In the first article (Scarabelli and Nardini, 2019), basic principles of the anaesthesia of small mammals were discussed. In this second article, a general overview of drugs commonly employed in the perioperative period is provided, together with protocols frequently used in the different species. In addition to anaesthesia of healthy animals, particular attention is paid to anaesthesia of animals with clinical conditions commonly encountered in clinical practice; examples include ferrets with insulinoma, rabbits with chronic kidney disease and guinea pigs with hepatic lipidosis (HL).
Pre-anaesthetic medications
Sedatives
The most commonly used sedative drugs in small mammals are alpha-2 agonists (medetomidine and dexmedetomidine) and benzodiazepines. The use of acepromazine in combination with other drugs has been reported (Amarpal et al, 2010; Perez-Rivero and Rendon-Franco, 2014) but it is not routinely used by the authors mainly due to its unpredictable sedative effects, long duration of action and vasodilatory properties.
Alpha-2 agonists
Medetomidine and dexmedetomidine have both sedative and analgesic properties and are the authors' drugs of choice when sedation is required in healthy animals. Advantages of alpha-2 agonists include good sedation and muscle relaxation; the option of subcutaneous (SC) or intramuscular (IM) administration; the possibility of reversal; their anaesthetic-sparing effect; possible neuroprotective effects; and organ-protective effects against ischaemic and hypoxic injury (Mahmoud and Mason, 2015). The main side effects are an increase in systemic vascular resistance (SVR) and reflective reduction in heart rate followed by a centrally-mediated bradycardia accompanied by normal or slightly increased/decreased SVR, depending on the species. In rabbits, the SVR remains increased during dexmedetomidine infusions (Sazuka et al, 2012), whereas no studies have been performed in other small mammals. Regarding the respiratory system, medetomidine has been reported to decrease the arterial partial pressure of oxygen in dogs (Raekallio et al, 2009) and dexmedetomidine to affect the respiratory response to carbon dioxide in rabbits (Chang et al, 2009). The effects of alpha-2 agonists on gastrointestinal (GI) motility have been extensively studied both in human and veterinary medicine, leading to controversial results. For example, medetomidine has been reported to decrease motility of the gastric antrum and duodenum in dogs (Nakamura et al, 1997); dexmedetomidine has been reported to inhibit peristalsis in the guinea pig small intestine in vitro in a concentration-dependent manner (Herbert et al, 2002) but also, more recently, to improve gastrointestinal motility after laparoscopic resection of colorectal cancer in people (Chen et al, 2016).
Other side effects of alpha-2 agonists include hyperglycaemia and enhanced diuresis. The authors generally use dexmedetomidine at doses of 25–35 μg/kg IM, ±opioids±ketamine.
Benzodiazepines
Benzodiazepines have sedative and muscle-relaxant effects with minimal cardiovascular and respiratory depression; they do not provide analgesia. Midazolam, compared with diazepam, has the advantage that it can be administered IM. In the authors' practice, midazolam (0.5–1 mg/kg) is used alone in paediatric, geriatric and compromised patients when sedation is required, whereas an opioid is added if more painful procedures have to be performed. In healthy animals, benzodiazepines are not generally sufficient to provide reliable sedation, but they can be added to alpha-2 agonists in order to reduce the dose of the latter.
Opioids
Opioids are the mainstay for perioperative analgesia in humans, dogs and cats; their use in rabbits has always been controversial due to their effects on GI motility. Opioid receptors are highly expressed at GI level (Fickel et al, 1997) and endogenous endorphins activation of mu-opioid receptors has been reported to inhibit GI motility (Kurz and Sessler, 2003). Clinically, opioid-related ileus is a well-recognised problem in human medicine (Kurz and Sessler, 2003) and it has also been extensively investigated and discussed in horses.
In rabbits, morphine has been reported to inhibit the small intestine's contractility in vitro (Feng et al, 2008) whereas buprenorphine is the opioid more commonly evaluated in clinical studies. Deflers et al (2018) reported that a single IM dose of 100 μg/kg of buprenorphine did not affect GI motility in 15 rabbits, whereas Martin-Flores et al (2017) reported that a dose of 50 μg/kg twice a day given SC decreased food intake, water consumption and prolonged GI transit time in healthy rabbits. Another study (Schnellbacher et al, 2017) compared the effects of buprenorphine and lidocaine in rabbits undergoing ovariohysterectomy and reported that animals receiving buprenorphine had lower GI motility, food intake and faecal output. Evenson and Mans (2018) evaluated analgesic effects and safety of three doses of hydromorphone in chinchillas: only the higher dose (2 mg/kg) provided pain relief but food intake and faecal output were decreased.
Despite this controversy about opioids and GI motility, it is authors' common practice to use opioids as analgesics in rabbits and small mammals; it is important to remember that pain itself will lead to adrenergic stimulation, hence reducing gastrointestinal motility. For surgical procedures or severe pain, the authors' drug of choice is methadone at doses of 0.2–0.3 mg/kg IM or IV, whereas for mild pain buprenorphine is used at 0.01–0.03 mg/kg IM or IV. Butorphanol 0.3–0.4 mg/kg is used to enhance the activity of an alpha-2 agonist or benzodiazepine when sedation is required for nonpainful procedures such as radiography or computed tomography. For postoperative analgesia, in order to avoid overdosing, at least in rabbits, mouse and rats, grimace scales (pain scales based on facial changes) are used to assess the degree of pain and analgesic requirements.
Ketamine
This is commonly used in small mammals in association with sedatives and opioids. Depending on the dose, it can be used either for premedication or for induction of general anaesthesia. Immobilisation and analgesia are the main advantages offered by ketamine whereas poor muscle relaxation is the main disadvantage; the latter can be overcome by adding benzodiazepines or alpha-2 agonists. Several studies have evaluated the use of ketamine in association with other drugs in different species (e.g. Grint and Murison, 2008; Schernthaner et al, 2011; Fox et al, 2016); it is authors' common practice to use ketamine (4–5 mg/kg) IM with an alpha-2 agonist and/or a benzodiazepine for premedication. Higher doses can be used to induce general anaesthesia.
Induction agents
General anaesthesia in small mammals can be induced with inhalant anaesthetics, either by mask or in an induction chamber, or with injectable agents, particularly propofol and alfaxalone.
When induction with inhalants is performed, sevoflurane is the agent of choice due to minimal or zero pungent odour; moreover, compared with isoflurane, it has a lower blood-gas partition coefficient, leading to quicker induction. Induction with inhalants is easy and quick to perform but it is important to remember that, if general anaesthesia is maintained only with inhalants, no analgesia will be provided; additional drugs must be administered for painful procedures (Wegner, 2012).
In the authors' usual practice, induction of general anaesthesia with inhalants is performed only in premedicated animals or in smaller mammals, such as gerbils, hamster, rats and chinchillas, for short non-painful diagnostic procedures; in rabbits, ferrets and guinea pigs, induction with injectable agents is usually the first choice. Note that in rabbits, inhalation induction can be stressful, particularly if animals are not deeply sedated, and can lead to bradycardia, apnoea, hypercapnia and acidosis (Flecknell et al, 1999).
Among injectable agents, propofol is commonly used, above all in rabbits, but recently alfaxalone has been licensed in this species as well. The main advantage of alfaxalone (Alfaxan, Jurox), compared with propofol, is that it can be used also IM even if in rabbits it is not presently licensed for this route. When used IV, a dose-dependent respiratory depression/apnoea is commonly reported (Grint et al, 2008; Gil et al, 2012). The Summary of Product Characteristics (SPC) of Alfaxan suggests that rabbits should be pre-oxygenated before induction of general anaesthesia and that oxygen is provided throughout the all anaesthetic procedure. Licensed doses are 4 mg/kg in premedicated and 5 mg/kg in non-premedicated animals even if it is authors opinion that lower doses (1–2 mg/kg) are generally efficacious. In the authors' experience, however, propofol is still the agent of choice for induction of general anaesthesia in rabbits due to a lower incidence of respiratory depression and better induction quality — this is possibly due to the familiarity of the authors with the use of this drug. One of the main limitations when propofol is used in smaller animals is that an intravenous (IV) catheter is needed and this is not always possible to place before, or even after, induction of general anaesthesia. Moreover, apnoea may still occur, therefore the clinician should be confident with endotracheal intubation. Respiratory depression is dose-related and can be decreased with slow administration of the drug. One study (D'Ovidio et al, 2018) evaluated the sedative effects of 5 mg/kg alfaxalone IM in guinea pigs: the authors conclude that it represents a valuable sedation protocol for healthy subjects undergoing minor non-invasive procedures.
Maintenance of anaesthesia
Volatile anaesthetics are commonly used for maintenance of anaesthesia; the main advantage is that they allow a better control of the depth of anaesthesia compared with injectable agents. However, for short, minor procedures, injectable agents used in premedication, such as ketamine at high doses and alpha-2 agonists, may be adequate. It is important to remember that oxygen should always be administered either by mask or by endotracheal intubation every time an animal is sedated/anaesthetised.
Endotracheal intubation
Details of endotracheal intubation (ETI) for various species are provided here. As described previously (Scarabelli and Nardini, 2019), placing of a supraglottic airway device may also be used when a species-specific version is available (V-gels (Docsinnovent) are available for rabbits and for guinea pigs). A tight-fitting face mask may be adequate for some short procedures.
Rabbit
As mentioned in part 1 (Scarabelli and Nardini, 2019), before intubation it is fundamental to clean the rabbit's mouth in order to remove food residues that could be otherwise pushed inside the trachea and inhaled. In order to achieve ETI, a suitable depth of anaesthesia should be induced and lidocaine (spray or the injectable preparation instilled topically) should be used to desensitise the larynx.
Regardless of the method that is to be used for ETI (endoscopically guided, blind or otoscopically guided), the rabbit should be placed in sternal recumbency with the neck and head extended dorsally and cranially but not completely vertical (Figure 1). Uncuffed endotracheal tubes (ETTs) of 2–4.4 mm internal diameter should be available.

- Endoscopically guided ETI: a 2.7 mm endoscope at 30° or 0° can be used. If feasible (if the internal diameter of the ETT is large enough), it can be inserted into the ETT and used as a guide to visualise the glottis and as a stylet to facilitate the ETT insertion. For ETT of smaller diameters a ‘side by side’, technique is used, the endoscope being used solely to visualise the glottis.
- Blind ETI: the ETT is inserted into the mouth and the breath sounds of the rabbit or the presence of condensation on the ETT itself are used to guide the insertion.
- Otoscopic ETI: for direct visualisation of the larynx. The otoscope is inserted into the side of the patient's mouth, then moved centrally and used to elevate the soft palate and visualise the glottis. An ETT can be inserted through the cone of the otoscope or with a ‘side by side’ technique.
In the author's experience, endoscopically guided ETI allows insertion of a larger diameter ETT, thus decreasing airway resistance and the work of breathing for the rabbit and allowing more accurate and reliable monitoring of end-tidal carbon dioxide.
Ferret
Direct visualisation of the larynx can be easily achieved with a laryngoscope and appropriate size straight blade. Local anaesthetic is sprayed on to the glottis and the uncuffed ETT can be inserted. 2–3 mm ID ETT should be available.
Guinea pig
As already mentioned in the first part of the article, due to anatomical features, ETI in guinea pigs is more complicated than in rabbits. Although techniques have been described in research settings (Kramer et al, 1998; Nambiar et al, 2007) and otoscope-guided intubation with the aid of a stylet is feasible, it is authors' common practice to perform endoscopically guided intubation. The animal is positioned in sternal recumbency on a tabletop mouth-gag positioner, with the neck and head extended and mouth opened; the end of the breathing system may be placed over the nares during the procedure to provide oxygen (Figure 2). The endoscope is inserted into the mouth, advanced in a caudo-dorsal direction first and moved ventrally later until the palatal ostium is seen; the ETT is inserted with an ‘over the endoscope’ or ‘side by side’ technique, depending on the size of the ETT and endoscope available. For guinea pigs, 8 Fr urinary catheters and 2–2.5 mm internal diameter ETTs should be available.
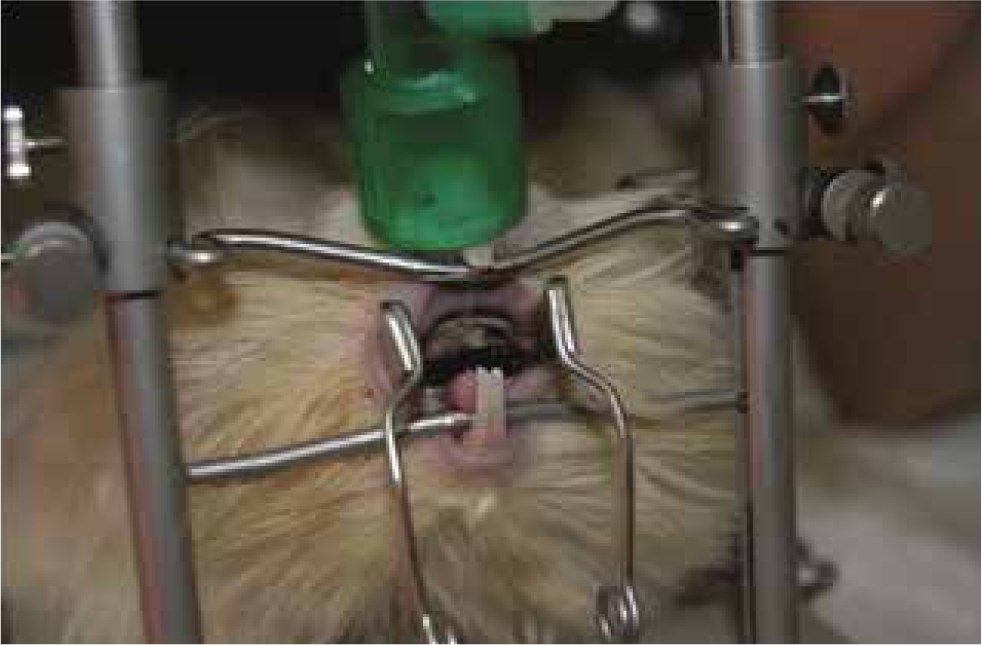
Chinchilla
As with guinea pigs, ETI in chinchillas is more difficult than in rabbits. It can be performed blindly but, in the authors' opinion, the endoscopically guided technique is easier and quicker to perform. A side-by-side technique is used.
Rat
In rats, ETI is difficult mainly due to the extremely small size of the anatomical structures. Tomasello et al (2016) reported that they quickly and successfully intubated 94.5% of 70 rats with the animals in dorsal recumbency and the aid of a neonatal laryngoscope blade and an operating table. Blind (Cheong et al, 2010) and endoscopically guided (Clary et al, 2004) techniques have been described also. At the authors' institution, rats are intubated in dorsal recumbency, with the head and neck extended; an otoscope is used to visualise the glottis, a guide wire is inserted through the otoscope cone, the otoscope is removed, the ETT is slide onto the guidewire and into the glottis, then the guidewire is removed. Oxygen is provided through the nostrils during the procedure (Figure 3). For rats, 14–16 G IV catheters should be available for use as ETTs.
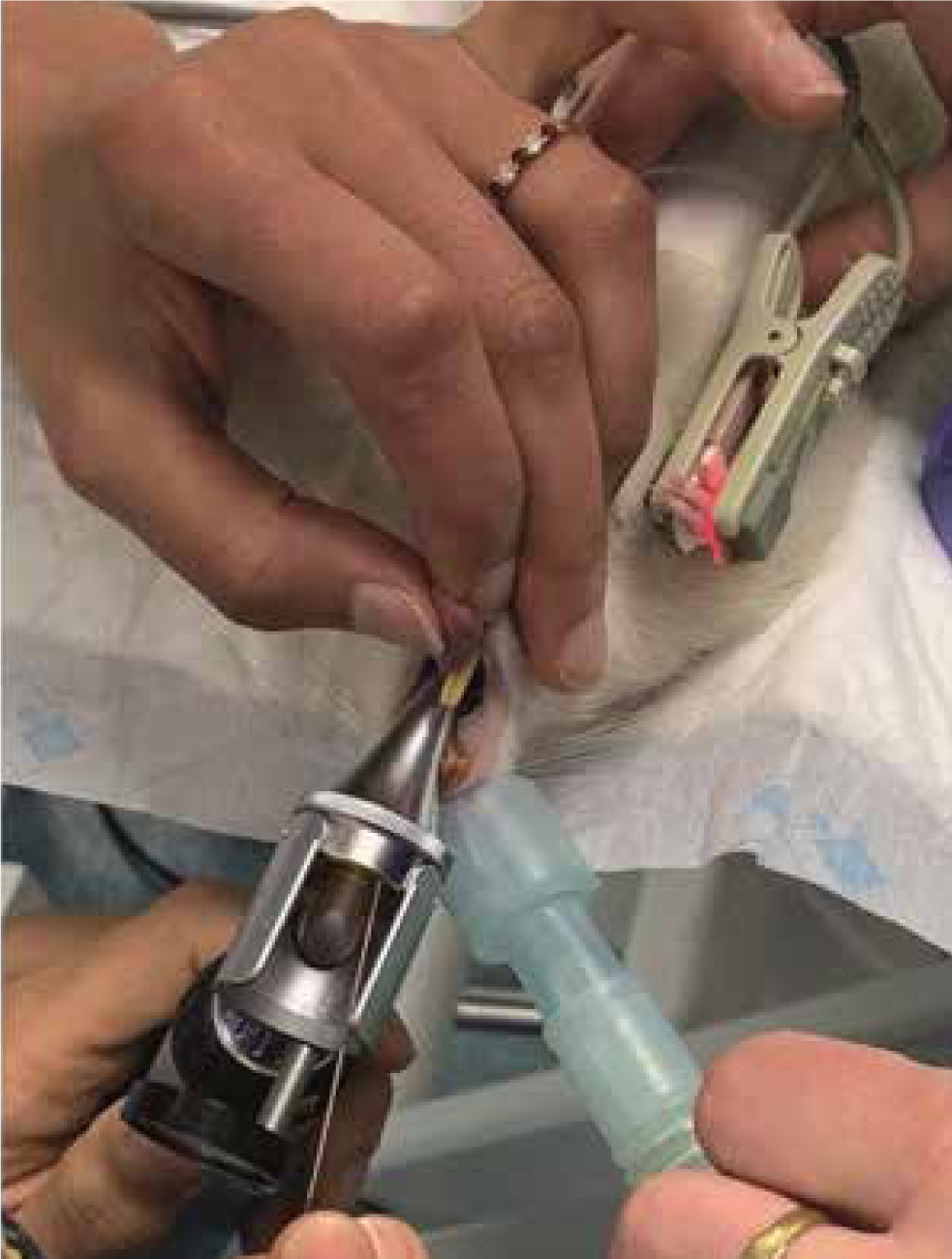
Analgesia
Opioids, ketamine and alpha-2 agonists provide analgesia acting at different levels; other classes of analgesic drugs that are used perioperatively are non-steroidal anti-inflammatory drugs (NSAIDs) and local anaesthetics.
The most commonly used NSAIDs in small mammals include meloxicam, carprofen and ketoprofen. Various doses and intervals can be found in the literature and an extensive review of the topic is beyond the scope of this article. At the authors' institution the most commonly used NSAID is meloxicam at doses of 0.5 mg/kg once a day in rabbits, guinea pig and chinchillas; in ferrets 0.2 mg/kg once a day is generally administered whereas higher doses (1 mg/kg) are used in rats and gerbils.
Of the local anaesthetics, lidocaine and bupivacaine are the most commonly used. Lidocaine has quick onset (1–2 minutes) and a short duration (40–60 minutes) of action; the main disadvantage is that no postoperative analgesia will be provided if the local block is performed intraoperatively.
Bupivacaine has a slower onset (up to 40 minutes) and a longer duration of action (4–6 hours) and it is usually preferred for more prolonged procedures and to provide postoperative analgesia. Maximum doses used by the authors are 2 mg/kg for lidocaine and 1 mg/kg for bupivacaine.
Anaesthesia for common diseases/procedures
The following section discusses considerations and preferred anaesthetic protocols at authors' hospital for clinical conditions commonly encountered in practice.
Healthy patients
Rabbits, guinea pigs, chinchillas, gerbils, rats and mouse may be presented to our clinics for routine surgeries such as ovariohysterectomy, castration or removal of small masses. Such patients are generally systemically healthy (American Society of Anesthesiologists classification I–II). For these procedures, several anaesthetic protocols have been described in the literature and the choice mostly depend on the clinician's familiarity with specific classes of drugs. At authors' institution, the most common anaesthetic protocols are as follows.
Rabbits, guinea pigs, ferrets
- Premedication: methadone 0.2 mg/kg plus dexmedetomidine 20–30 μg/kg plus ketamine 3–5 mg/kg all in the same syringe, IM
- Non-steroidal anti-inflammatory drugs once the animal is sedated
- Preoxygenation for 5 minutes while an IV catheter is placed
- Induction with propofol to effect
- Maintenance with sevoflurane in 100% oxygen via ETT.
Chinchillas, gerbils, rats
Although an anaesthetic protocol similar to the one described in rabbits could be used, the authors are less familiar with ETI in these species. Therefore, for short procedures and in healthy animals, we generally prefer, after premedication and administration of NSAIDs, to induce and maintain general anaesthesia with sevoflurane in 100% oxygen via a tight-fitting face mask or via the end of the anaesthetic breathing system placed over the nares.
Recently (Saldanha et al, 2019), the use of the transversus abdominis plane block has been described in chinchillas; this block is used in human and veterinary medicine to provide analgesia for abdominal surgeries. It is authors' opinion that the use of this loco-regional anaesthesia (LRA) technique has a great potential in small mammals in order to decrease opioid requirements.
Dental procedures in rabbits
Rabbits are commonly presented to our practices for dental diseases. The clinical condition of the animal will depend upon the severity of the disease, ranging from healthy animals to anorexic and debilitated patients. The anaesthetic protocol will be influenced by the animal's clinical condition and the type of procedure that has to be performed. In general, when performing dental procedures, LRA is of paramount importance. Local blocks for dentistry include:
- Rostral infraorbital nerve block. Provides analgesia for the upper incisor teeth, upper lip and surrounding soft tissue. The landmark is the infraorbital foramen on the lateral aspect of the skull (Figure 4).
- Mental nerve block. Provides analgesia for the cranial part of the mandible and lower lip. The landmark is the mental foramen just in front of the first premolar (Figure 5).
- Inferior alveolar nerve block. Provides analgesia for the mandibular check teeth and adjacent soft tissues. The nerve enters the mandibular foramen on the medial aspect of the mandible. The authors perform this block with peripheral nerve stimulator (PNS) guidance; the needle is introduced at the level of the temporomandibular joint and advanced in a rostromedial direction until contraction of the masticatory muscles is seen.
- Maxillary nerve block. Due to the limited access to oral cavity, an intraoral approach cannot be performed in rabbits. The maxillary nerve can be blocked with the rabbit in lateral recumbency, inserting a 25–27 G needle just caudal to the facial tuberosity and ventral to the zygomatic arch in a dorsomedial direction (Figure 6) (Campoy 2018).
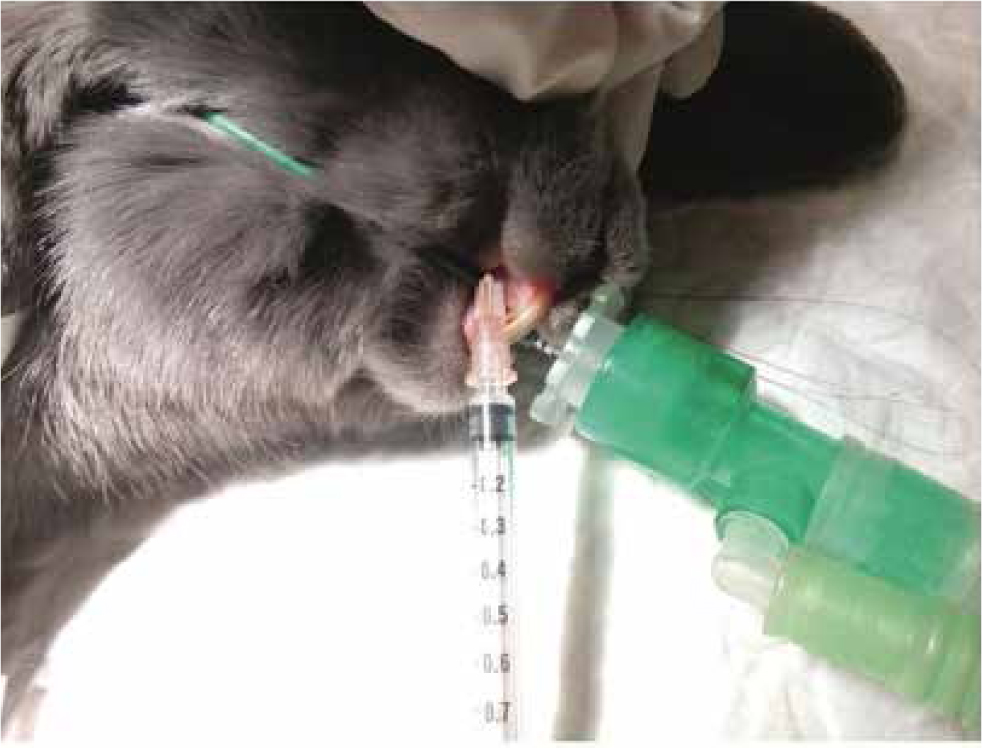
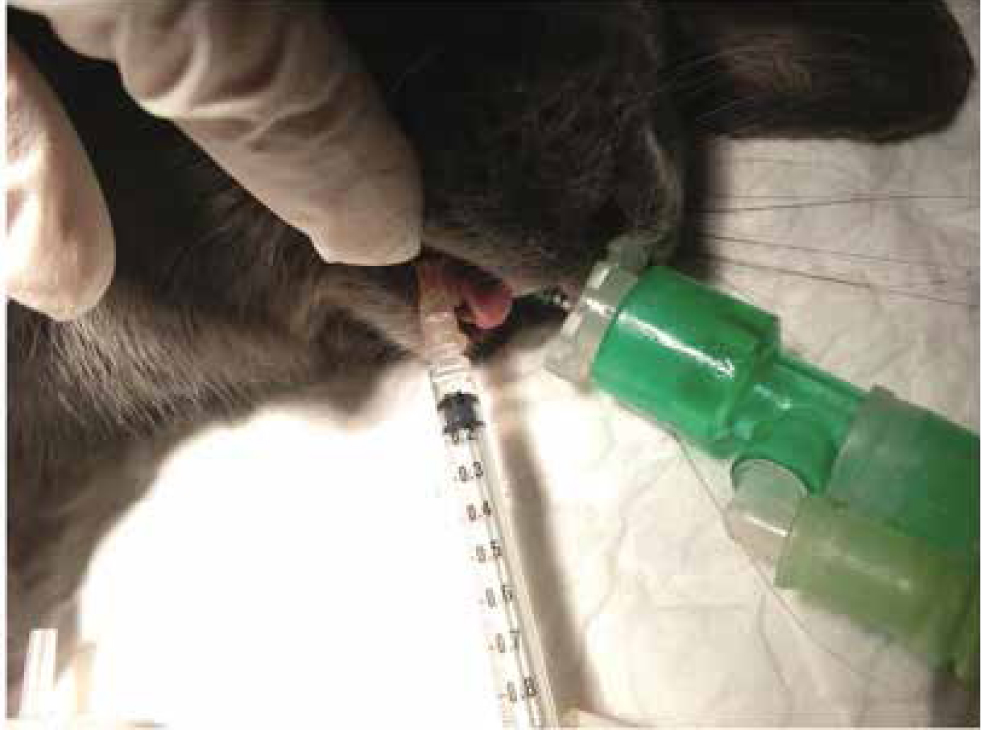
Ear surgery in rabbits
Ear disorders are common in rabbits, with otitis externa and otitis media being the most clinically relevant. Often medical management is unable to completely solve the problem and a surgical approach is needed (Cosmos et al, 2016). As for dentistry, LRA is fundamental. For surgeries at the level of the external ear canal and pinna, three nerves should be blocked: the auriculotemporal, the caudal auricular and the greater auricular. The auriculotemporal and the caudal auricular nerves can be blocked under PNS guidance with one injection inserting the needle between the caudo-dorsal border or the coronoid process of the mandible and the external acoustic meatus until caudal displacement of the pinna is observed as muscular response (Otero and Portela, 2019). For total ear canal ablation with or without bulla osteotomy, another technique has been described by D'Urso et al (2018): the mandibular nerve at the level of the temporomandibular joint, and the auriculopalpebral nerves are blocked under PNS guidance.
Rabbits with chronic renal disease
When anaesthetising rabbits with kidney disease, general principles applied to other species can be followed.
- Renal function should be assessed: blood urea nitrogen (BUN), creatinine, urine specific gravity and urine proteins level can be measured. It is important to bear in mind that BUN in rabbits is greatly influenced by extra-renal factors such as circadian rhythm, stress, protein intake, nutrition status of the animal, intestinal absorption and activity of the caecal flora (Jenkins, 2008). Moreover, trace of proteins can be present also in the urine of healthy young and adult rabbit.
- Stabilise the patient before surgery for elective procedures
- Multiple administration or constant rate infusion of drugs with renal excretion should be avoided. Ketamine kinetics have been reported to be altered in rabbits with experimentally-induced renal impairment (Pedraz et al, 1985)
- Non-steroidal anti-inflammatory drugs should be used with caution. It is authors' opinion that in severe renal impairment they are better avoided whereas in mild and stable renal disease, if deemed necessary, a single administration of a therapeutic dose (0.5 mg/kg SID) can be given postoperatively if the patient is haemodynamically stable
- Monitor blood pressure and avoid intraoperative hypotension in order to maintain kidney perfusion. Use drugs with MAC-sparing effects to minimise cardiovascular side effects of inhalant anaesthetics. LRA is the author's first choice; alternatively, fentanyl as a CRI at 10–20 μg/kg/h IV can be used intraoperatively
- Adjust intraoperative fluid therapy based on patients' hydration status and expected fluid losses
- Avoid hypoxia and hypercapnia.
Ferrets with insulinomas
Insulinomas are insulin-secreting tumours of the pancreatic beta cells commonly reported in ferrets. Clinical history varies from unremarkable to weakness, ataxia, seizure, coma and death due to hypoglycaemia. Treatment can be medical or surgical, therefore ferrets with insulinomas may be anaesthetised for procedures unrelated to the disease or for pancreatic nodulectomies or partial pancreatectomies. General considerations include:
- Avoid fasting the animal
- Stabilise the patient before the procedure (prednisone, diazoxide)
- Serial monitoring of blood glucose levels before and during general anaesthesia. Measure blood glucose during tumour manipulation that can lead to insulin spikes and hypoglycaemia
- Supplement fluid therapy intraoperatively with 5% glucose — usually higher concentrations are not needed
- Appropriate pain management. In dogs has been reported that pain-related sympathetic stimulation can cause hyperglycaemia, tumour stimulation and rebound hypoglycaemia (Goutal et al, 2012)
- Use of alpha-2 agonists. Alpha-2 agonists have an inhibitory effect on pancreatic beta cells thus decreasing plasma insulin concentration. In dogs with insulinomas undergoing surgery, the use of medetomidine in premedication assisted in maintaining blood glucose concentration, decreased the amount of dextrose used during anaesthesia and maintained more stable blood pressure (Guedes and Rude, 2013). Despite the lack of studies in ferrets with insulinomas, it is authors opinion that alpha-2 agonists can be used in these cases, bearing in mind that blood glucose monitoring remains of paramount importance
- Avoid hypotension and hypoxaemia to decrease the risk of postoperative pancreatitis after pancreatic surgery.
Ferrets with cardiac disease
Cardiac disease is common in pet ferrets; dilated cardiomyopathy (DCM) and, where it occurs, heartworm (Dirofilaria immitis) infection are the most frequent but hypertrophic cardiomyopathy and acquired valvular disease have been reported also. Clinical presentation may be similar to dogs and cats, although hind limb weakness may be a prominent feature (Wagner, 2009). Radiography and echocardiography are useful tools in the diagnosis of the disease and to evaluate cardiac function before anaesthesia.
From an anaesthetist's point of view, it is important to know the underlying cardiac pathology in order to choose the most appropriate anaesthetic protocol and the anaesthetic goals that we want to achieve during the anaesthetic event. For example, in ferrets with DCM, the main concerns are reduction in myocardial contractility and arrhythmias. Anaesthetic goals include maintain preload, avoid increase in afterload, maintain or increase contractility, avoid tachycardia with increased myocardial oxygen demand and control arrhythmias (Scarabelli and Bradbrook, 2016). Ketamine and alpha-2 agonist should therefore be avoided in these patients.
When cardiac disease is suspected, one of the authors' favourite protocols is to use methadone 0.2 mg/kg+midazolam 0.3 mg/kg IM for premedication, followed by induction with propofol and maintenance with inhalants in oxygen. If further intraoperative analgesia is required, a fentanyl CRI at 5–10 μg/kg/h is the authors' first choice.
Guinea pigs with hepatic lpidosis
Hepatic lipidosis (HL) is a common sequela in anorexic guinea pigs and can be seen as soon as 12 hours after the beginning of anorexia. This must be considered if an anorexic animal has to be anaesthetised. In these animals:
- Check liver enzymes, albumin levels, blood glucose and electrolytes
- Stabilise the patient as much as possible before anaesthesia with supportive therapy
- Ideally drugs with hepatic metabolism should be avoided; if avoidance is not possible, reduce doses and increase dosing intervals. It is preferable to use drugs that have an antagonist (e.g. alpha-2 agonists).
To the authors' knowledge there are no studies investigating drugs pharmacokinetics in guinea pigs with HL. In humans, dogs and cats, propofol is one of the drugs of choice due to its extrahepatic metabolism. In humans, there is no evidence of impaired elimination of propofol in patients with cirrhosis of the liver and it has been successfully used in cats with HL for feeding tube placement (Posner et al, 2008).
As an analgesic, remifentanil would be the drug of choice, being the only opioid that is not metabolised by the liver but by non-specific tissue and plasma esterases. There are no clinical reports investigating the use of remifentanil in guinea pigs, but the drug has been safely used in research (Kojima et al, 2013; Schorghuber et al, 2014).
Conclusions
Balanced general anaesthesia entails the administration of different types of drugs in order to use less of each one and thus reduce side effects. This concept should be applied, whenever possible, also in small mammals. Several drug combinations can be used according to the patient conditions, the procedure that need to be performed and the clinician's familiarity and knowledge of drugs, mechanism of action and possible side effects.
KEY POINTS
- Alpha-2 agonists, benzodiazepine and ketamine are the most commonly used drugs for premedication/induction of general anaesthesia in small mammals.
- Inhalant anaesthetics are generally used for maintenance of general anaesthesia.
- Multimodal analgesia should be provided.
- Opioids should be used when moderate/intense pain is expected: pain induces gastrointestinal stasis.
- Loco-regional anaesthesia, when possible, should be the first choice to provide analgesia.
- Choose the anaesthetic protocol and perioperative management based on the patient's clinical condition.


