The Syrian or Golden hamster (Mesocricetus auratus) is the best-known species in the pet trade. Dwarf species such as the Russian Dwarf (Phodopus sungorous), Campbell's dwarf (Phodopus campbelli), Roborovski (Phodopus roborovskii) and the Chinese Hamster (Critetulus griseus) are also popular. Their lifespan is up to 3 years and gastrointestinal disease is common in these species, although there is a paucity of information in the literature regarding how to approach these cases.
Anatomy and physiology
Hamsters’ dental formula is 2 I1/1, C0/0, P0/0, M3/3. Their incisors are continuously growing and coloured yellow because of iron deposits in the enamel. The crowns of the molar teeth can retain food, so hamsters are susceptible to dental caries. Hamsters have large, non-glandular cheek pouches invaginating from the oral mucosa, which extend to the scapulae and are used for transporting food, bedding and young (O'Malley, 2005). Their contents must be taken into account when obtaining an accurate bodyweight. Hamsters have a large non glandular forestomach, leading into the main glandular stomach, separated by a muscular-like sphincter. The contents of these compartments do not mix. Oesophageal and stomach anatomy means, like other rodents, hamsters cannot vomit. The caecum is sacculated, containing bacterial microflora including Bacteroides spp. and Lactobacillus spp., plus a few coliforms and clostridia (O'Malley, 2005). The liver is divided into four lobes and the gall bladder is located in the ventral median lobe.
Nutrition
Hamsters are omnivorous. A complete pelleted diet should be the predominant food offered, supplemented with small amounts of fresh fruit and vegetables, for example leafy greens, broccoli, herbs, apples or pears. Sugary treats should be avoided, but small pieces of boiled egg or mealworms can be offered for additional protein (Keeble, 2009). Hay can be offered to increase fibre intake (Johnson, 2012). Muesli mixes should be avoided as they may encourage selective feeding, leading to dietary imbalance and obesity (Keeble, 2009; Sayers and Smith, 2010; Lennox and Bauck, 2012). Wood should be provided to encourage gnawing behaviour for enrichment and to help wear the teeth. Fresh water should always be available; sipper bottles are usually preferred. Food is carried in the cheek pouches and often cached in the corners of the cage. This food should be regularly removed to prevent spoiling. Sudden diet changes commonly precipitate diarrhoea in hamsters, so any new foods should be introduced gradually.
Oral cavity diseases
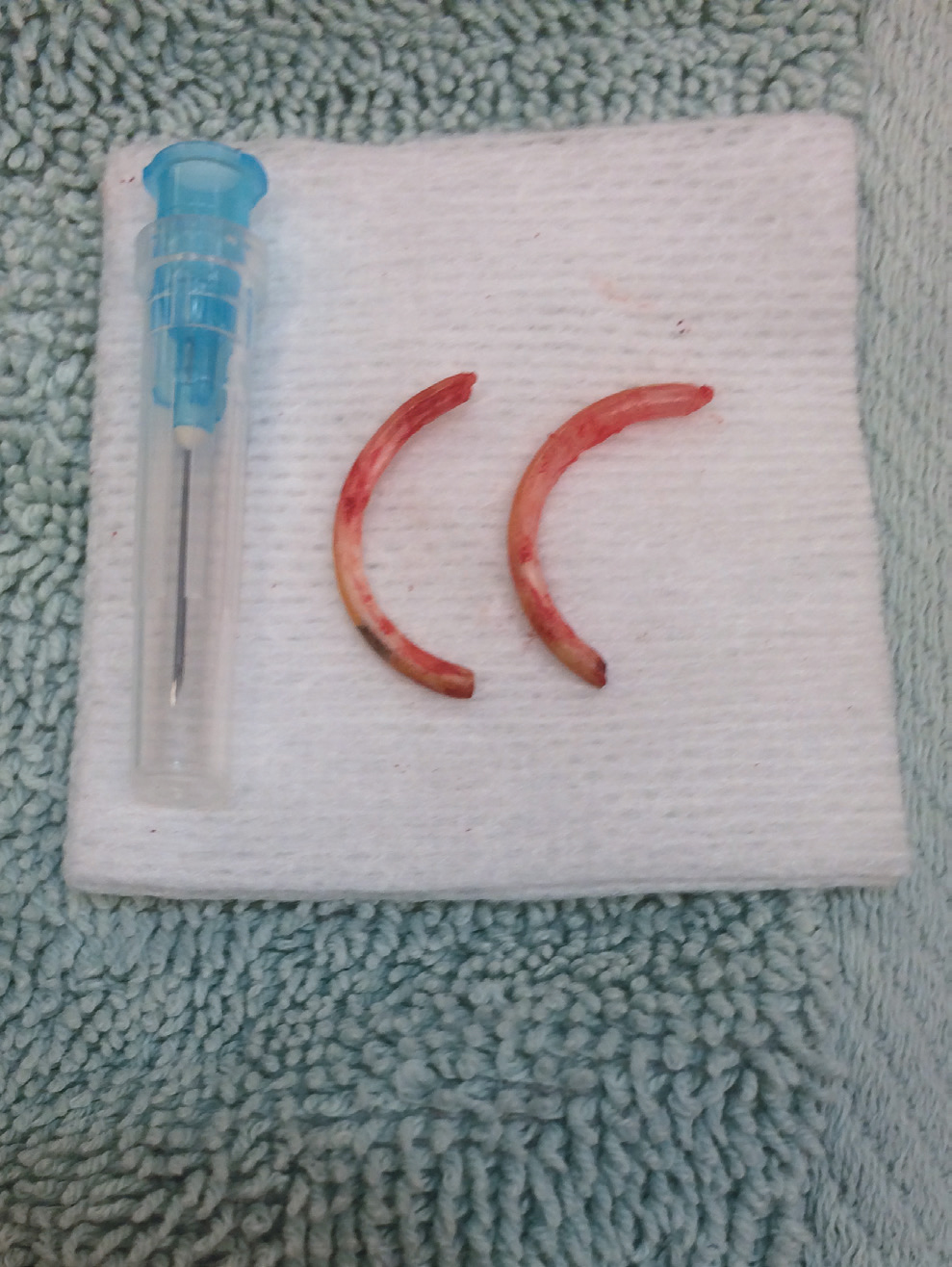
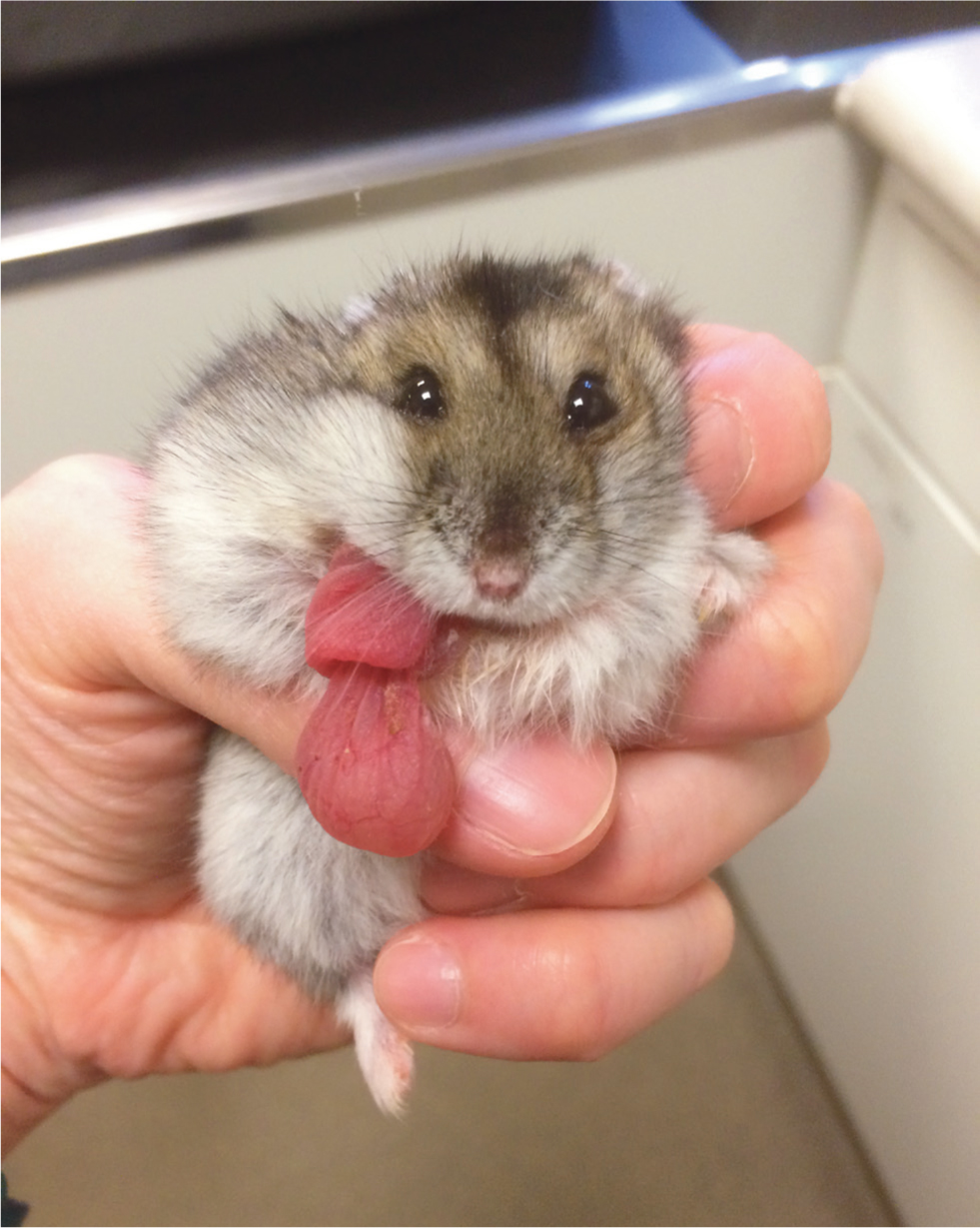
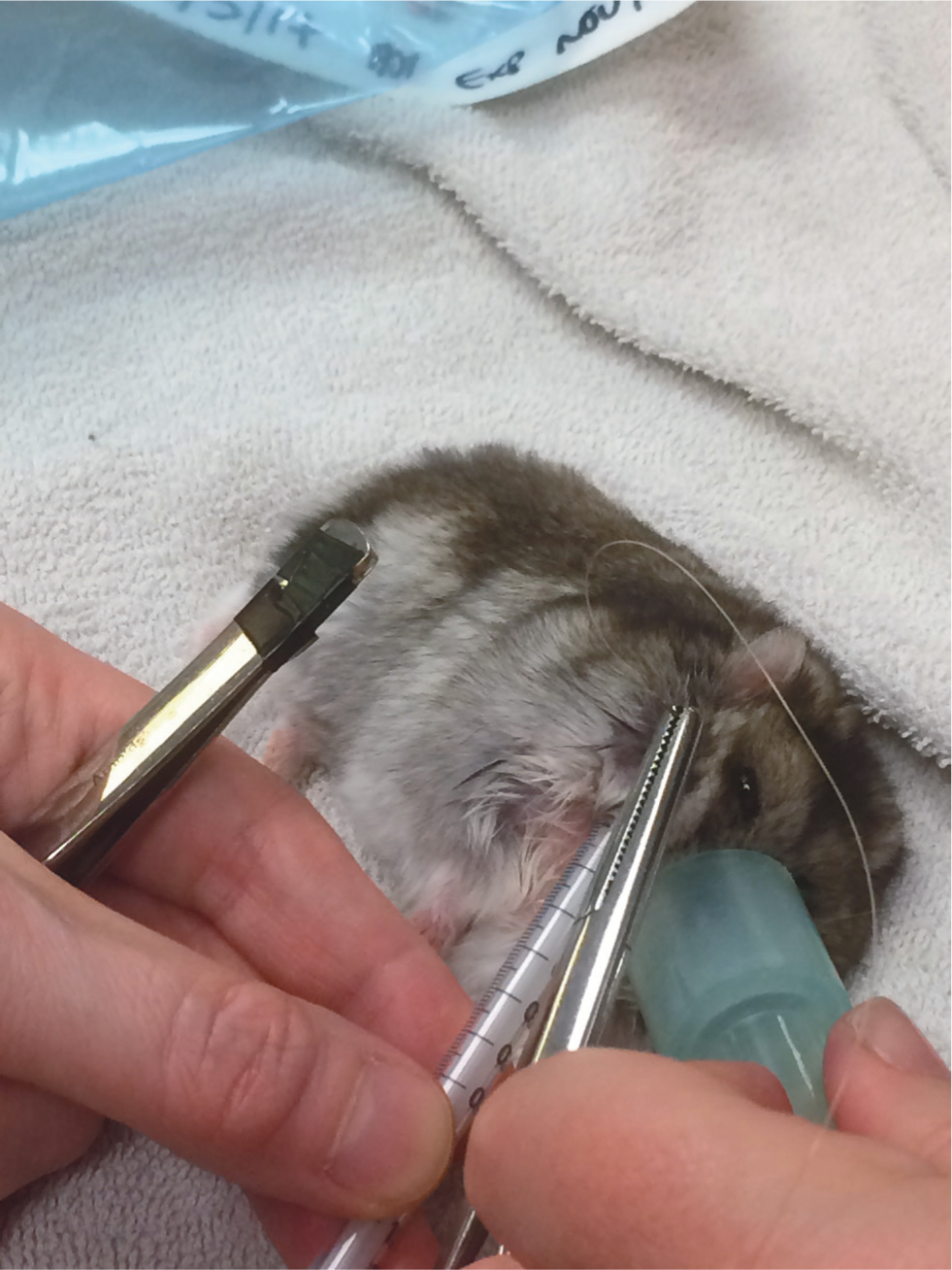
Oral cavity disorders of particular note are overgrown incisors, which may need regular trimming. This can be caused by a lack of wear, for example a lack of chewing opportunity, or by trauma, such as bar chewing or trauma from a fall. Incisor reduction should be performed with a low speed burr or cutting disc rather than clippers, to avoid longitudinal fractures of the teeth. Extraction is possible but challenging given the extensive reserve crowns and fragile jaw (Böhmer, 2015) (Figure 1). Particular care must be taken to avoid fracturing the jaw or damaging the apices of the molars which lie in close proximity. Cheek pouches may become impacted, necessitating manual evacuation and flushing. Eversion of the cheek pouch may occur and has a dramatic appearance (Figure 2). Providing the tissue remains viable, the pouch may be gently replaced under anaesthesia, with 1–2 sutures placed to hold it in position. This is most easily performed with a plastic 1ml syringe inserted into the replaced pouch, used to ‘bounce’ the needle off to ensure a full thickness bite of skin and pouch (Figure 3). Cheek pouch impaction and eversion are often linked to dental disease, so a full dental examination should be performed and treatment directed to the underlying cause.
Approach to the hamster with diarrhoea
Diarrhoea is commonly seen in hamsters and is often colloquially referred to as ‘wet tail’. This is not technically correct, as the term wet tail actually refers to infection with Lawsonia intracellularis. The following suggests a logical approach to the investigation of these cases. Specific disease conditions are discussed.
History
A thorough history is essential and should be structured as for other mammal species. Particular attention should be paid to signalment, diet and husbandry. This includes any recent changes to diet, group size, disease in conspecifics, any historic disease in the colony (for example, if the animal came from a breeding establishment), duration of clinical signs and any treatments administered by the owner.
Clinical examination
This can be challenging in small species, but a systematic approach will help ensure that nothing is missed. A small towel can be used to assist handling of more fractious individuals. Weight, body condition and condition of the coat should first be assessed, along with hydration status. When measuring bodyweight, the contents of the cheek pouches should be taken into account. Severely dehydrated hamsters will appear unkept, with sunken flanks and a marked skin tent. Incisors can be assessed by gently parting the lips and cheek pouch filling can be visually observed and palpated. Thoracic auscultation is done with a paediatric or neonatal stethoscope head. Abdominal palpation will reveal the presence of thickened intestinal loops, gaseous distension or masses. The perineal region should be observed for wetness or staining and, if present, further examination should differentiate diarrhoea from polyuria or vulval discharge. Prolapses can be easily seen if present.
Initial supportive care
This will depend on the severity of clinical signs, but the following should be considered:
Warmth
Collapsed animals may be hypothermic and may benefit from gentle active warming in an incubator. Caution should be taken not to cause heat stress and ideally a heat gradient should be provided, where possible.
Fluid therapy
This is generally provided with crystalloids given via the subcutaneous route, based on a maintenance rate of 75–100 ml/kg/day (Lichtenberger and Lennox, 2012). Intravenous access can be challenging because of the animals’ size, so the intraosseous (IO) route should be considered for collapsed or severely dehydrated animals. The proximal tibia or proximal femur are the preferred IO access sites and a spinal needle or 22–27 gauge hypodermic needle may be used (Lennox and Bauck, 2012). Fluid deficit and ongoing losses should be added to the calculated maintenance rate. Fluids should be warmed to body temperature before administration and the addition of hyaluronidase can increase the speed of absorption from the subcutaneous space.
Analgesia
Hamsters in pain often adopt a hunched posture and reduced movement around their enclosure. In the presence of gastrointestinal disease and possible dehydration, non-steroidal anti-inflammatory drugs should be avoided. However, an opioid such as buprenorphine is appropriate if analgesia is required. Assessment of pain in rodents can be challenging, but analgesia should not be overlooked. The off-licence use of many drugs, used routinely in dogs and cats, has been reported (Miller and Richardson, 2011). An up-to-date exotics formulary should be consulted for dosing and medications should be diluted before use if necessary. The use of maropitant citrate is anecdotally reported for the treatment of visceral pain and inflammation in rabbits (Le, 2017), but there are no published reports of its use in hamsters and a study in mice showed that maropitant did not have an anti-inflammatory effect, and induced intestinal hypomotility (Mikawa et al, 2015). Further research is required before its use can be recommended.
Nutritional support
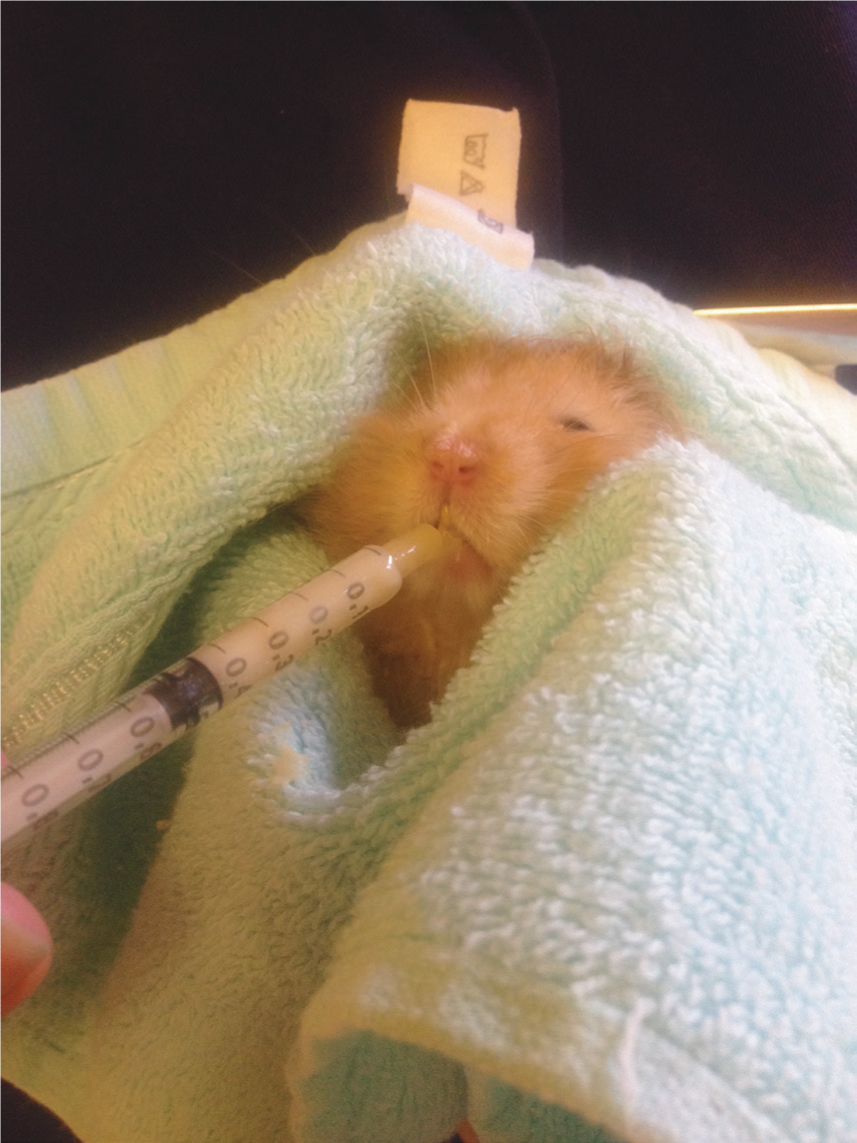
Animals should always have access to their usual diet. If anorexic, syringe feeding with a commercial liquid diet such as Lafeber Emeraid for omnivores may be required (Figure 4). Oral electrolytes and glucose can also be administered.
Diagnostics
Despite the small size of hamsters, venepuncture is possible. Use of the lateral saphenous, jugular, cranial vena cava and femoral vein have all been reported (McKeon et al, 2020). Chemical restraint should be considered, especially if the cranial vena cava is to be sampled. As a rule of thumb, the maximum volume of blood obtained should be no more than 1% of bodyweight. A glucose measurement, blood smear examination and packed cell volume, and total protein should be performed as a minimum.
Faecal parasitology (wet smear and flotation) and gram stain should be performed in all cases. Culture and sensitivity may also be indicated. If pinworms are suspected, then a sticky tape preparation from the perianal region can be assessed for presence of eggs (Ward, 2009).
Radiography and abdominal ultrasound can also be informative. Anaesthesia will usually be required to achieve adequate positioning. The administration of positive contrast may be helpful if obstruction or intussusception is suspected. If results of imaging are suspicious, exploratory laparotomy should be considered. Post-mortem examination should always be offered if the patient fails to recover. This is particularly important in a disease outbreak in a colony.
Common conditions
Drug-associated enteritis
The gastrointestinal flora are predominantly gram-positive organisms (Lactobacillus spp., Bacteroides spp.) (O'Malley, 2005). Hamsters are therefore prone to dysbiosis following administration of certain antibiotics. The PLACE rule is a useful mnemonic to remember which ones to avoid (Hedley, 2018) and is shown in Table 1. Use of these drugs may disrupt the normal flora leading to overgrowth of coliforms and clostridium difficile (CD). In the event of dysbiosis, treatment should include discontinuation of the antibiotic and supportive care with fluids and nutritional support. Probiotics are anecdotally recommended (Ward, 2009) and may be beneficial, although there is little definitive evidence for their use. Hamsters are used as a model for CD-associated diarrhoea in human patients, and a study showed that when pre-treated with Lactobacillus spp. and the prebiotic xylitol, hamsters were resistant to experimental infection with CD (Rätsep et al, 2017). Faecal microbiota treatment (transfaunation) has been successful in treating gastroenteritis caused by various conditions in laboratory mice (Lawley et al, 2012; Tian et al, 2016) and this technique has been anecdotally reported in hamsters (Ward, 2009) There is also evidence for the use of bovine immunoglobulin to protect against CD infection in hamsters (Lyerly et al, 1991). Overall prognosis is often poor once clinical signs have developed.
Table 1. The PLACE mnemonic of antibiotics that may result in serious side effects in hamsters (Hedley, 2018)
| Penicillins |
| Lincosamides |
| Aminoglycosides |
| Cephalosporins |
| Erythromycin |
Bacterial diseases
Bacterial diseases in hamsters are often associated with them being immunocompromised (Fiskett, 2011). This may be caused by stress from transport, overcrowding, poor environmental conditions, nutritional compromise (which can be secondary to dental disease), or co-morbidities. All of these factors must be considered in the approach to a clinical case.
Diarrhoea is common in hamsters and is often referred to as ‘wet tail’. Although this is not a medical term, ‘wet tail’ is a commonly used colloquial term referring to a specific disease called proliferative ileitis, caused by a bacterial infection with Lawsonia intracellularis (Fiskett, 2011). This is a gram-negative curved rod, spread via the feco-oral route. The disease is particularly common in breeding colonies, with juvenile hamsters at 4–8 weeks old most commonly affected, and mortality may approach 90% (Fiskett, 2011). Adult hamsters may be affected, if severely stressed, but mainly act as a reservoir for infection. Animals may present with acute, profuse haemorrhagic diarrhoea with death occurring within 24–48 hours. A more chronic form of the disease presents as diarrhoea, stunted growth, palpably thickened intestinal loops and enlarged mesenteric lymph nodes. Intussusception and rectal prolapse may also be features. Postmortem examination usually reveals a thickened, dilated and hyperaemic distal ileum and caecum. Diagnosis is based on typical presenting signs and clinical examination, with confirmation requiring histopathology. Treatment has variable success and includes aggressive supportive care with warmth, fluid therapy and nutritional support, in combination with analgesia, antibiosis and oral bismuth subsalicylate. Suitable empiric antibiotic choices for these species include trimethoprim/sulphonamide, tetracyclines or metronidazole (Ward, 2009). Control strategies aim to eliminate the pathogen with strict hygiene and isolation of affected individuals, combined with the optimisation of husbandry to reduce stress.
Helicobacter spp. and Campylobacter spp. have been isolated from the intestinal tracts of hamsters with clinical signs of gastrointestinal disease and in asymptomatic animals (Nagamine et al, 2015). Both have potential zoonotic potential. Salmonella typhimurium may cause enteritis and usually progresses to systemic infection, septicaemia and death. The disease is uncommon in hamsters but does also have zoonotic potential. Antibiotic therapy should be based on culture and sensitivity testing, however the empiric suggestions above are suitable while awaiting results.
Tyzzer's disease, caused by Clostridium piliforme, is reported in hamsters and may be associated with high mortality. Infection is usually considered to be opportunistic, secondary to severe stress such as overcrowding, poor hygiene and inappropriate diet. Clinical signs include acute pale yellow, watery diarrhoea, lethargy, dehydration and death. Treatment is as for other gastrointestinal bacterial infections.
Parasitic diseases
Giardiasis in hamsters ranges from subclinical to chronic wasting with diarrhoea. Treatment is with metronidazole or fenbendazole but the infection can be difficult to eliminate (Ward, 2009). Spironucleus (Hexamita) muris is also found, but is generally considered non-pathogenic.
Several species of tapeworm affect hamsters, most notably Rodentolepis (formerly Hymenolepis) nana, the dwarf tapeworm. This organism has both a direct and indirect lifecycle and has zoonotic potential. Most infections are asymptomatic, but clinical signs associated with heavy infestations include poor weight gain, abdominal distention and diarrhoea (Ward, 2009). Diagnosis is by faecal flotation and treatment is with praziquantel (Huynh and Pignon, 2013).
Hamsters are commonly infected with the mouse pinworm Syphacia obvelata, although they have their own, Syphacia mesocriceti. The nematodes are generally found in the caecum, and, to a lesser extent, the colon. Syphacia has a direct life cycle with eggs deposited around the anus. In immunocompetent animals, infestation does not result in overt clinical disease. Clinical disease signs attributed to the pinworm include rectal prolapse, poor hair coat and cachexia (Huynh and Pignon, 2013). Diagnosis is via faecal flotation or transparent tape slide test of the animal's anal area. The recommended treatment for hamster pinworms is fenbendazole, 20–50 mg/kg orally once a day for 5 days, or ivermectin, 2 mg/kg topically once (Huynh and Pignon, 2013). Environmental decontamination is also required.
Neoplasia
The incidence of alimentary neoplasia is low and most reported tumours are benign, such as gastric squamous papillomas and intestinal adenomas, although cases of small intestinal lymphosarcoma have also been reported (Greenacre, 2004; Ward, 2009; Johnson, 2012). Intestinal carcinomas and small intestinal lymphoma are reported and metastasis may occur. Presenting clinical signs of alimentary neoplasia include reduced appetite, weight loss and palpable abdominal masses (Brown and Donnelly, 2012). Solitary neoplasms may be surgically resected and for diffuse disease, chemotherapy protocols are anecdotally reported (Bennett, 2012).
Surgical conditions
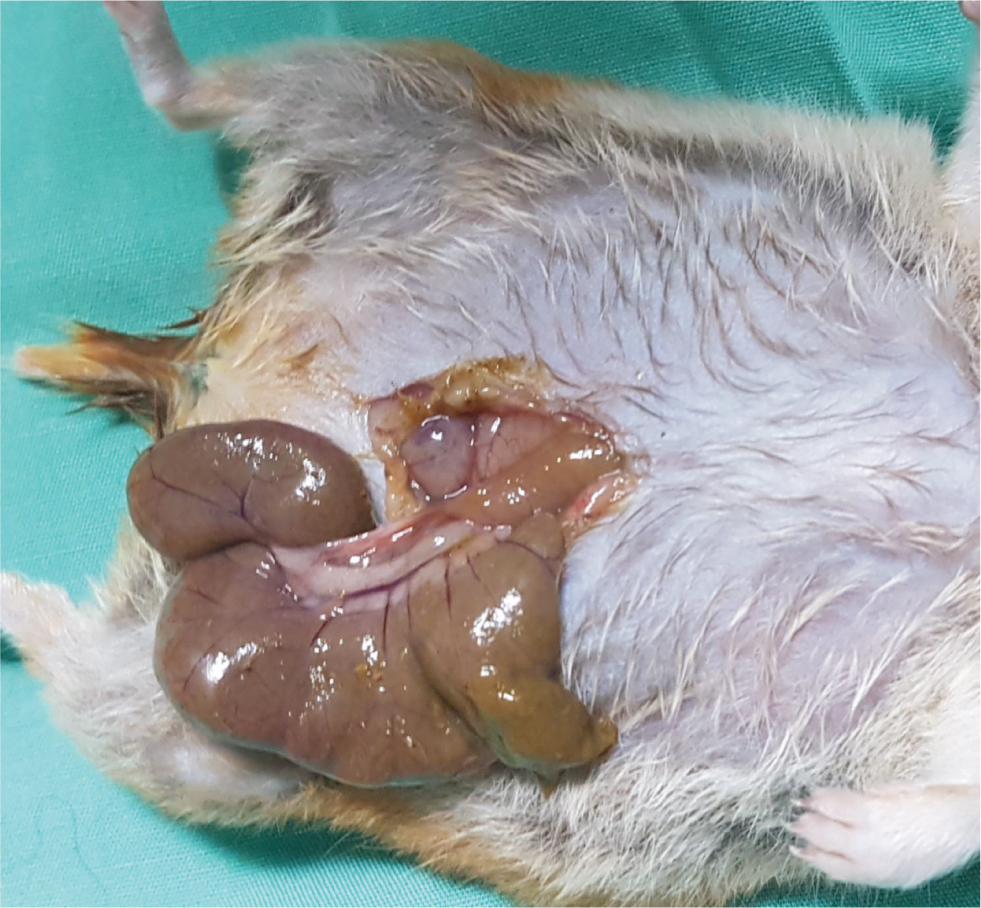
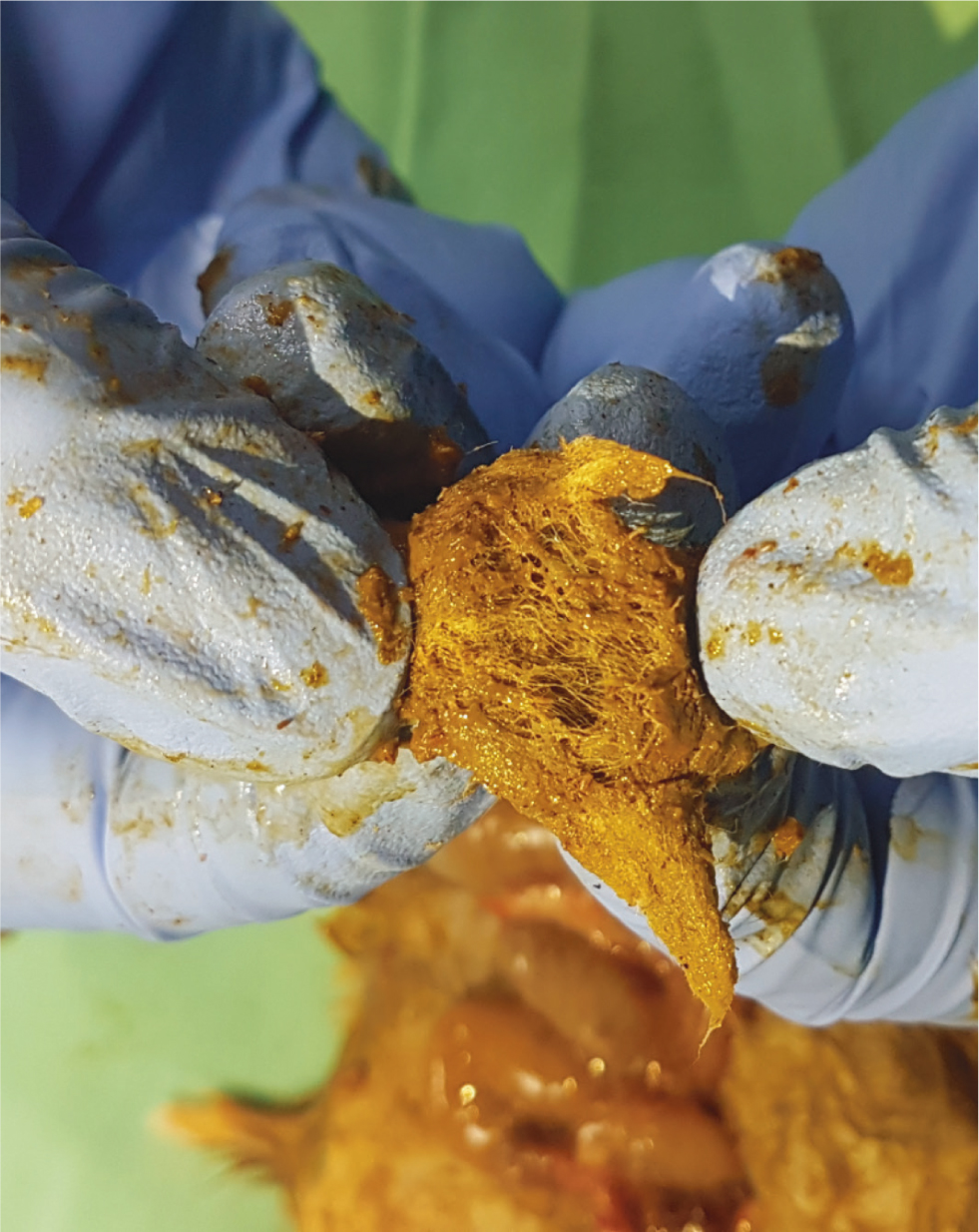
Intestinal obstruction may occur following ingestion of bedding material. In general, paper bedding or shavings are recommended (Keeble, 2009). A depth of at least 40 cm allows burrowing behaviour and improves welfare of Golden hamsters (Hauzenberger et al, 2006). Inappropriate bedding can lead to intestinal impaction and obstruction. Figure 5 shows a dilated large intestine found at postmortem of a hamster presenting initially with diarrhoea, followed by reduced/absent faecal production, dehydration and abdominal pain. Figure 6 shows kapok bedding material recovered from the dilated loop of bowel. Intestinal obstruction should be considered a differential diagnosis in hamsters with gastrointestinal signs and questions regarding bedding provided should be included in the history of each case.
Rectal prolapse is not uncommon and may be comprised of a partial or complete prolapse or a prolapsed intussusception. Causes include anything leading to enteritis, hypermotility or straining, for example proliferative ileitis, enteritis, heavy parasite burdens, intussusception or intestinal obstruction. Partial prolapses may be replaced and a purse-string suture placed to retain while the underlying cause is addressed, although larger prolapses or intussusception require surgical treatment.
Summary
Gastrointestinal disease is common in pet hamsters. Many conditions are related to husbandry deficits, inappropriate nutrition, sudden diet change or poor dental health, while others are related to previous drug administration. A logical approach to the case, comprising a detailed evaluation of the history and identification of potential predisposing factors, is important to assist with treatment and prevention of these conditions. Intensive supportive care is oft en required, alongside carrying out further diagnostics to allow targeted treatment which will enhance success with these cases.
KEY POINTS
- Gastrointestinal disease is a common presentation in hamster species, and their small size and temperament can make some aspects of diagnosis and treatment challenging.
- Diarrhoea is particularly common and sudden dietary change is a common inciting cause. A common bacterial cause is Lawsonia intracellularis (the cause of ‘wet tail’) and a less common cause is Clostridium piliforme (the cause of Tyzzer's disease). Drug induced dysbiosis and neoplasia are also seen regularly.
- Supportive treatment of diarrhoea includes warmth, fluids, analgesia and nutritional support, whilst underlying causes are identified and addressed.
- Following a full history and clinical examination, further diagnostic steps include faecal parasitology and microbiology, biochemistry and haematology, abdominal ultrasound and radiography (with or without positive contrast).
- Cheek pouch prolapse is occasionally seen and can be corrected by replacing the pouch and placing an anchoring suture. Other indications for surgery include gastrointestinal obstruction, intussusception and rectal prolapse.


