Brachycephalic obstructive airway syndrome is a chronic, lifelong, hereditary, respiratory condition related to the shortened skull and muzzle confirmation of brachycephalic patients. Kennel Club registrations of brachycephalic dogs saw a marked increase over the past decades with increases of 193%, 3104% and 96% of pugs, French bulldogs and bulldogs respectively between 2004 and 2017 (British Veterinary Association, 2021). More recent data show that registrations of bulldogs continue to climb, while that of pugs and French bulldogs have reduced from their peak but are still very high (Kennel Club, 2023), despite ongoing campaigns to highlight the health concerns related to the breeds (RSPCA, 2022) and reduce their use in media advertising (Blue Cross, 2021). Around 50% of extreme brachycephalic breeds including the pug, French bulldog and bulldog are functionally affected by brachycephalic obstructive airway syndrome (Liu et al, 2016). Approximately 20% of brachycephalic patients are presented to primary care practice for an upper respiratory tract disorder within their lifetime (O'Neill et al, 2015). However, given that around 60% of brachycephalic dog owners may not perceive their dog has a breathing problem (Packer et al, 2012; Liu et al, 2015), it is likely many more of these patients are suffering from brachycephalic obstructive airway syndrome without intervention. This article reviews the current understanding around the respiratory component of this complex condition, alongside the diagnostic and therapeutic options available.
Pathogenesis
Many decades of selective breeding has resulted in dogs with mediolateral widening of the skull and rostrocaudal shortening of the muzzle without a proportional reduction in soft tissues of the face. This leads to relative narrowing of upper respiratory tract (Ekenstedt et al, 2020).
Primary respiratory abnormalities
Stenotic nares occur because of a deformation in the wing of the nostril, involving a relative oversize of the alar fold that contacts the nasal septum from the lateral aspect and an immobility of the wing of the nostril at exercise. This results in a marked increase in airflow resistance (Ekenstedt et al, 2020; Krainer and Dupré, 2023). It has been estimated that around half of French bulldogs have stenotic nares (Liu et al, 2017a), and the increase to airflow resistance from open or mildly stenotic nares to moderate and severely stenotic is 10 times and 29 times respectively (Khoa et al, 2021). This resistance can then prevent appropriate saturation and humidification of inspired air, meaning these patients also experience poor thermoregulation (Khoa et al, 2021).
Soft palate hyperplasia takes the form of both elongation beyond the tip of the epiglottis, resulting in obstruction of the rima glotidis (Ekenstedt et al, 2020), and pathological thickening via mucous gland hyperplasia and oedema accompanied by a reduction in muscle mass because of necrosis and degeneration (Pichetto et al, 2011; Crosse et al, 2015). Up to 87% of patients with brachycephalic obstructive airway syndrome have an elongated soft palate (Riecks et al, 2007), with soft palate thickness correlating with the severity of clinical signs or brachycephalic obstructive airway syndrome (Grand and Bureau, 2011). Nasopharyngeal space is still dependant on the relationship between head size and these soft tissue structures; French bulldogs have thicker soft palates than pugs, but pugs still have a smaller relative nasopharyngeal airway space despite this (Heidenreich et al, 2016).
Aberrant turbinates and mucosal hypertrophy of this nasal tissue also contributes to reduced airflow through the nasal cavity (Ekenstedt et al, 2020). Between 21% and 77% of brachycephalic patients have aberrant turbinates extending into the nasopharyngeal meatus, causing narrowing of this airspace (Oechtering et al, 2007; Ginn et al, 2008; Auger et al, 2016). Pugs appear to be overrepresented within brachycephalic patients (Oechtering et al, 2007; Ginn et al, 2008). Unfortunately, the exact functional impact that this excess tissue has on these patients is unclear, as they are also seen in healthy unaffected brachycephalic patients (Vilaplana Grosso et al, 2015) and normocephalic patients without nasal disease (Auger et al, 2016).
Tracheal hypoplasia is characterised by small and rigid tracheal cartilage rings with a shortened or absent dorsal tracheal membrane (Ekenstedt et al, 2020). It is defined as a ratio of the tracheal diameter to the thoracic inlet of less than 0.12 in bulldogs, less than 0.16 in other brachycephalic breeds and less than 0.2 in non-brachycephalic breeds (Harvey and Fink, 1982; Regier et al, 2020; Krainer and Dupré, 2023). It is primarily noted in English bulldogs and screw tailed brachycephalic breeds (Komsta et al, 2019), with between 54% and 70% of bulldogs with brachycephalic obstructive airway disease having this component (Harvey and Fink, 1982; Coyne and Fingland, 1992; Riecks et al, 2007; Regier et al, 2020). Tracheal hypoplasia has not been shown to have a clinical impact in the absence of concurrent respiratory or cardiac disease (Coyne and Fingland, 1992), and does not seem to be improved by surgery to alleviate brachycephalic obstructive airway disorder (Regier et al, 2020). This is in contrast to an increase in tracheal diameter seen following resolution of bronchopneumonia (Clarke et al, 2011).
Macroglossia, an enlarged and thickened tongue relative to skull size, has also been noted as a primary factor as it not only reduces airway space but also further displaces the soft palate dorsally (Jones et al, 2020; Siedenburg and Dupré, 2021). However, little attention is given to this area as surgical options to address this are lacking, and the effect of tongue size is difficult to fully quantify as the position of the tongue changes dynamically with breathing.
Secondary respiratory abnormalities
Everted tonsils are found in up to 56% of patients with brachycephalic obstructive airway disorder and are suspected to occur as a result of increased negative pressure during inspiration (Fasanella et al, 2010). Excised tonsils show inflammatory changes and oedema (Belch et al, 2017) and may well contribute to reduction in airway space.
Laryngeal collapse is described in three stages. Typically, stage 1 involves the eversion of the laryngeal saccules, stage 2 involves the medial displacement of the cuneiform process of the arytenoid cartilage and stage 3 involves the collapse of the corniculate process of the arytenoid cartilage with loss of the dorsal arch of the rima glottidis (Leonard, 1960). This is thought to occur as a result of increased negative pressure during inspiration (Koch et al, 2003; Pink et al, 2006; Fasanella et al, 2010) combined with degenerative histological changes of the arytenoid cartilage resulting in mechanical weakness and possibly chondromalacia. This means they are less able to withstand the negative pressures generated in inspiration (Tokunaga et al, 2020). In some studies, laryngeal collapse has been shown to be present in up to 97% of cases; however, pugs appear to be particularly over-represented (De Lorenzi et al, 2009; Liu et al, 2019).
Tracheal and bronchial collapse is another common finding, with one study identifying it in 87% of patients with brachycephalic obstructive airway syndrome (De Lorenzi et al, 2009). It is suspected to occur through similar mechanisms to laryngeal collapse and has been shown to correlate with the severity of laryngeal collapse, but it has not been shown to impact post surgical outcome regardless of severity (De Lorenzi et al, 2009).
Clinical signs
The signs exhibited by these patients can vary greatly dependant on the severity of their disease and the degree to which each primary or secondary abnormality is present. The vast majority of patients present with respiratory noise, respiratory effort, exercise intolerance, sleep apnoea, heat intolerance or in extreme cases, cyanosis and collapse (Hendricks et al, 1987; Hendricks, 1992; Kenny et al, 2022). Other commonly noted signs are gastrointestinal in nature, with regurgitation and vomiting frequently reported and often worsening at exercise or excitement (Poncet et al, 2005; Kenny et al, 2022). Occasionally, these patients can also present with signs related to middle ear effusions including pruritis, head shaking, vestibular signs or head and neck pain (Schuenemann et al, 2022).
Diagnostics
Conformational risk factors for brachycephalic obstructive airway syndrome include obesity, stenotic nostrils, thick neck girth and relative short muzzle length (Packer et al, 2015; Liu et al, 2017a). These initial aspects, along with a clinical history and physical examination, may well be sufficient to form a clinical diagnosis of brachycephalic obstructive airway syndrome.
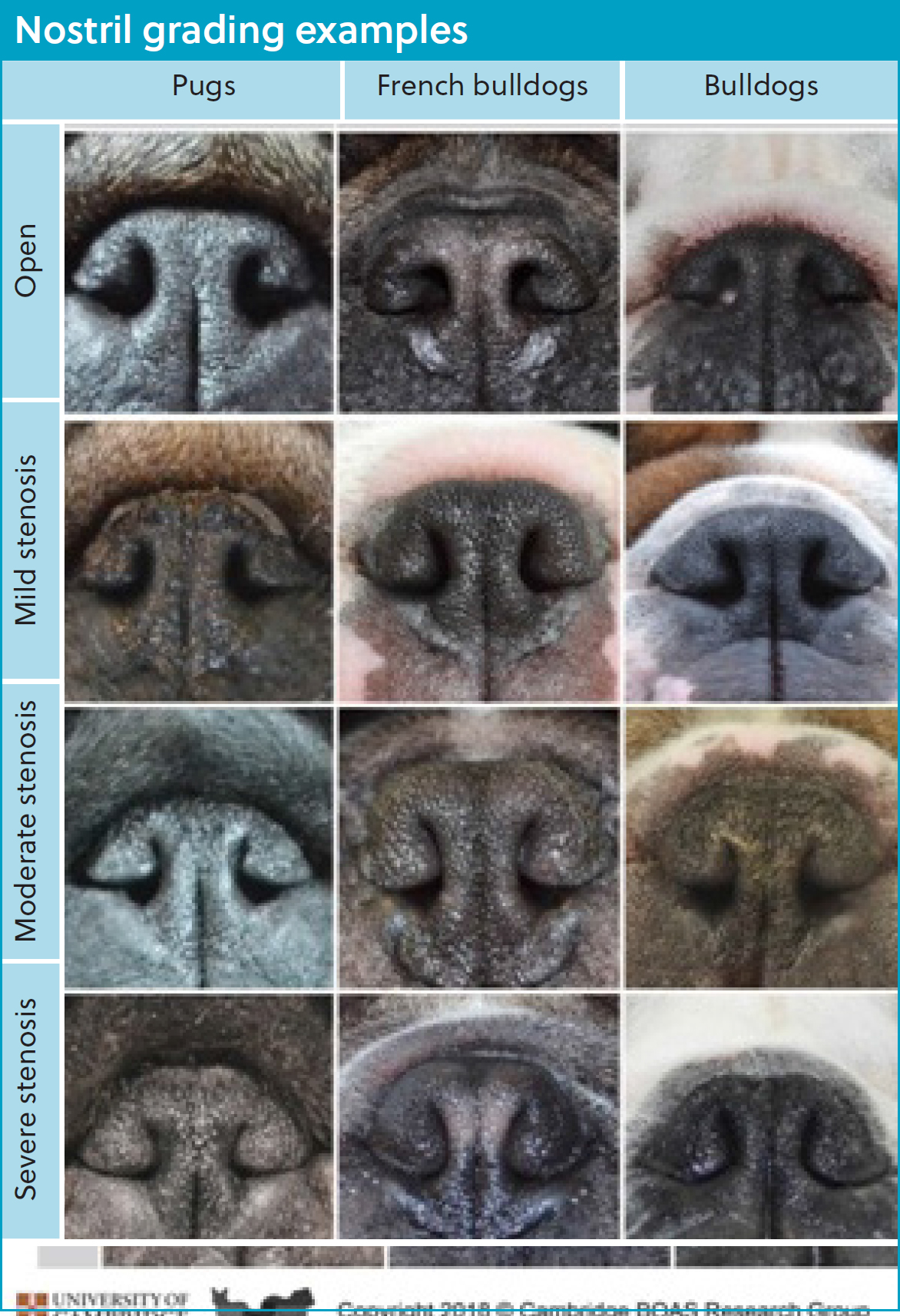
The nares can be easily assessed on physical examination and subjectively classified from open to severely stenosed based on the grading system proposed by Liu et al (2017a) (Figure 1). Dogs with moderately to severely stenosed nostrils are significantly more likely to experience brachycephalic obstructive airway syndrome (Liu et al, 2017a).
Whole-body barometric plethysmography, which measures the changes in airflow and pressure within a sealed chamber that the patient is kept within, has been shown to be 97% sensitive and 93% specific for stratification of patients into whether they are functionally affected by brachycephalic obstructive airway syndrome or not. It allows the user to generate a brachycephalic obstructive airway syndrome index for the patient, which is predictive for brachycephalic obstructive airway syndrome (Liu et al, 2015; 2016). While this is potentially the best currrently available tool for objectively assessing these patients, it is not readily available.
An exercise tolerance test, validated using whole-body barometric plethysmography, was developed to assess the functional impact, and requirement for surgery in patients with brachycephalic obstructive airway syndrome (Riggs et al, 2019). This test involves pharyngolaryngeal auscultation before activity and immediately after a 3 minute trot. The assessor grades patients on the amount of respiratory noise and effort alongside any signs of dyspnoea, cyanosis or collapse (Liu et al, 2015). This increases the sensitivity of clinical examination for a diagnosis of brachycephalic obstructive airway syndrome to 93.3% following the trot test (Riggs et al, 2019). Other exercise tests of set durations or distances have been proposed but not validated with whole-body barometric plethysmography, so although they are likely to increase the sensitivity of diagnosis similarly, it is not known to what degree (Lilja-Maula et al, 2017; Villedieu et al, 2019). Other grading systems which assess respiratory changes alongside anatomical abnormalities visualised at endoscopy have been described (Bernaerts et al, 2010), as well as systems that assess frequency of gastrointestinal and respiratory signs (Poncet et al, 2005). These rubrics are potentially not as useful given the requirement for anaesthesia and endoscopy in the former, and the reliance on client recollection or recognition in the latter.
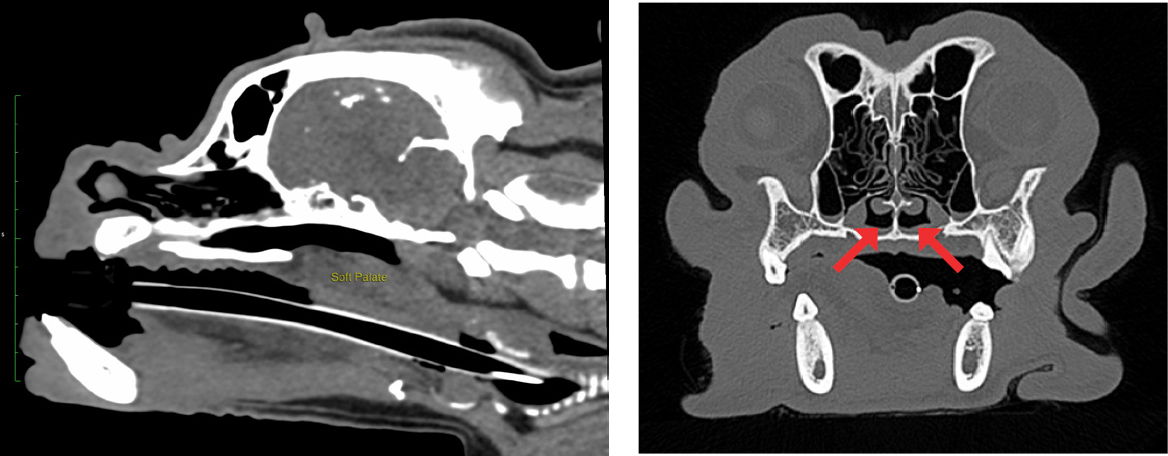
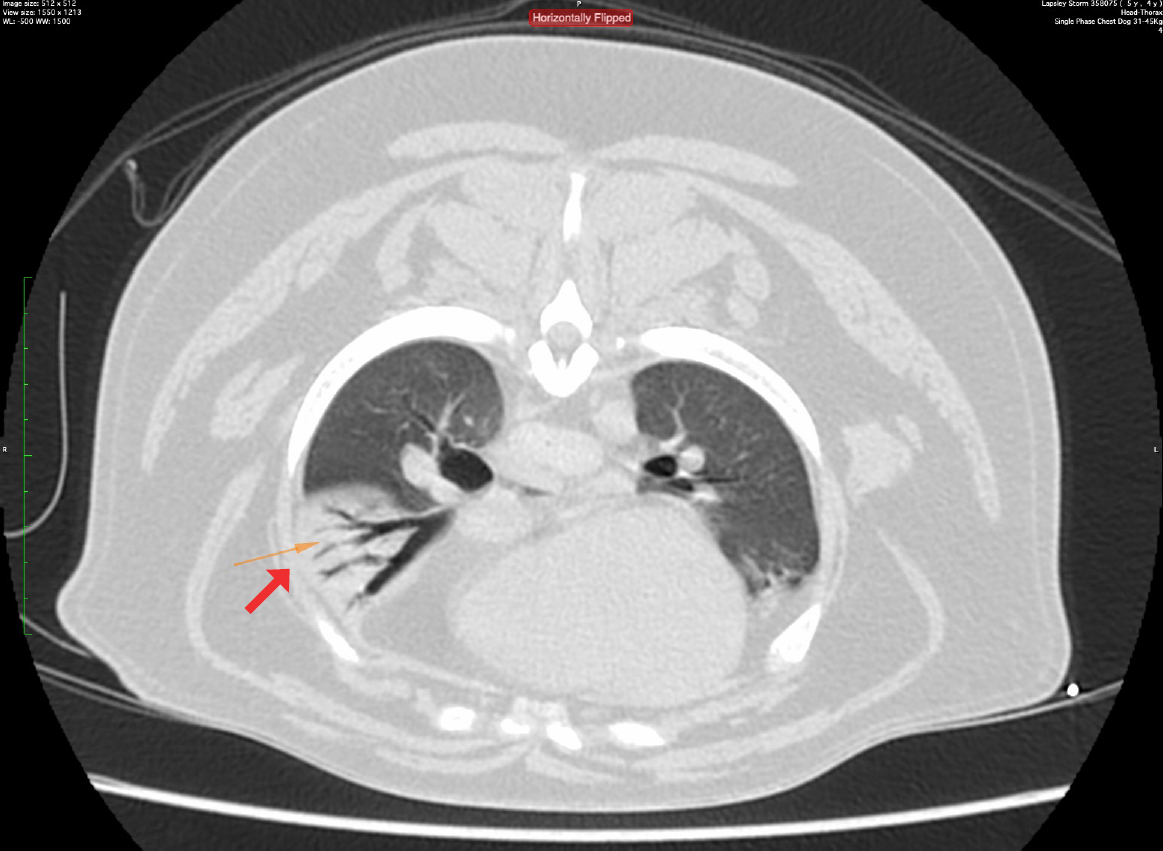
Diagnostic imaging can be used to assess other aspects of brachycephalic obstructive airway syndrome that are not readily appreciated outwardly. Radiography has been recommended to assess cardiopulmonary comobidities (Harvey and Fink, 1982; Hendricks, 1992; Dupré and Heidenreich, 2016) and pharyngeal soft tissue (Hendricks, 1992). However, another study has highlighted that luminal diameters of the pharynx can change dramatically between inspiration and expiration in clinically healthy breachycephalic dogs (Noh et al, 2023), so interpretation may be challenging. Fluoroscopy can be used to assess pharyngeal and bronchial collapse (Johnson et al, 2015; Rubin et al, 2015) and is considered to be the most sensitive assessment for oesophageal dysfunction and sliding hiatial hernia (Pollard, 2012; Pollard et al, 2017; Luciani et al, 2022). However, this is rarely available outside of specialist centres. Computed tomography is increasingly available to veterinary surgeons and can be effectively used to assess the nasal, oral and pharyngeal abnormalities of brachycephalic obstructive airway syndrome (Grand and Bureau, 2011; Vilaplana Grosso et al, 2015; Auger et al, 2016; Siedenburg and Dupré, 2021) (Figure 2). It also has higher sensitivity for detecting aspiration pneumonia than radiography (Syrjälä et al, 1998; Constantinescu et al, 2023), so would be a preferred modality to screen for this (Figure 3).
Endoscopy in the form of retrograde rhinoscopy and tracheobronchoscopy may also be used in order to document features of brachycephalic obstructive airway syndrome including caudal turbinates, larygneal granulomas and airway collapse (Bernaerts et al, 2010). It has also been suggested that endoscopy may be more effective in evaluating tracheal hypoplasia than radiography or computed tomography (Kaye et al, 2015). Endoscopy also allows for the concurrent examination of gastro-oesophageal disease such as strictures, oesophagitis and hiatial hernias (Poncet et al, 2005). However, endoscopy has been shown to be inferior to fluoroscopy in assessment of hiatial hernias and gastro-oesophageal reflux in patients with brachycephalic obstructive airway syndrome (Eivers et al, 2019).
Treatment options
The maintenance of a lean bodyweight is an important part of managing brachycephalic obstructive airway syndrome regardless of surgery. Obesity increases the severity of clinical signs related to brachycephalic obstructive airway disorder by 1.9 times (Liu et al, 2016) and has been shown to be a risk factor for brachycephalic obstructive airway syndrome (Packer et al, 2015). This is likely because of a combination of fat deposition in the tissues surrounding the airways and a reduced tidal volume, which has been demonstrated in non-brachycephalic obese dogs (Manens et al, 2012).
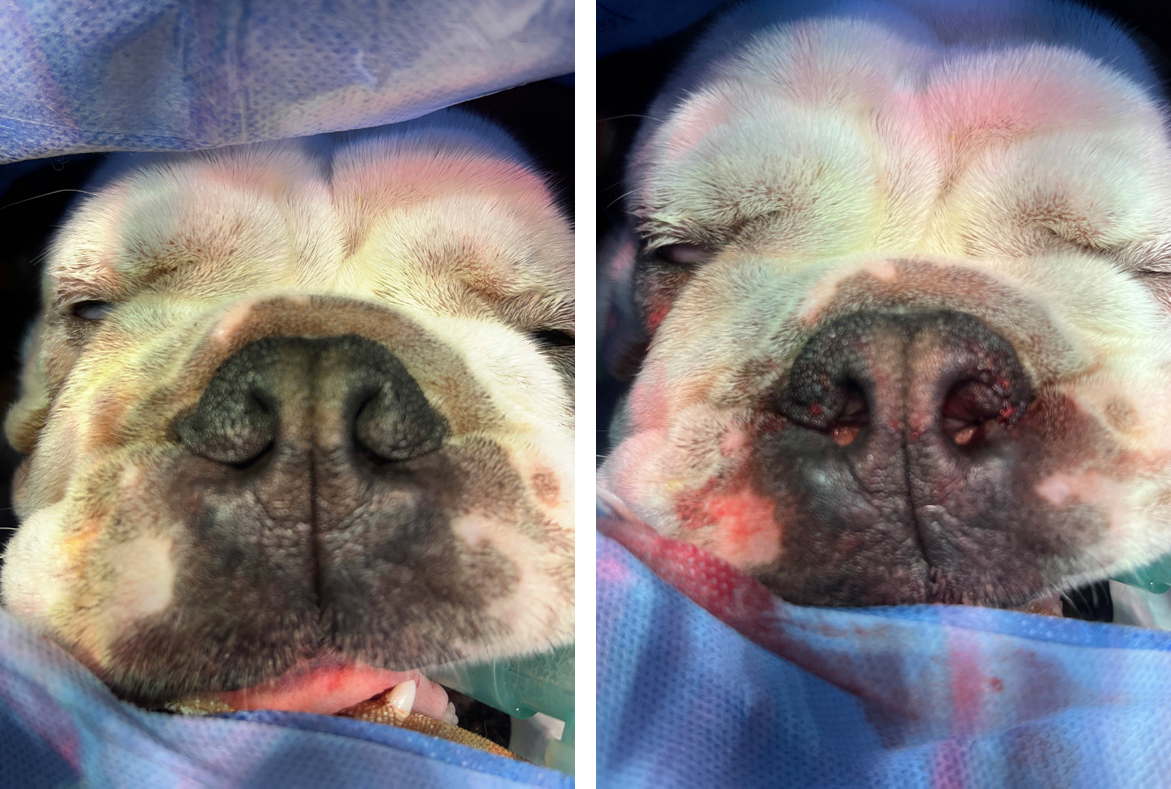
Rhinoplasty is recommended for the management of stenotic nares in patients with brachycephalic obstructive airway syndrome. Several techniques have been described to remove different parts of the skin and alar cartilage. The originally described Trader's technique (Trader, 1949) involved resection of the alar fold without the use of sutures. Other techniques involve wedge alaroplasty (Figure 3) in various formats (Monnet, 2003), the use of punch biopsies (Trostel and Frankel, 2010) or CO2 laser (Mendes Junior et al, 2021). Comparisons of rhinoplasty techniques in silicone models have shown that ala vestibuloplasty increases the cross-sectional area of the nares substantially more than that of vertical or horizontal wedge resections (Franklin et al, 2024). Rhinoplasty alone is rarely indicated, and it is unclear how much of an impact this procedure alone has on the functional capacity of patients with brachycephalic obstructive airway syndrome. However, owners report marked reductions in reported clinical signs of brachycephalic obstructive airway syndrome with this intervention alone (Huck et al, 2008; Mendes Junior, 2021). In contrast, wedge rhinoplasty in French bulldogs has not been shown to substantially reduce airflow resistance in the upper airways, suggesting this surgical intervention may not have a meaningful effect. When combined with other surgical treatments for brachycephalic obstructive airway syndrome, rhinoplasty may aid in the improvement of the syndrome (Liu et al, 2017b; Villedieu et al, 2019).
Soft palate resection is recommended for the management of hyperplastic soft palates. The use of various surgical instrumentation has been described to remove this excess tissue, including traditional sharp dissection (Harvey, 1982a), harmonic shears (Michelsen, 2011), CO2 lasers (Clark and Sinibaldi, 1994) and bipolar sealing devices (Brdecka et al, 2008). Although outcomes appear to be similar regardless of instrumentation, both surgical time and haemostasis are improved with the use of advanced surgical equipment (Davidson et al, 2001; Kirsch et al, 2019; Conte et al, 2022; Gilman et al, 2023). Peripheral tissue injury through thermal necrosis has also been noted; however, this did not result in increased tissue oedema so is unlikely to be clinically significant when compared with sharp dissection (Conte et al, 2022). These techniques only aim to shorten the soft palate, and various techniques have been developed in order to reduce the overall thickness of the palate as well as the length in an attempt to further increase the oropharyngeal space present. These techniques include the folding flap palatoplasty (Findji and Dupre, 2008) (Figure 5), H-platoplasty (Carabalona et al, 2021) and split staphlectomy (Holloway et al, 2022). These procedures do not carry any greater risk of morbidity or mortality than the more traditional techniques (Carabalona et al, 2021; Miller et al, 2024). To date, there have not been any reliable comparisons between staphlectomy techniques in terms of validated outcomes. One study found modified multilevel surgery involving a folding flap palatoplasty was associated with better outcomes than traditional cut and stitch staphlectomy (Liu et al, 2017b), but it is difficult to assess the degree of the impact as differing surgical techniques were also used on the nares and larynx.
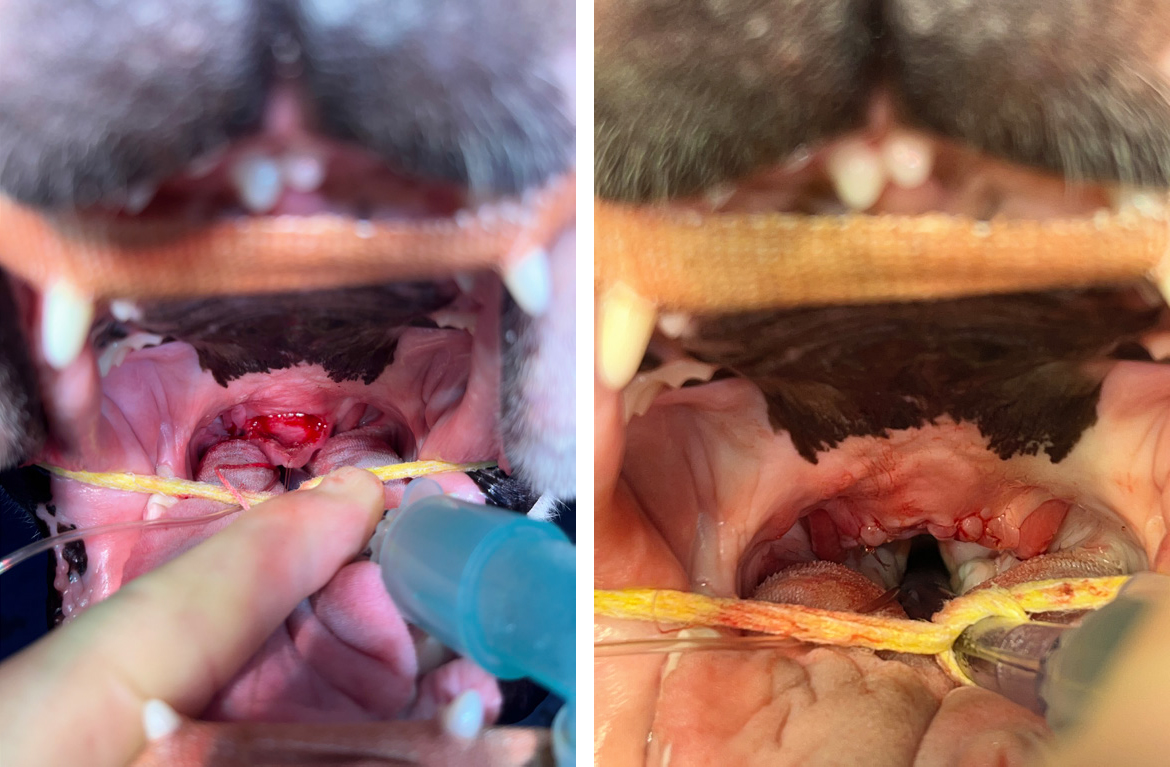
Everted laryngeal saccule excision has been traditionally described through the use of cup biopsy forceps (Leonard, 1957) and scissors (Harvey, 1982b; Packer and Tivers, 2015; Liu et al, 2017b). The surgery has also been described with the use of harmonic scalpels (Shin et al, 2022) and CO2 laser (Arza, 2019). Given the relative avascularity of this tissue, the additional haemostasis provided by these techniques does not seem necessary. The benefit of saccule resection still remains unclear as outcomes appear similar in patients undergoing surgery for brachycephalic obstructive airway syndrome whether sacculectomy is performed or not (Poncet et al, 2006; Riecks et al, 2007). However, it is hard to assess on its own merit, as it is typically performed alongside other procedures. Some studies have reported an increased risk of postoperative morbidity following surgery for brachycephalic obstructive airway syndrome when sacculectomy is performed (Hughes et al, 2018), but this has not been a consistent finding (Riecks et al, 2007; Fasanella et al, 2010). Given that saccule eversion is a secondary effect of increased airway pressures, there was a belief that management of primary abnormalities may result in resolution. However, saccule eversion has been shown to persist long term in the face of these surgical corrections (Cantatore et al, 2012). Therefore, it is still clinician dependant as to whether this procedure is felt warranted.
Everted tonsils have also been suggested as a surgical target to increase the overall airway space (Fasanella et al, 2010). However, similar to the laryngeal saccules, the overall benefit of removal is unclear and it may not be necessary (Poncet et al, 2006). Removal has been described with the use of harmonic scalpels, bipolar forceps and metzenbaum scissors (Liu et al, 2017b).
Laryngeal collapse is a more challenging condition to manage surgically in patients with brachycephalic obstructive airway syndrome. Patients with moderate to severe laryngeal collapse can still benefit from management of their primary and secondary abnormalities (Torrez and Hunt, 2006; Riecks et al, 2007). Therefore, surgical management of the laryngeal collapse is typically reserved for patients who have not responded sufficiently to the aforementioned surgical interventions (Packer and Tivers, 2015). Surgical techniques including cricoarytenoid lateralisation combined with thyroarytenoid caudolateralisation (White, 2012), arytenoidetectomy (Wykes, 1991), laryngeal tieback (Johnston and Tobias, 2017) and partial cunieformectomy (Liu et al, 2017b) have been shown to be possible options for the management of laryngeal collapse in patients with brachycephalic obstructive airway disorder that have continued to have life-threatening respiratory compromise. Nevertheless, the prognosis is still guarded and severe complications, including aspiration pneumonia, are common (Packer and Tivers, 2015). The alternative is to place a permanent tracheostomy tube in order to bypass the end stage upper respiratory tract obstruction (Occhipinti and Hauptman, 2014). This is not without risk – major complications and death have been reported in around 50% of patients (Occhipinti and Hauptman, 2014; Gobbetti et al, 2018). Patients that did survive were reported to have a good quality of life and much extended lifespan.
A final treatment modality that has been described more recently is the laser ablation of caudal aberrant turbinates (Schuenemann et al, 2017). This is designed to remove the intranasal obstruction which previous surgical techniques have not been able to address. It has been objectively shown to improve brachycephalic obstructive airway syndrome in patients that have already had conventional multilevel surgery but have not significantly improved (Liu et al, 2019). Although long-term follow up is not available to fully assess the benefit of this procedure, the vast majority of patients show re-growth of conchae by 6 months postoperatively. However, the airway was less obstructed than pre-sur-gery (Schuenemann and Oechtering, 2014).
The respiratory function grading scheme, run by the Kennel Club in association with University of Cambridge, is a way for veterinary surgeons to be trained in the respiratory grading of these patients and allows them to reach and advise breeders of brachycephalic dogs. This aims to reduce the overall population of dogs with brachycephalic obstructive airway syndrome by improving the breed as a whole. Unfortunately, as of 2022, only around 3% of French bulldogs, 15% of pugs and 7% of bulldogs registered with the Kennel Club in that year underwent respiratory function grading (Kennel Club, 2022). There is much work ahead to continue to educate and improve the welfare of these breeds.
The prognosis for patients with brachycephalic obstructive airway syndrome is variable. Although surgery commonly results in owner-reported improvements in up to 94% of patients, with many of these being excellent or good (Poncet et al, 2006; Torrez and Hunt, 2006; Riecks et al, 2007), caution must still be exercised in veterinary ability to make meaningful changes to these patients' airways. Although varying forms of multilevel surgery have objectively shown to improve these patients' conditions in terms of oxidative stress (Erjavec et al, 2021) and brachycephalic obstructive airway syndrome index (Liu et al, 2017a; 2019), it has also been identified that between 50–68% of undergoing surgery would still be objectively classified as clinically affected (Liu et al, 2017b; 2019).
Conclusions
An understanding of the anatomy and resultant pathophysiology is helpful when managing these patients. Appropriate diagnosis of clinically affected patients can be easily achieved without the need of advanced diagnostics, although these can be helpful in further defining the relevant conditions. Surgical treatments exist to target most of the various anatomical abnormalities, but clarity on the optimal techniques and combinations of surgeries is still lacking. The best hope to significantly improve the health and welfare of brachycephalic breeds is to continue to educate owners, breeders, veterinary professionals and the general public, such that the un-der-diagnosis of affected dogs can be avoided, appropriate treatment can be provided, dogs with brachycephalic obstructive airway syndrome are not bred and owners purchasing a brachycephalic dog are made aware of this condition.
KEY POINTS
- Brachycephalic obstructive airway syndrome is a complex, lifelong condition that primarily affects the respiratory system but can have wide ranging impacts on the brachycephalic patient.
- Knowledge of the aberrant anatomical features present in brachycephalic patients is key in understanding the pathophysiology behind the respiratory challenges they cause.
- Diagnosis and stratification of patients with brachycephalic obstructive airway syndrome that are respiratory functionally affected and non-affected can be straightforward with the use of an exercise tolerance test and clinical examination.
- Many other diagnostic techniques exist to further detail the components of brachycephalic obstructive airway syndrome that are present.
- A wide variety of surgical procedures are described to address the different anatomical components of the respiratory system thought to play a part in brachycephalic obstructive airway syndrome. However, it still unclear which of these provide optimal outcomes and how best to perform them.
- The prognosis for brachycephalic obstructive airway syndrome is guarded. Despite frequent owner-reported improvements, many of these patients will continue to be functionally affected in spite of surgical intervention.


