Insulinomas are the most common pancreatic tumours in dogs. Both insulin and hypoglycaemia provide negative feedback for insulin secretion; however, neoplastic β-cells may secrete insulin in an uncontrolled fashion due to the lack of a response to this negative feedback. The excessive insulin secretion leads to hyperinsulinaemia-induced hypoglycaemia (Tobin, 1999).
Although the exact incidence of canine insulinoma has not been established, insulinomas are most commonly seen in middle-sized to large dog breeds including German Shepherds, Irish Setters, Boxers, Poodles, Golden Retrievers, Labrador Retrievers and Collies. Nonetheless, insulinomas have also been reported in smaller breeds like West Highland White Terriers (Mehlhaff, 1985; Leifer, 1986; Caywood, 1988). The average age of diagnosis for dogs with insulinomas, based on data from eight publications encompassing 214 dogs, is approximately 9 years, ranging from 3 to 15 years (Buishand, 2022).
Primary canine insulinomas typically manifest as nodular tumours with a diameter of less than 2.5 cm. These tumours primarily occur in the left or right pancreatic limb, rather than in the pancreatic corpus. Multiple primary tumours have been reported in up to 14% of cases. Despite the absence of definitive histological criteria for malignancy, insulinomas are overwhelmingly associated with metastasis and are regarded as malignant in over 95% of cases. At the time of diagnosis, 40–50% of dogs with insulinomas have visible metastases, primarily affecting the abdominal lymph nodes, the liver or both (Mehlhaff, 1985; Buishand, 2010).
Clinical signs
The most common clinical signs associated with canine insulinomas are as follows, along with the corresponding percentage of cases (Dunn et al, 1993; Ryan et al, 2021):
- Seizures (52%)
- Generalised weakness (42%)
- Posterior paresis (33%)
- Collapse (28%)
- Muscle fasciculations (19%)
- Ataxia (18%)
- Polyphagia (7%)
- Polydypsia and polyuria (6%).
The clinical signs of canine insulinomas typically manifest intermittently, as affected dogs usually exhibit an absence of clinical signs between hypoglycaemic episodes. Events which trigger increased glucose use or reduced glucose availability increase the likelihood of a hypoglycaemia event, for example periods of fasting, exercise, excitement or stress during the early stages of disease. The severity of clinical signs is influenced by the nadir of glucose, the rate at which blood glucose concentration decreases, the duration of hypoglycaemia and whether the individual has experienced previous episodes of hypoglycaemia. A gradual decline in blood glucose concentration over an extended period to 2 mmol/L (normal reference: 4.2–5.8 mmol/L) is less likely to trigger clinical signs of hypoglycaemia compared to a rapid drop to the same level within a few hours. In cases where hypoglycaemia is severe and prolonged, there is a risk of developing cerebral cortical laminar necrosis, which can lead to coma and, ultimately, death (Buishand, 2022).
Diagnosis
Historically, a presumptive diagnosis of canine insulinoma relied on signalment and clinical history, along with the presence of Whipple's triad. This triad consists of three components: the presence of hypoglycaemia, clinical symptoms associated with hypoglycaemia and relief of symptoms following glucose administration or feeding. In cases where there is a clinical suspicion of insulinoma, but the dog does not exhibit hypoglycaemia upon presentation (for example, due to increased catecholamines during hospitalisation or lack of excessive insulin secretion at that time), it may be necessary to fast the dog to demonstrate spontaneous hypoglycaemia. However, careful glycaemic monitoring is crucial during fasting, as blood glucose can decrease before hypoglycaemic signs become apparent, potentially leading to rapid and severe symptoms in dogs with insulinoma. In most cases, fasting dogs with insulinomas will demonstrate hypoglycaemia within 24 hours (Leifer, 1986).
While Whipple's triad encompasses various causes of hypoglycaemia, the next step in the diagnostic process involves ruling out differential diagnoses. In elderly dogs, common differential diagnoses for hypoglycaemia, apart from insulinoma, include spurious laboratory results, xylitol intoxication, hypoadrenocorticism, hepatic insufficiency, portosystemic shunts, sepsis and non-pancreatic neoplasms that produce incompletely processed insulin-like growth factors (such as hepatocellular carcinoma, leiomyosarcoma, metastatic mammary carcinoma and lymphoma) (Zini et al, 2007; Polansky et al, 2018). Less common differentials encompass juvenile hypoglycaemia, hunting dog hypoglycaemia, glycogen storage disease, glucagon deficiency, severe primary renal glucosuria and nesidioblastosis. Additionally, iatrogenic causes of hypoglycaemia can arise from drug administration, such as insulin and sulfonylureas (Buishand, 2022).
A key component of a diagnosis of an insulinoma is to determine whether hypoglycaemia occurs and if this is associated with inappropriate insulin secretion. Therefore, measuring insulin concentration at the time of hypoglycaemia is essential. Serum fructosamine may be decreased in patients with insulinoma. However, fructosamine measurement varies widely between laboratories and the true sensitivity and specificity of this test for insulinoma is unknown (Loste et al, 2001; Mellanby and Herrtage, 2002). Therefore, whilst decreased fructosamine may increase suspicion, it is not diagnostic for an insulinoma. In cases of canine insulinoma, circulating insulin concentration typically falls within or above the reference range (2-21 μU/mL). The characteristic feature of a canine insulinoma is the simultaneous occurrence of blood glucose levels below 3.5 mmol/L and plasma insulin levels above 10 μU/mL. While plasma insulin concentrations above the upper limit of the reference range are observed in 56–83% of dogs with insulinomas (Fernandez et al, 2009; Madarame et al, 2009), insulin levels can be within the reference range. However, in the presence of blood glucose below 3.5 mmol/L, plasma insulin concentration should be negligible due to hypoglycaemic negative feedback. Failure to exhibit this response indicates inappropriate insulin secretion (Buishand and Kirpensteijn, 2012). The next step would be to investigate whether a pancreatic mass is identifiable.
Diagnostic imaging
It is crucial to make every effort to maximise the likelihood of identifying a pancreatic mass if present in an individual. Knowing the specific location of the insulinoma prior to surgery helps guide the selection between open and laparoscopic pancreatic surgery, as well as the choice between enucleation or resection through partial pancreatectomy.
Insulinoma staging follows the World Health Organization's TNM (tumour, node, metastasis) system (Owen, 1980). Canine insulinoma are classified into three stages: T1N0M0 (stage I), T1N1M0 (stage II), and T0N0M1, T1N0M1, or T1N1M1 (stage III). The TNM stage serves as an important prognostic factor, providing valuable information to clients for making informed decisions about treatment options (Caywood, 1988).
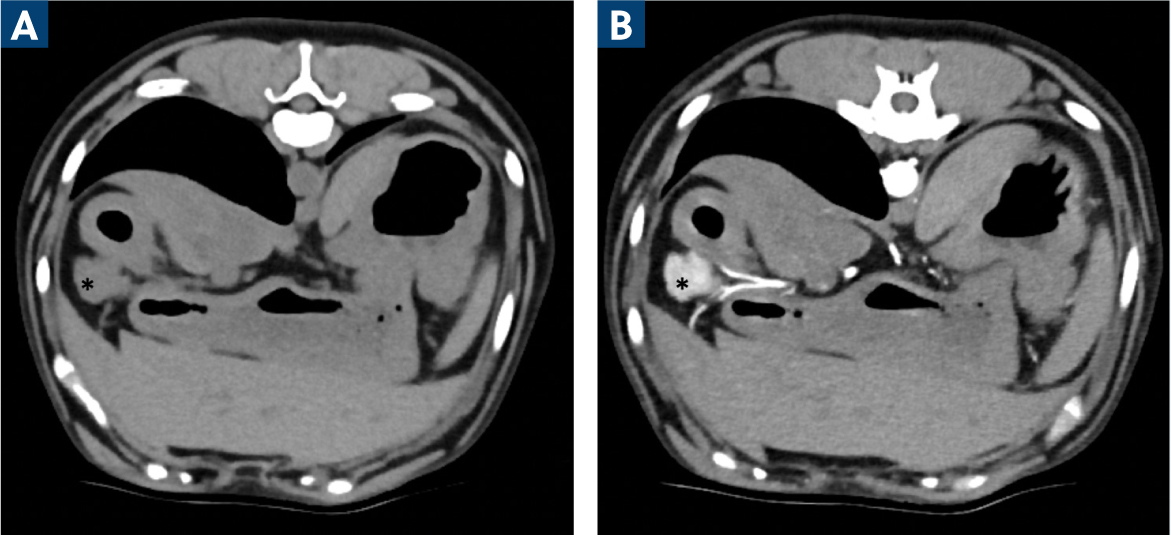
A contrast-enhanced computed tomography scan (CECT) is considered the gold-standard method for detecting and localising canine insulinomas (Figure 1). In a case series of 27 dogs with insulinomas, CECT demonstrated a high sensitivity of 96% in detecting primary insulinomas. The sensitivity of CECT scans in detecting lymph node metastases was 67%, and the sensitivity for detecting liver metastases was 75% (Buishand et al, 2018).
While ultrasonography is commonly available and used in general veterinary practice, its sensitivity in detecting canine insulinomas is low. Only approximately one-third of pancreatic insulinomas are visualised using ultrasound and in one study, none of five abdominal insulinoma lymph node metastases were detected with ultrasonography (Robben, 2005). Therefore, the author's institution does not use ultrasonography as the imaging modality of choice for detecting canine insulinomas. Ultrasonography proves to be useful if ultrasound-guided fine needle aspiration aspirates or biopsies are deemed appropriate from any liver lesions, regional lymph nodes and any enlarged lymph nodes detected on CECT to facilitate accurate pre-operative TNM staging.
Medical management
Typically, surgical treatment is the most effective approach for canine insulinoma. Insulinoma resection can lead to extended periods without disease recurrence and improved survival times. Concurrent medical treatment may also be necessary for many dogs at some stage of their management.
Emergency treatment
Immediate treatment is essential for dogs experiencing an acute hypoglycaemic crisis, which can lead to severe and sudden seizures. The aim is to minimise the duration of hypoglycaemia and reduce the risk of irreversible brain damage.
Initially, a slow intravenous infusion of 1 mL/kg of 20% glucose over 5–10 minutes should be administered to stabilise the patient. If the dog responds well, a small meal can be provided, and long-term medical treatment should be initiated. In cases of uncontrollable hypoglycaemic seizures, a continuous rate infusion of 2.5–5% glucose at a rate of 3–4 mL/kg/h should be initiated. Additionally, dexamethasone at a dose of 0.5–1 mg/kg can be added to the intravenous fluids and administered over a 6-hour period. This can be repeated every 12–24 hours if necessary (Rijnberk, 2010; Goutal, 2012).
Most dogs will respond to the emergency treatment outlined previously; however, it is crucial to seek urgent specialist advice if the dog's blood glucose concentration fails to normalise or if seizures persist despite the normalisation of blood glucose levels. Persistent hypoglycaemia may require medical treatments such as glucagon and urgent surgical resection of the insulinoma. A low dose of medetomidine or dexmedetomidine may also aid short-term glycaemic control. Persistent seizure activity may require diazepam, levetiracetam, phenobarbital loading, propofol or a combination of these.
Long-term management
Dogs diagnosed with insulinoma should follow a feeding regimen consisting of four to six meals throughout the day. Their diet should be rich in proteins, fats, and complex carbohydrates, while avoiding simple carbohydrates. This type of diet helps reduce postprandial hyperglycaemia (Goutal, 2012), thereby minimising the stimulation of insulin release from the insulinoma. Physical exercise should be limited to short lead walks to prevent clinical hypoglycaemia. If clinical signs persist despite frequent feedings and restricted activity, additional medications may be necessary.
Glucocorticoids, like prednisolone, promote hepatic gluconeogenesis and glycogenolysis, while counteracting the effects of insulin at the cellular level. The recommended initial dose of prednisolone is 0.25 mg/kg administered orally twice daily. Dosages exceeding 1 mg/kg twice daily are known to suppress the immune system and will likely cause iatrogenic Cushing's syndrome (Elie, 1995; Steiner, 1996).
Diazoxide inhibits pancreatic insulin release, stimulates hepatic gluconeogenesis and glycogenolysis and reduces glucose uptake by tissues. The recommended starting dose of diazoxide is 5 mg/kg administered orally twice daily, which can be gradually increased up to 30 mg/kg twice daily if necessary (Steiner, 1996). Contrain-dications for diazoxide use in dogs include liver, kidney or heart failure. Side effects are rare, although reduced appetite and vomiting have been reported (Elie, 1995; Steiner, 1996). Diazoxide availability is often limited, and long-term treatment can be costly.
Recently, three small retrospective studies have explored the use of toceranib phosphate (marketed as Palladia, a receptor tyrosine kinase inhibitor licensed for canine mast cell tumours) in the treatment of canine insulinomas (Flesner, 2019; Alonso-Miguel, 2021; Sheppard-Olivares, 2022). Long-term glycaemic control was observed in some dogs receiving toceranib phosphate. However, the specific contribution of toceranib phosphate to this effect is yet to be determined, because it is important to note that these studies had limitations, including their retrospective nature and the small size of the study groups, which introduced heterogeneity. The Royal Veterinary College is currently leading a prospective multi-institutional clinical study to precisely assess the effectiveness of toceranib in improving the quality of life and extending the lifespan of dogs with insulinomas. This study is open to dogs with TNM stage II and III insulinoma, or recurrent insulinoma and enrolment will continue until June 2025. Enrolment is not blinded or randomised, and clients and treating veterinarians have full control over treatment group allocation. If you are interested to learn more about this study, because you think you might have seen a dog that would qualify for enrolment, please contact researchers at the Royal Veterinary College for more information: https://www.rvc.ac.uk/research/projects/toceranib-phosphate-therapy
Surgical therapy
Anaesthetic considerations
The authors recommend the following protocol for preparing dogs with insulinoma for anaesthesia and surgery. To prevent fasting hypoglycaemia, dry food should be withheld for 12 hours before surgery, and canned food can be given up to 6 hours prior. Dogs experiencing clinical hypoglycaemia should be provided easily digestible liquid food preparations up to 1–2 hours before surgery. In the immediate pre-operative period, if clinical signs occur, a slow intravenous administration of 1–5 mL of 50% dextrose is recommended over 10 minutes. At induction, the blood glucose concentration should be assessed. When glucose is >3.0 mmol/L no correction of the glucose concentration is required, but the blood glucose concentration should be monitored every hour. If glucose <3.0 mmol/L, 5% dextrose supplementation should be started intravenously at 1 mg/kg/min and the blood glucose concentration should be monitored every 15 minutes. The 5% dextrose continuous rate infusion should be stopped when the blood glucose concentration is >3.0 mmol/L, upon resection of the insulinoma or both. Insulin has a short half-life and normoglycaemia should be restored within minutes when all insulinoma cells are excised, or hyperglycaemia is induced. If a dog remains hypoglycaemic at 5–10 minutes after insulinoma excision, this indicates that insulinoma resection is incomplete and this warrants further exploration of the pancreas, abdominal lymph nodes and liver to identify and resect remaining insulinoma tissue (Comas Collgros, 2022).
Surgical techniques
Regardless of the pre-operative imaging results, a thorough assessment of the entire pancreas should be performed during surgery to locate the insulinoma. The surgical technique for excising the insulinoma depends on its location within the pancreas. If the insulinoma is found in or near the pancreatic corpus, a local enucleation can be performed (Figure 2). Care must be taken to avoid damaging the pancreatic ducts and pancreaticoduodenal arteries during local enucleation.

For insulinomas located in the right or left pancreatic limb, a partial pancreatectomy is the preferred surgery. There are two common techniques used for partial pancreatectomy: the suture-fracture method and bipolar vessel sealing (Wouters, 2011). The suture-fracture technique involves encircling the pancreas with sutures placed proximal to the insulinoma. By tightening the ligatures, the pancreatic parenchyma is crushed, and the portion of the pancreas distal to the ligatures, including the insulinoma, is excised. Alternatively, bipolar vessel sealant devices can be used for secure and rapid haemostasis during partial pancreatectomy, eliminating the need for sutures on the pancreas. The bipolar vessel sealing technique is preferred as it improves surgical performance and is especially beneficial for hard-to-reach lesions (Wouters, 2011).
Laparoscopy has emerged as an advancement in surgical technique for resecting canine insulinomas (Mcclaran, 2017; Nimwegen, 2021). Laparoscopic partial pancreatectomy can be performed on dogs with insulinomas located in the distal two-thirds of the right or left pancreatic limb. The approach (ventral or flank) depends on the specific location of the insulinomas. Laparoscopic resection of selected abdominal lymph node metastases is also possible, but a laparoscopic approach is contraindicated in cases where there are extensive lymph node or liver metastases.
During surgery, metastatic disease is assessed by gross inspection of the abdominal lymph nodes and liver, as well as by monitoring blood glucose levels upon insulinoma resection as outlined above. A recent case report described the use of indocyanine green near-infrared lymphography to detect sentinel lymph nodes in a dog with insulinoma (Nolff, 2023). Eight sentinel lymph nodes were detected and resected, six of which would not have been detectable through visualisation or palpation during surgery. Although histopathology demonstrated that all resected lymph nodes were negative for insulinoma metastases, indocyanine green near-infrared lymphography might offer a more objective approach to abdominal lymph node resection in dogs with insulinoma and in the future, it should be investigated whether this technique improves the clinical outcome of dogs with insulinoma. Until then, any macroscopically enlarged lymph nodes should be excised and debulking of liver metastases is necessary to enhance the effectiveness of adjuvant medical therapy.
Post-operative complications
Approximately 10% of dogs may develop acute pancreatitis following surgical removal of an insulinoma. While the complete form of pancreatitis is observed in only 1 out of 10 dogs, 27% of dogs may experience inappetence and 24% may experience vomiting after the procedure (Hixon, 2019). In cases of post-operative pancreatitis, hospitalised dogs will receive supportive care, including intravenous crystalloid fluids, analgesia, anti-emetic and antinausea medications. Fortunately, most dogs recover within a few days with treatment.
Up to a third of dogs exhibit blood glucose levels above the normal reference range after surgical removal of an insulinoma. This hyperglycaemia is typically transient and occurs as the remaining healthy β-cells in the pancreas regain their normal function. The duration and significance of this hyperglycaemia in human and veterinary medicine are not fully understood, but studies suggest that it resolves on its own in about 90% of cases within 3–9 days (Nockel et al, 2018). In some cases, dogs may require treatment with exogenous insulin if they develop persistent hyperglycaemia beyond the immediate post-operative period, as hyperglycaemia itself will contribute to β-cell toxicity. However, it is important to note that permanent diabetes mellitus requiring long-term insulin treatment is uncommon and has been reported in only about 6% of dogs following surgical removal of insulinomas (Del Busto, 2020).
Prognosis
Combining medical therapy with surgery in dogs with insulinoma has shown to significantly improve the prognosis compared to medical treatment alone. Dogs treated solely with medical therapy have been reported to have a median survival time of 4 months (ranging from 0 to 18 months) (Buishand, 2022). In contrast, recent studies have reported median survival times of around 2.5 years for dogs who received combined surgery and medical therapy, with some cases surviving up to 5 years (Cleland, 2021). Metastasectomy is performed in humans and has been performed in dogs who experience a period of disease-free clinical signs but who later present with disease recurrence and one or two regions of surgically resectable metastatic disease are identified. Other significant prognostic factors include the TNM stage, post-operative blood glucose levels and the histopathological Ki-67 index (Buishand et al, 2010).
Conclusions
Despite the implementation of current multimodal treatment protocols, the long-term prognosis for canine insulinoma remains guarded in most cases. This is primarily due to the potential regrowth of the tumour and the presence of micrometastases that were not detected during surgery. Future studies should focus on establishing novel adjuvant treatments for canine insulinomas. Biobanking programmes, like the Royal Veterinary College Cancer Biobank that systematically collect insulinoma samples and paired blood samples, function as important resources for researchers to unravel the complex genetic and molecular underpinnings of canine insulinoma (Royal Veterinary College, 2022). This knowledge will not only enhance understanding of the disease but also opens doors to innovative treatments and personalised care.
KEY POINTS
- Insulinomas are characterised by uncontrolled secretion of insulin leading to hyperinsulinaemia-induced hypoglycaemia.
- Diagnosis of insulinomas involves the demonstration of the simultaneous occurrence of blood glucose levels below 3.5 mmol/L and plasma insulin levels above 10 μU/mL.
- Contrast enhanced computed tomography scans are the preferred imaging modality for precise tumour, node, metastasis staging of canine insulinomas.
- Medical management of insulinomas involves a feeding regimen with frequent meals, a diet rich in proteins and complex carbohydrates and medications such as glucocorticoids and diazoxide to control blood glucose levels.
- Surgical therapy is the most effective treatment for insulinomas, with techniques including partial pancreatectomy, local enucleation and laparoscopic surgery.


