Canine atopic dermatitis is a chronic, pruritic, inflammatory and hereditary disease that affects around 10% of the patients under veterinary care (Hillier and Griffin, 2001). The International Committee on Allergic Diseases of Animals has recently proposed a change in definition to a ‘predominantly T-cell driven inflammatory skin disease involving an interplay between skin barrier abnormalities, allergen sensitisation and microbial dysbiosis’ (Tamamoto-Mochizuki et al, 2023a). Canine atopic dermatitis is very similar to human atopic dermatitis, most commonly referred to as eczema, and usage of this word rather than ‘allergy’ can help owners understand the chronicity of disease and the need for lifetime management.
When considering the number of dogs seen daily in companion animal practice, this 10% reflects a large number of patients and their owners living with a condition that impacts both pet and caregiver quality of life (Noli et al, 2011). Over the last few years, a change has been seen to the face of canine atopic dermatitis. Previously, this condition was most often seen in in Boxers, Staffordshire Bull Terriers and West Highland White Terriers, whereas now, French Bulldogs, designer Cavapoo and Cockapoo's, alongside the ever-faithful Labrador Retriever are seen more frequently (Bizikova et al, 2015).
This article reviews current knowledge around the pathogenesis of canine atopic dermatitis, changes in diagnostics and treatment options available and what considerations should be taken into account when devising a treatment plan for a patient.
Pathogenesis
The previous definition of canine atopic dermatitis was of a ‘genetically predisposed inflammatory and pruritic allergic skin disease with characteristic clinical features associated with immunoglobulin E antibodies most commonly directed towards environmental allergens’ (Halliwell, 2006). As mentioned earlier, the International Committee on Allergic Diseases of Animals have recently proposed expanding the definition of canine atopic dermatitis to better reflect the complexity of pathogenesis, with the intertwining of the environment, genetics and microbiome. It is seemingly impossible to detangle this complex web to determine the ‘which came first’ concept that has not yet been understood. It is known that skin barrier dysfunction exists, either as cause or consequence, with abnormalities in keratinocytes and cornified lipid envelope development as well as antimicrobial peptide function and release (Santoro et al, 2015). There are aberrations with both the innate and adaptive immune system resulting in T-helper 2, T-helper 17, T-helper 22 and T-regulartory cell dysregulation (Jassies-van der Lee et al, 2014; Olivry et al, 2016; Rostaher et al, 2018). The resultant effect is the clinical signs of canine atopic dermatitis frequently encountered in clinics.
Clinical signs
Most patients with canine atopic dermatitis will first present with clinical signs from 6 months to 3 years of age. Favrot's criteria (Table 1) can be used as a handy tool in clinic however, there are exceptions to this, and this table should not be solely relied on (Favrot et al, 2010).
Table 1. Favrot's Criteria: Match 5 out of 8 criteria, sensitivity and specificity are 85% and 79% respectively. Match 6 out of 8, sensitivity reduces to 58% and specificity increases to 89% (Favrot et al, 2010).
| 1. | Onset of signs under 3 years of age |
| 2. | Dog living mostly indoors |
| 3. | Glucocorticoid-responsive pruritus |
| 4. | Pruritus sine materia at onset (pruritus without lesions at onset) |
| 5. | Affected front feet |
| 6. | Affected ear pinnae |
| 7. | Non-affected ear margins |
| 8. | Non-affected dorso-lumbar area |
Some of the exceptions to this are the patients that only present with recurrent otitis, but on closer examination may be found to have additional clinical signs such as pedal pruritus. One study assessed breed specific differences in a variety of breeds including Boxers, Jack Russell Terriers and Labradors (Wilhem et al, 2011). This study noted that 80–100% of assessed German Shepherds showed ventral abdominal pruritus, assessed Shar Pei's showed both dorsal lumbar (40–60%) and hindlimb pruritus (60–80%) and 40–60% of assessed West Highland White Terriers demonstrated dorsal lumbar pruritus. These aforementioned patterns would have traditionally been associated with flea allergic dermatitis and show the importance of appreciating that breed variation occurs.
These differences in patterns also likely reflect alterations in both the endotype (pathogenesis and genetics) and phenotype (clinical presentation). Further research is needed in this area to better understand endotype and phenotype development in these patients so that therapeutics can be tailored more effectively.
History tips
So, when might an owner present their dog to the veterinary practice? The following are commonly encountered concerns:
- Scratching or licking keeping the owner up at night especially if the pet sleeps in the same room as them
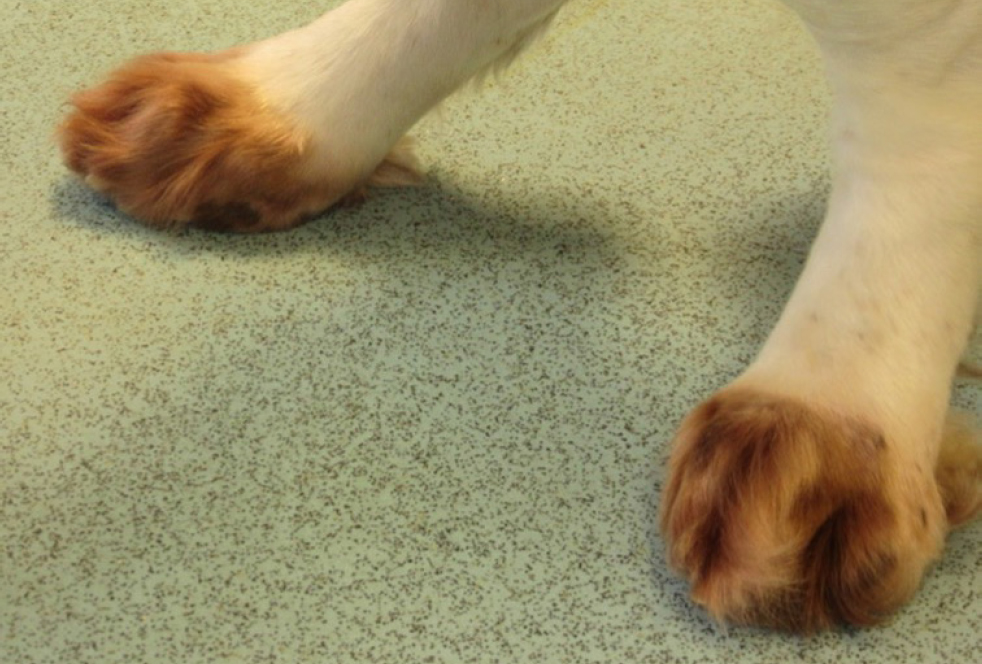
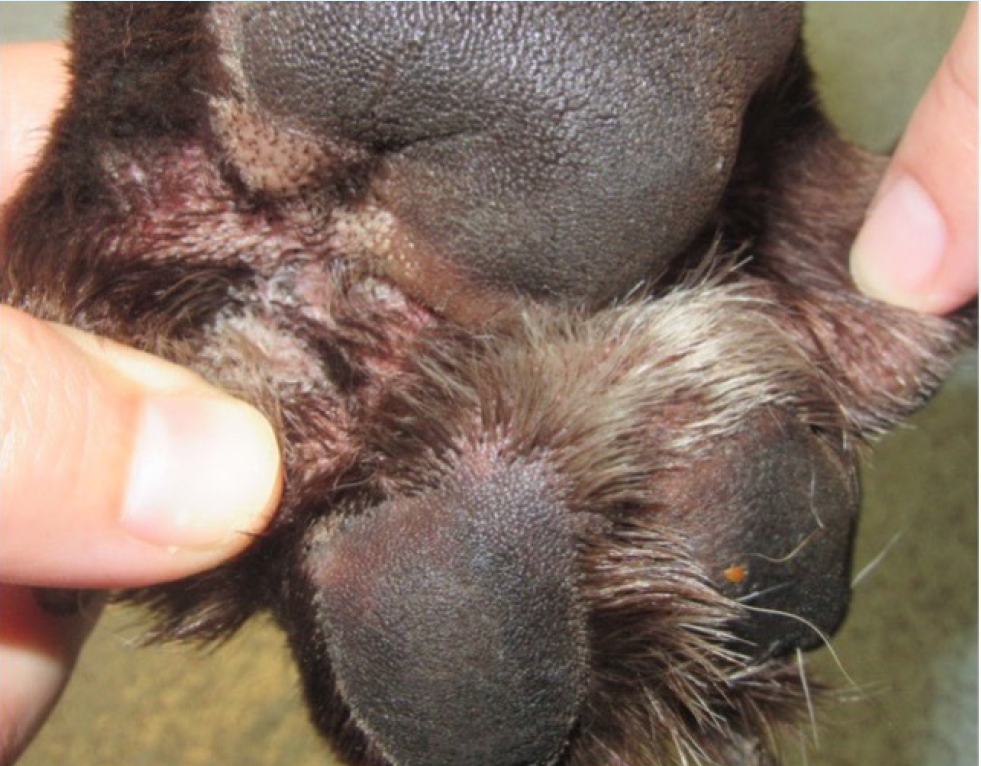
- Development of alopecia/noticing hair loss/bald spots/crust/thickened skin/change in coat colour especially on the paws in light colour coated animals
- Malodour
- In patients with ear disease – head shaking, scratching, ‘heat’ in the ears, pinnal erythema, malodour, discharge from the ear or into their house when the patient scratches, pain/discomfort when patted or when another pet comes close
- Groomer has noted a change.
Pertinent questions to ask, especially when short on time, can include:
- What, when, where; is there a why?
- What have they tried before coming in?
- Ectoparasite control – what, when was it last used, interval in use, other pets
- Any zoonosis or contagion
- Any changes at home – usually asking regarding renovations and the like – problem in patients with house dust mite sensitisations; the dog is very unlikely to be ‘allergic’ to the new carpet but all of the dust or dust mites ‘kicked up’ into the environment during renovations has caused a significant problem
- Kennels, dog walker and travel history
- Diet – not just in case food allergy is present but also safety issues like bones and raw food, should barrier nurse, be cognisant of the risk of carrying antimicrobial resistant microbes and challenges that can occur with use of immunosuppressive medication. Diet is also important in young dogs or those with perennial clinical signs
- Bowel movements – number of times per day, consistency, borborygmi and flatulence are all important suggestions of food playing a role in disease.
For those in practice who are keen on dermatology, development of a questionnaire to cases that are coming in for a dermatology specific consult (for example, those patients that are having recurrent episodes of otitis, pyoderma or pruritus) can either be emailed before the consult or handed to them on arrival. This can significantly expedite the conversation in a 20 minute consultation. For those that have shorter consult times, it is important to charge appropriately for these extended consultations. Most practice management systems allow attachment of clinical images to the patient record which can be used as a reference of lesion progression or regression.
Itch scores can be another handy tool not only for clients and clinicians to monitor response to therapy, but also useful when clinician continuity cannot be guaranteed. There are two regularly used scores; Hill et al's (2007) pruritic visual analogue scale is most commonly recognised as a 10 centimetre scale with descriptors to which owners are asked to mark on the scale the level of pruritus correlates most with their pets clinical signs (Hill et al, 2007). The mark should then be measured from the base in centimetres. The other scale is a verbal numerical scale from 0–10 with verbal ratings of mild, moderate and severe (Strzok et al, 2022). Both scales are independently validated with the latter study demonstrating that they are not interchangeable. Finally, there are lesion severity scores including canine atopic dermatitis extent and severity index (CADESI) with 04 (CADESI-04) being the latest version. These indices can be useful in referral settings, however, in general practice they are time consuming.
Diagnostics
These are very important to ensure a different condition masquerading as canine atopic dermatitis (such as Sarcoptes scabiei spp. infestation) is not missed. The role and importance of cytology, skin scrape and trichogram cannot be downplayed.
1. Cytology
The type of infection, if any, must be determined. Vets should not be reaching for antimicrobials if there is no indication for infection (which, in canine atopic dermatitis patients, is really dysbiosis of the microbiome). Cytology can be collected using a cotton bud from exudative areas such as the ears and lip folds; acetate tape from the paws and impression smears from the skin directly under a freshly lifted crust. Samples can then be quickly air dried (usually less than 1 minute) before being stained with modified Romanowky stain (Diff-Quik®; Rapi-Diff®), dried, immersion oil and a cover slip applied for cotton bud and impression smears and assessed under microscope. Acetate tape samples do not require a cover slip and immersion oil can be applied directly to the top of the sample for examination under a x100 magnification lens.
2. Skin scrape
Use rounded scissors to gently clip any hair from the sample area. Squeeze the skin gently before applying liquid paraffin to the skin and blunted scalpel blade. Holding the skin taut, scrape at a 45° angle along the direction of hair growth before scooping any scraped material and transferring to a glass slide with a few drops of liquid paraffin present on the surface.
3. Trichogram
Collect 100–200 hairs (not necessarily all at once) from representative areas using forceps, which can be protected with drip tubing, and place on to a glass slide with liquid paraffin applied to it. Try to keep all hairs in the same alignment (for example, roots to the right and tips to the left) to allow easy assessment for hair cycle stage, ecto-parasites including Demodex spp., dermatophytosis along the length of the hair or evidence of self-trauma on the hair tips.
Once ectoparasites have been ruled out and any infections (which may or may not be associated with underlying canine atopic dermatitis) have been managed, the baseline itch can be established. It is also important to consider whether or not blanket ectoparasite treatment or a treatment trial is needed in all patients. There is somewhat of a disconnect between companion and large animal practice, whereby responsible use of endectocides is much lauded within in the latter, yet in small animal practice mass use can be commonplace. It should be considered whose benefit this is for and what the environmental implications of widespread use are.
Elimination diets to determine the role of food in disease are necessary in many, but not all, patients. Those with perennial (all year round) clinical signs, young dogs (under 1 year of age) and slightly older dogs with recent onset of pruritus (over 6 years) are those that benefit most from elimination diet. Additional questions as mentioned above including bowel habits can help increase the index of suspicion. Elimination diets should not be considered in patients with seasonal-only clinical signs, those in which the pattern of disease is undetermined (except in the aforementioned age ranges) nor performed at a time of year in which the patient is known to flare.
Currently it is advised to feed a hydrolysed diet for 6–8 weeks and the time of this diet can be shortened using appropriate, short acting anti-pruritic medication such as glucocorticoids or oclacitinib for the first 2–3 weeks of the elimination diet followed by a short 3–5 day course if required (Fischer et al, 2021). If the patient's clinical signs remain controlled on an elimination diet for 2 weeks without anti-pruritic therapy and relapse upon challenge with previous diet, then this is highly suggestive of food playing a role in disease. Ideally, the patient should be challenged more than once to confirm food reaction. Once clinical signs are controlled again on an elimination diet for 2 weeks, the clinician should scrutinise the previously offered diets, treats and the like to identify suspected offending allergens. The patient can be challenged with home cooked single protein sources such as chicken or white fish. Approximately 50% of patients will flare by day 5 of consumption of an offending protein although up to 2 weeks is reported (Olivry and Mueller, 2020). If the patient tolerates a protein for 2 weeks without recurrence of clinical signs, then this protein can be considered safe and further proteins trialled. The aim is to then be able to identify commercial diets that the patient can be maintained on long term although some clients may opt to continue the hydrolysed diet for the duration of the dog's life.
It is worth noting that a comprehensive diet history is required to ensure that the parent molecule of the hydrolysed diet has not previously been fed. A small-scale study found 40% of chicken-sensitised patients still reacted to a hydrolysed chickenbased diet, with the same cohort not reacting to a poultry feather hydrolysed diet (Bizikova and Olivry, 2016). There is currently no evidence supporting the ability of allergen serology testing to correctly identify patients with an adverse food reaction given high variability, poor reliability and correlation to clinical signs. As such, an elimination diet followed by re-challenge remains most appropriate.
The reader is directed towards the comprehensive open access critically appraised topic reviews on adverse food reaction available from BioMedCentral Veterinary Research available here: https://www.biomedcentral.com/collections/catsfoodreactions
Treatment options
Following history, clinical examination and diagnostics, the veterinarian will hopefully be better informed about the owner's concerns, distribution and severity of clinical lesions as well as presence and type of any secondary infection or overgrowth that it evident.
Treatment of secondary overgrowth, infection and dysbiosis
It is important to treat secondary infections and/or overgrowths appropriately. Successful treatment can often significantly impact the itch score (for example, an itch score of 7/10 with significant secondary Malassezia overgrowth that drops to 4/10 once effectively treated (2/10 or less is considered normal)).
Topical products are usually all that is required especially in Malassezia overgrowth, surface and superficial infections. They are a helpful adjunct in deep pyoderma cases. There is good evidence for the use of 2–4% chlorhexidine containing products in bacterial pyoderma (Santoro, 2023). There is strong evidence for 2% miconazole/2% chlorhexidine shampoo for Malassezia dermatitis and moderate evidence for 3% chlorhexidine shampoo with insufficient evidence currently for use of other products such as foams, sprays and wipes (Bond et al, 2020).
Systemic antimicrobials are only used in certain circumstances and their use should be guided by clinical examination, cytological findings, microbial culture and susceptibility testing where appropriate. So, when is it appropriate to consider culture and susceptibility testing? Evidence of rods in a patient with deep pyoderma, previous use of systemic antimicrobials (within the previous year) and poor response to recent course of antimicrobial therapy can all be indicators of antimicrobial resistant infection. The reader is directed towards the open access articles by Beco et al regarding antimicrobial choices (Beco et al, 2013a; 2013b). Use of systemic treatments for Malassezia dermatitis should be restricted to those cases where compliance (patient and owner) is limited such as owners with mobility concerns or aggressive patients to help reduce the risk of development of resistance.
Therapeutics for the management of canine atopic dermatitis
Once infection and overgrowth are suitably controlled, the clinician can target the underlying atopic dermatitis. On occasion, there is benefit to treating both at the same time (within reason). Mirroring the current trend in human atopic dermatitis management, vets now aim to start patients on reactive or induction treatment in the hope that this induces remission before deciding on a proactive or maintenance treatment plan that works for both the patient and owner to manage this long term disease (Olivry and Banovic, 2019). Therapeutics used at these separate stages may be the same but at differing doses, such as glucocorticoids, or they may be completely agents such as glucocorticoids to induce remission and long term oclacitinib for proactive or maintenance treatment.
So, what factors should veterinarians consider to help choose the most appropriate therapeutic?
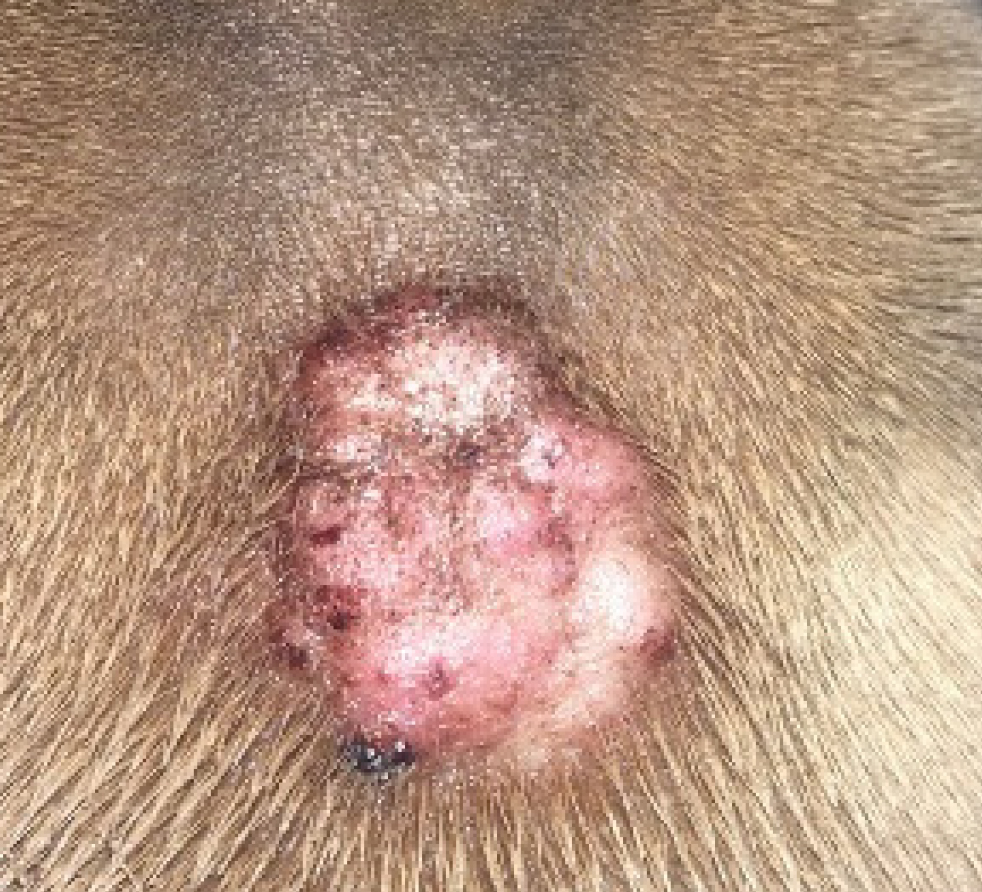
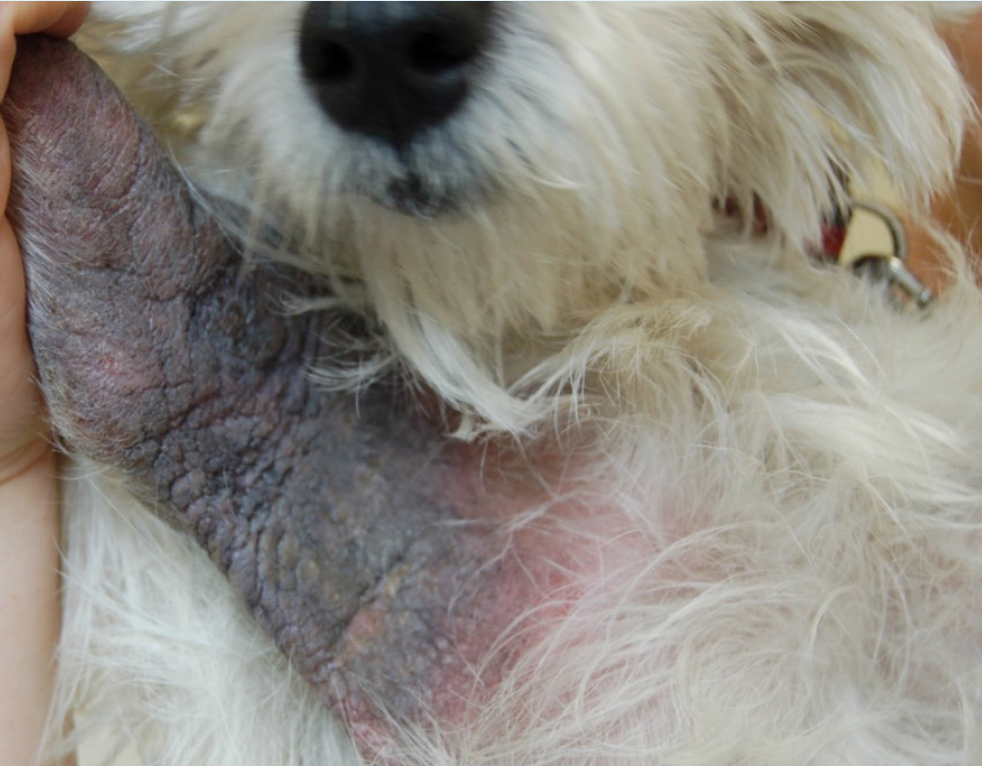
- Level of inflammation, lichenification, hyperpigmentation or chronic change present. It is also important to note that some patients can show a combination of both acute and chronic lesions in different body sites. For example, in patients with minimal clinical evidence of inflammation and lack of chronic change, lokivetmab or oclacitinib can often be most appropriate versus patients with severe, chronic change such as the West Highland White Terrier in Figure 2.
- Systemic versus topical. For patients with generalised dis ease, systemic therapy is usually most appropriate. For patients with predominant recurrent otitis, regular ear cleaning and application of topical glucocorticoids sufficiently controls disease and prevents flares.
- Compliance of both the patient and client. It is important to consider clients work/home life as well as other commitments as those on shift work may struggle with medications that require regular intervals.
- Adverse effects, side effects or contraindications. For example, in patients with a previous history of neoplasia, both ciclosporin and oclacitinib would be considered inappropriate without sufficient informed consent.
- Cost. Regardless of veterinarians perceptions, cost is not often the deciding factor for clients however, they should remain cognisant of this – especially with the cost of living crisis and veterinary practices in the UK under review by the Competitions and Marketing Authority at the time of writing.
It is important to discuss these factors with clients so that they feel empowered with their choice – this will hopefully also help with compliance. When presenting options for patients, it is important to ensure interventions are considered to manage immune dysfunction, underlying allergy or sensitisation component and ideally, management of barrier function.
Systemic agents (in order of breadth of targeting with widest breadth first)
The following systemic agents are listed in order of breadth of targeting, with those that have a broad action (target multiple inflammatory cells, signalling pathways and such) listed first before those with a narrow target (like a single cytokine) mentioned last.
a. Glucocorticoids
Glucocorticoid treatment, systemic or topical, remains the mainstay of management of canine atopic dermatitis for many patients. Use results in a reduction in production of inflammatory cells and mediators, thus effectively controlling inflammation and pruritus (Saridomichelakis and Olivry, 2016). Glucocorticoids are fast acting and inexpensive. However, adverse effects can include polyuria, polydipsia, polyphagia, behaviour change, muscle wastage, weight gain and skin atrophy amongst others (Koch et al, 2012). Glucocorticoids can be considered for reactive and proactive or maintenance management of canine atopic dermatitis. If long term glucocorticoid treatment is to be considered, tapering to determine the most effective dose should be performed (Olivry et al, 2015). Adjuncts to glucocorticoid therapy including antihistamines (off licence in the UK) and/or essential fatty acids can be utilised to allow dose reduction (Paradis et al, 1991; Saevik et al, 2004). Systemic and/or topical glucocorticoids remain the mainstay of therapeutics for both induction and maintenance of atopic otitis and should be used as part of the multimodal approach.
b. Ciclosporin
Ciclosporin is a systemic calcineurin inhibitor licensed for use in canine atopic dermatitis patients. It acts to prevent gene transcription resulting in a reduction in cytokines including interleukin-2, interleukin-4 and interferon gamma, thus reducing the number and activity of antigen presenting cells and mast cells and reduction in immunoglobulin E-producing B cells (Forsythe and Paterson, 2014). Ciclosporin takes several weeks for maximum effect, so it is inappropriate to use during a flare or for reactive therapy. It can be combined with oral glucocorticoids or oclacitinib for a short period of time until effective, usually as a 2–3 week tapering course (Dip et al, 2013; Panteri et al, 2016).
Short term side effects can include vomiting and diarrhoea, which are usually self-limiting. Gingival hyperplasia and excessive hair growth can occur in a small number of patients (Nuttall et al, 2014). As ciclosporin utilises the P450 cytochrome enzyme system, there are known drug interactions which prescribing clinicians should be familiar with. Rare opportunistic infections can be seen on ciclosporin, and it is advisable to counsel owners to monitor for development of single or multiple nodules as well as draining tracts. There is no evidence to suggest regular biochemical, haematological analysis or urinalysis is required in patients on long term ciclosporin unless there is a clinical suspicion. Long term treatment can usually be tapered by reducing frequency of dosing (rather than total dose) to find the most effective dosing regimen for each patient which subsequently lowers the cost.
c. Oclacitinib
Oclacitinib is a Janus kinase inhibitor, predominantly Janus ki-nase-1, licensed for treatment of clinical signs of canine atopic dermatitis as well as pruritus associated with allergic dermatitis in dogs over 12 months of age. By blocking the Janus kinase/signal transducers and activators of transcription pathway, it reduces production of key cytokines including interleukin-2, interleukin-4, interleukin-13 and interleukin-31. It is as fast acting as glucocorticoids, effective with a good safety profile and it has now been utilised in veterinary practice for 10 years.
Common side effects include vomiting, diarrhoea, otitis, lethargy, weight gain and pyoderma (Cosgrove et al, 2015). It is contraindicated in patients with neoplasia, generalised demodicosis and serious infection, and patients should be monitored for the development of any cutaneous masses. Routine monitoring of biochemistry, haematology and urinalysis is advised as haematological and biochemical disturbances and proteinuria have been reported (Cosgrove et al, 2015; Simpson et al, 2017). An abstract published by Hill et al (2017) showed an increase in reported histiocytomas in patients on long term oclacitinib compared to treatment with ciclosporin (2.6% versus 0.6%). A recent study involving 660 dogs with 339 dogs on oclacitinib for at least 6 months with a minimum of 2 year follow up with age and breed matched controls (321 dogs) did not find that oclacitinib therapy posed an increased risk to neoplasia development (Lancellotti et al, 2020).
Oclacitinib can be used in both reactive or flare situations as well as a proactive or maintenance treatment. Given its mechanism of action and short half-life (4–6 hours), tapering of the dose interval is rarely possible but it can be possible to reduce the daily dose.
d. Lokivetmab
Lokivetmab (Cytopoint®) is a caninised interleukin-31 monoclonal antibody which acts to bind and neutralise interleukin-31, thought to be a major pruritogenic cytokine. The European licensed dose is 1-3.3 mg/kg to be given subcutaneously every 4 weeks with 2 mg/kg every 4–8 weeks.
It has been reported to reduce pruritus by 60% and lesion score by around 45% (Michels et al, 2016). A further study noted that when given proactively it prevented expected pruritus but not lesion development (Tamamoto-Mochizuki et al, 2019). A follow up study in a colony of Maltese-Beagles with spontaneous canine atopic dermatitis challenged with housedust mite extract, noted that sole interleukin-31 inhibition with lokivetmab was insufficient to prevent expression of other pro-inflammatory mediators (Tamamoto-Mochizuki et al, 2023b). Taken altogether, these studies illustrate why lokivetmab is not effective in all canine atopic dermatitis patients and why duration of activity can vary.
Lokivetmab can be used in some patients for reactive treatment; however, it is worth bearing in mind that there can be non-visual inflammation in the skin and that glucocorticoids or oclacitinib may be more appropriate first line therapeutics, and lokivetmab used for proactive or maintenance treatment. If patients do not respond to two injections of lokivetmab at an appropriate 4 week interval, this therapeutic is not going to successfully manage clinical signs and alternatives should be considered.
Topical agents
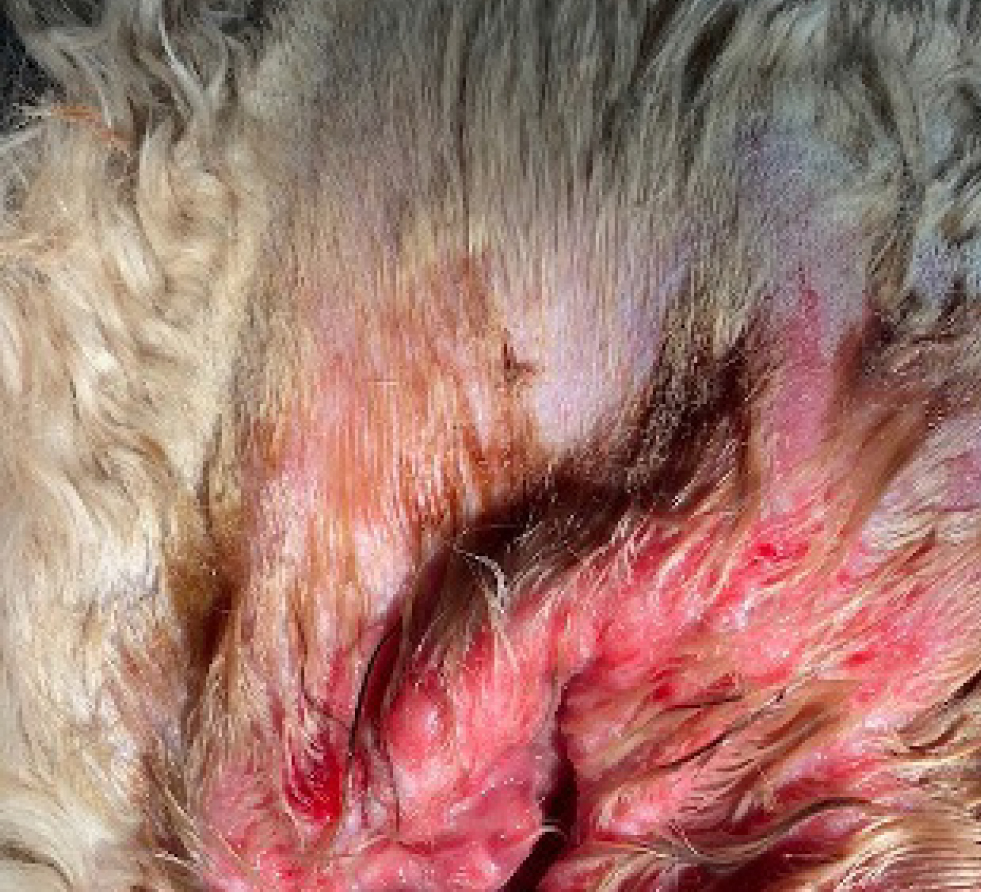
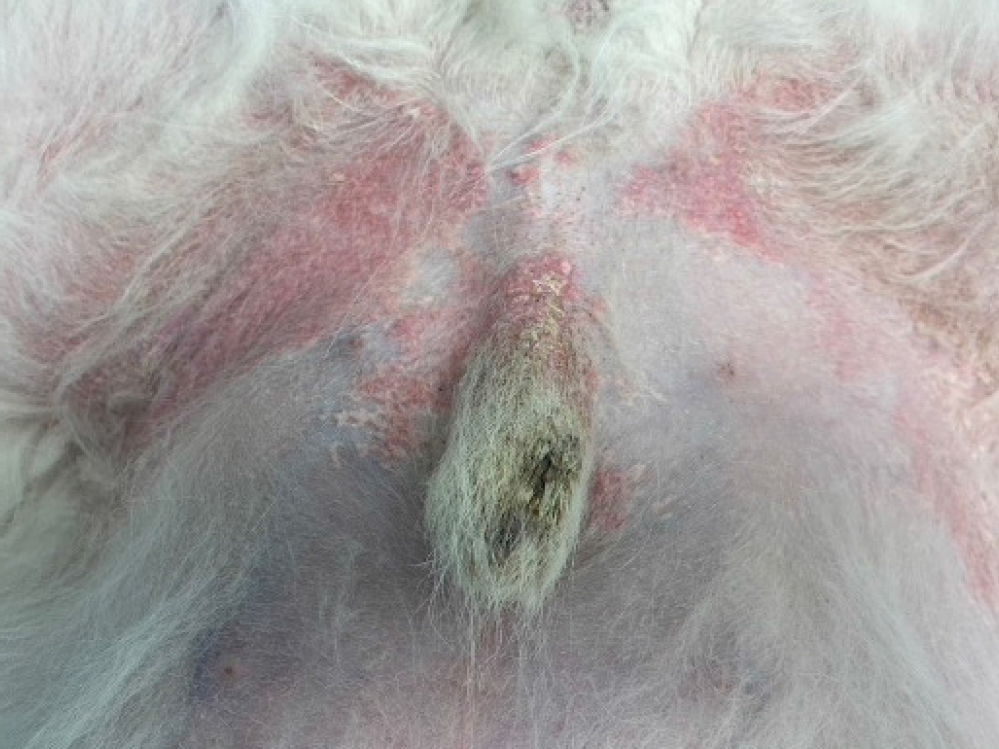
Topical agents are incredibly useful for management of localised areas such as the ears, axillae or paws. They can be utilised as part of a multimodal management plan for canine atopic dermatitis patients with minimal systemic side effects, although it is important to monitor for adverse effects including skin atrophy and calcinosis cutis which can occur with both systemic and topical therapy (Koch et al, 2012) (Figure 6).
a. Glucocorticoids
These are available as sole agents such as 0.0584% hydrocortisone aceponate. Proactive use of hydrocortisone aceponate applied to the skin on 2 consecutive days in patients with controlled atopic dermatitis was found to significantly reduce the length of time to flare (Lourenço et al, 2016). Hydrocortisone aceponate is largely metabolised in the skin (Nuttall et al, 2009). There was no alteration in adrenocorticotrophic hormone stimulation, biochemistry or haematology when hydrocortisone aceponate was applied daily for 70 days (Nuttall et al, 2009).
b. Tacrolimus
Tacrolimus (Protopic®) is a topical calcineurin inhibitor. It is as effective as topical glucocorticoids without the unacceptable adverse effects such as skin atrophy. However, it is expensive and application to haired skin is difficult. Use of this product is off licence in the UK.
Skin barrier management
This is an area currently under research in canine atopic dermatitis, especially as it is has not been determined if the noted changes are caused by or are a consequence of canine atopic dermatitis. Current recommendations are to use a combination of omega-3 and omega-6 fatty acids, and the optimum ratio is not known. Most premium dog foods are well fortified with these fatty acids.
Proactive management of the allergic component of canine atopic dermatitis
Allergen specific immunotherapy is the only therapeutic shown to help manage the allergic component of canine atopic dermatitis (Mueller, 2023). This treatment takes 3–6 months to start to become effective and therefore, is unsuitable for initial management of disease. Allergen specific immunotherapy comes in three main forms: intralymphatic, sublingual and subcutaneous. Allergen specific immunotherapy acts to induce production of T regulatory cells by interleukin-10 and transforming growth factor ß (Keppel et al, 2008).
Allergen testing for environmental allergens, either by allergen serology or intradermal testing, should only be performed by the clinician once the owner has decided that allergen specific immunotherapy is an option for both them and their pet not only in terms of finances but also compliance. It is otherwise impossible for patients to avoid inciting allergens. The method of choosing allergens for inclusion is unaffected by the mechanism of identification (serology or intradermal testing) but it is imperative that included allergens are determined based on the history of the patient.
Cross-reactive carbohydrate determinants are components of glycoproteins commonly found in the surface of plants and insects, and patients can develop immunoglobulin E to cross-reactive carbohydrate determinants (Levy and DeBoer, 2018). This can complicate allergen serology testing as anti-cross-reactive carbohydrate determinant immunoglobulin E is not clinically significant, as it does not crosslink antibody motifs resulting in degranulation of mast cells but can be detected on testing. Blocking anti-cross-reactive carbohydrate determinant immunoglobulin E can improve correlation between intradermal testing and allergen serology testing in patients with detectable levels of anti-cross-reactive carbohydrate determinant immunoglobulin E (Gedon et al, 2019). However, a further study showed no impact on allergen serology results however, this may have been impacted by use of ciclosporin and lokivetmab on levels of immunoglobulin E (Canning et al, 2021).
Cross reactions and co-sensitisations also exist, so it is unnecessary and costly to include all sensitisations (Buckley et al, 2013). Furthermore, as each bottle of allergen specific immunotherapy contains a set concentration of allergens, inclusion of many allergens can lower the concentration of each one and subsequently be insufficient to induce tolerance.
Intralymphatic allergen testing can be performed in clinic without ultrasound guidance with one injection (0.1 ml) given every 4 weeks for 6 months before changing to a subcutaneous protocol (Mueller et al, 2023). Sublingual immunotherapy is usually given twice daily starting with a low concentration of allergen and progressing to a higher concentration. Finally, subcutaneous immunotherapy, the more widely recognised form, typically starts with ever increasing increments with or without concentrations of allergen solution, usually either in an aqueous or alum-precipated format. There are pros and cons to each of these forms of allergen specific immunotherapy with a recent review by Mueller discussing these (Mueller, 2023). Allergen specific immunotherapy is an ever-evolving field and there will be further advancements to come over the next few years.
Conclusions
Canine atopic dermatitis is a common, chronic, pruritic skin disease that has an impact on both owners and patients quality of life, and affects approximately 10% of canine patients. Pathogenesis of the disease is complex and routine diagnostics should be performed in patients presenting with compatible clinical signs to rule out any conditions that mirror clinical signs. There are multiple therapeutic agents, both systemic and topical, available to manage this condition and the choice of multimodal therapy should be individualised to the patient and client. This is an area of constant research in veterinary dermatology which will hopefully help improve the quality of life of our canine patients.
KEY POINTS
- Canine atopic dermatitis is a complex multifactorial disease which requires multimodal therapy to induce remission (reactive therapy) before determining a long-term proactive plan.
- Canine atopic dermatitis is a dynamic disease which fluctuates, so both clinicians and owners should be aware that treatment options can alter at different time points and it can take time to determine the most appropriate treatment plan for that patient at each particular time point.
- It is imperative that secondary infection or dysbiosis is managed appropriately as this can be a common reason for flare ups of clinical signs.
- There are a multitude of therapeutics available that can be employed to control clinical signs.
- Regular rechecks should be employed to improve communication, management and compliance with the treatment regimen.


