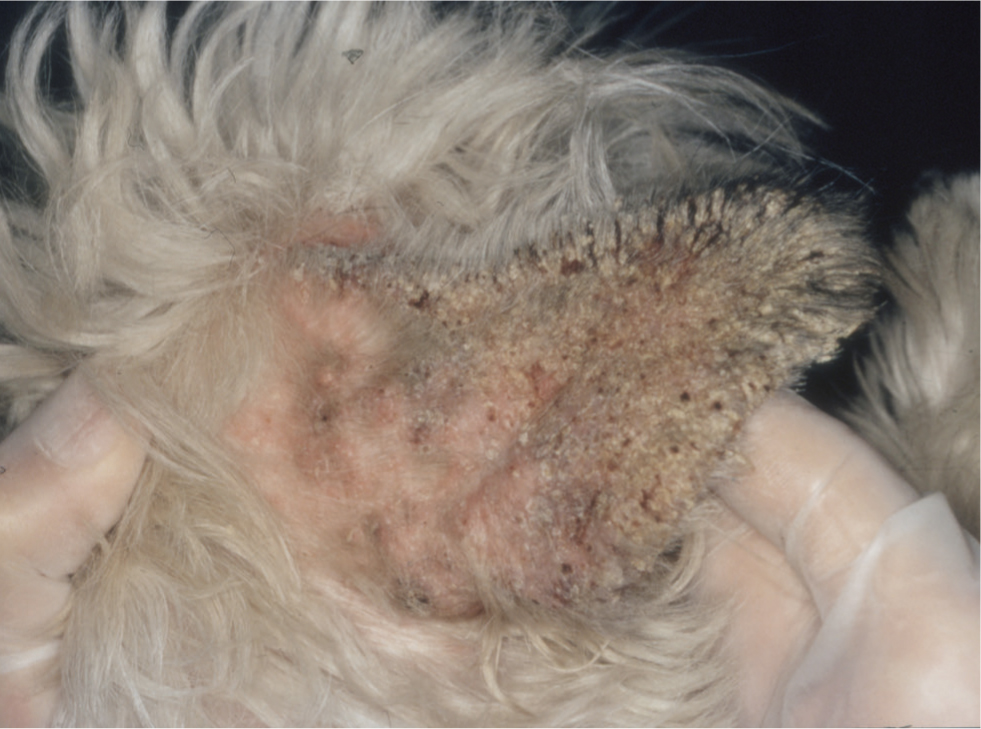
The classic lesion of sarcoptic mange is a crusted papule, which represents the site where the gravid female burrows into the skin to lay her eggs. In common with other infectious and ectoparasitic skin diseases, the lesions are often asymmetrical, differentiating them from allergic or immune-mediated dermatoses. As the disease progresses, the pinnal margins, elbows and hocks can become covered in heavy scale, and whole regions of the dog (such as the entire ventrum or the flanks) become erythematous and lichenified, as a consequence of repeated self-trauma. However, to differentiate the latter signs from those seen in cases of allergic skin disease, if one inspects the skin carefully, there are usually papules or papulocrustous lesions still present within these erythematous zones, particularly at their edges, and these are often the best sites to sample, as diagnostic material may not yet have been removed or dislodged from them by the animal's licking or scratching behaviour.
The definitive diagnosis of scabies relies on direct visualisation of mature or immature mites and/or their eggs and/or faeces in skin scrapings, skin biopsy specimens or faecal samples (Figures 11 and 12). Of the three, skin scraping is practised most frequently, yet mite detection rates as low as 20% are quoted in some texts. To optimise the chances of detecting mites, lesions should be selected discerningly. The most successful samples will be harvested from intact, undisturbed, crusted papules or areas of heavy scaling. Multiple skin scrapings using a size 10 scalpel blade should be performed and it is imperative that the entire epidermis is removed to effect capillary ooze, as only a proportion of the mite population resides on the surface of the skin.
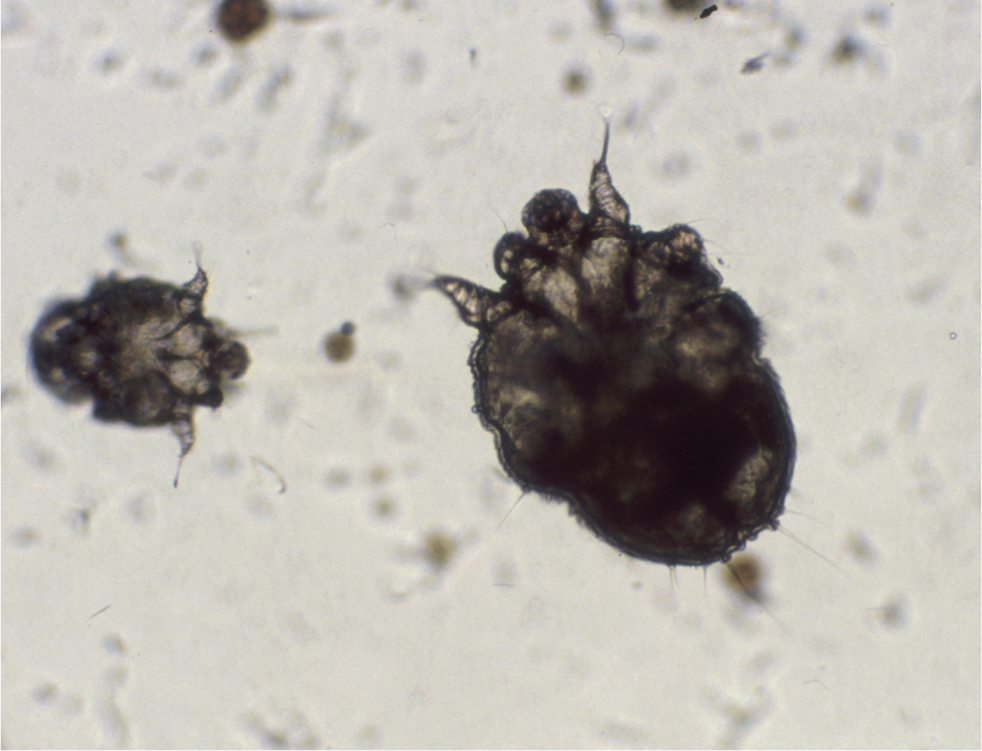
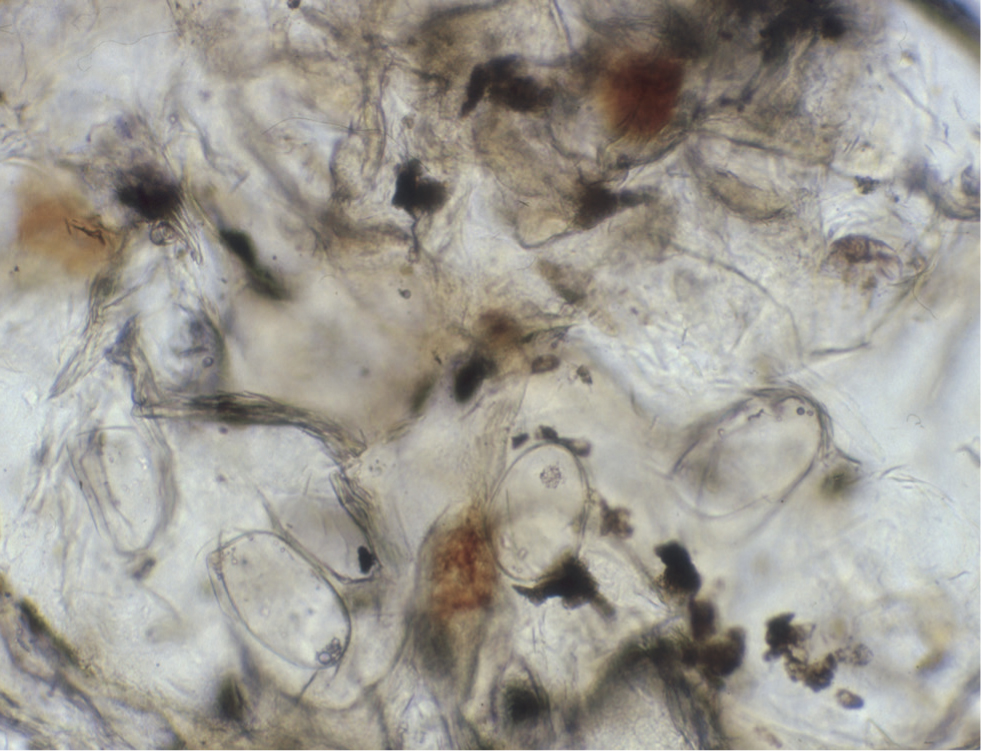
Dislodged material should be suspended in a few drops of liquid paraffin or mineral oil and a cover slip added, to both assist inspection beneath the microscope and to entrap the mites, as they can move through the liquid paraffin quite efficiently and are occasionally observed wandering off the slide. For the same reason, the samples should be examined as soon as possible, using light microscopy at scanning low power magnification (×40). Any areas of interest can be examined at high power (×100), and at this magnification it may be possible to make out aggregates of mite faeces (scybala) that are also diagnostic (Figure 12).
When mites elude microscopic detection, serum samples from suspected cases can be submitted for circulating anti-Sarcoptes IgG determination by enzyme-linked immunosorbent assay. This test was initially developed in Sweden (Bornstein and Zakrisson, 1993) and independent testing has reported sensitivity and specificity rates of 83% and 92% respectively (Curtis, 2001). Therefore, false negative results are possible and if the clinician remains suspicious of sarcoptic mange, a scabicidal therapeutic trial should be performed (this will be discussed further in part 2 of this review). Failure to respond to therapy should prompt a review of the major differential diagnoses for sarcoptic mange listed below:
- Flea bite hypersensitivity
- Atopic dermatitis
- Adverse cutaneous food reaction
- Superficial pyoderma
- Malassezia dermatitis
- Demodicosis
- Cheyletiellosis
- Pemphigus foliaceus.
Conclusions
The historical and clinical aspects of the common causes of canine mange, plus the approach to diagnosis, have been covered in part 1 of this series. Part 2 will discuss treatment and management options.
KEY POINTS
- The clinical signs of ectoparasitic skin diseases are often sudden in onset and so any patient (and particularly an older one with no previous history of skin disease) should be assessed for ectoparasitic infestation, if presenting with, for example, acute-onset pruritus.
- The maintenance and recent ectoparasitic control programme that an owner has in place should be discussed during the history-taking for all skin disease patients.
- Remember that ectoparasites do not typically cause symmetrical lesions/zones of pruritus, particularly early in the disease, so aim to establish this when asking the owner about the areas on the animal that are initially affected.
- Evidence of contagion between other co-habiting pets and/or the animal's owners provide vital clues about ectoparasitic diseases, but it should be remembered that lack of evidence of contagion does not rule out a parasitic disease.
- As time progresses, ectoparasitic infestations often spread to affect more of the skin surface area, whereas allergic skin diseases can remain confined to the same regions of the body.


