Circulatory shock is a syndrome characterised by inadequate tissue perfusion leading to insufficient oxygen and nutrient supply for cellular function. Systemic consequences of circulatory shock include endothelial dysfunction, vasoplegia, multiple organ dysfunction syndrome and eventual death if left untreated. Circulatory shock has been classically divided into four categories, each defined by the type of pathology leading to inadequate perfusion (Silverstein and Hopper, 2022). These categories are:
Other subcategories such as hypoxic shock (decreased oxygen content in arterial blood) and metabolic shock (inability to use delivered oxygen) are independent of circulatory dysfunction.
Vasodilatory shock caused by sepsis is the most common form of shock among critically ill human patients (De Backer et al, 2010; Gitz Holler et al, 2019). Although similar data do not exist in veterinary medicine, it remains a common cause of morbidity and mortality in critically ill small animals in the authors’ experience. Besides sepsis, vasodilatory shock may be caused by non-in-fectious aetiologies leading to systemic inflammation (ie systemic inflammatory response syndrome) such as burn injuries, acute pancreatitis or trauma. Other causes include, but are not limited to, anaphylaxis, toxicoses (eg nitrogen or carbon monoxide), liver failure and glucocorticoid deficiency (Landry and Oliver, 2001; Narayan and Petersen, 2022). Persistent, severe shock of any cause can also result in pathological vasodilation.
Regardless of the aetiology, resuscitation of patients with vasodilatory shock includes fluid therapy and pharmacological vasopressors to correct hypotension and increase systemic vascular resistance. The type and volume of administered fluids, as well as the choice of vasoactive agents, has considerably shifted over the past two decades, with high quality recommendations from large human clinical trials and the development of the Surviving Sepsis Campaign guidelines (Evans et al, 2021). Unfortunately, there is a dearth of similar evidence in veterinary medicine and many recommendations are adapted from human intensive care medicine. This article provides a contemporary review of vasodilatory shock, including its pathophysiology and treatment in small animal veterinary medicine.
Pathophysiology of vasodilatory shock
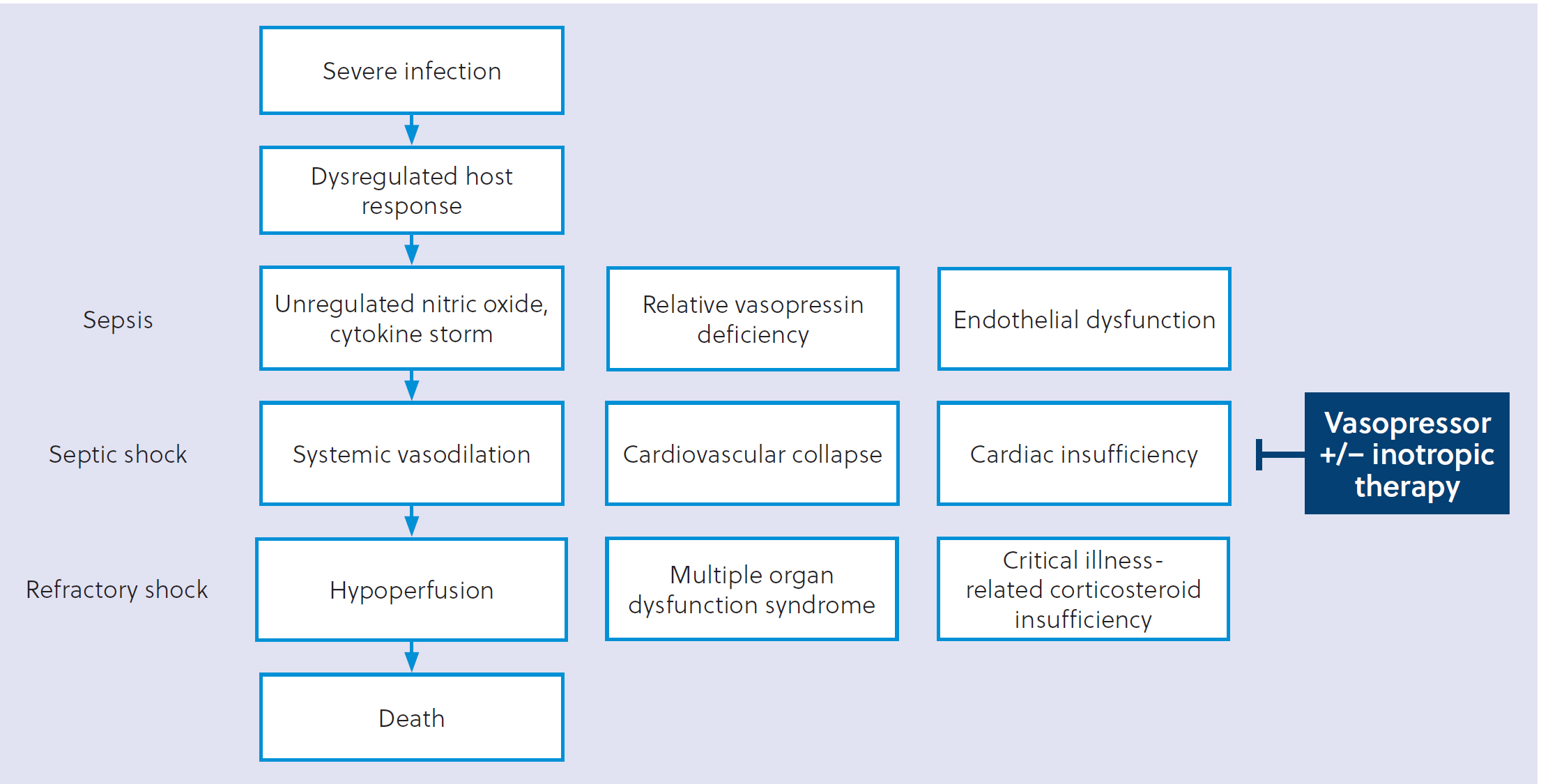
There are several common pathophysiological disturbances that result in vasodilatory shock; a brief review of these allows for a more complete understanding of the rationale of behind vasopressor selection. A comprehensive summary of detailed, aetiologyspecific mechanisms is beyond the scope of this review. Given the prevalence and available bodies of knowledge for different causes of vasodilatory shock, most of this section will describe the pathophysiology of sepsis and septic shock. A graphical overview of sepsis-induced vasodilatory shock can be found in Figure 1.
Anaphylaxis
Anaphylaxis is an acute, multiorgan, systemic allergic reaction that can progress to vasodilatory shock if unrecognised or untreated. The cardiovascular sequelae of anaphylaxis occur after a previously sensitised patient is re-exposed to the sensitising antigen (eg insect sting, environmental allergen, medication etc). This exposure leads to the rapid activation of intracellular signalling cascades that include generalised immunoglobulin E-mediated mast cell degranulation, the generation of arachidonic acid metabolites and a cascade of cytokine- and chemokine-mediated cardiovascular collapse (Peavy and Metcalfe, 2008). Mediators such as histamine, prostaglandin D2, platelet activating factor and tumour necrosis factor alpha increase vascular permeability and cause vasodilation that potentiate the shock state (Nuñez-Borque et al, 2022). Adrenaline is used as a first-line treatment because of its wide range of effects including alpha-adrenergic mediated vasoconstriction and beta-adrenergic actions that cause bronchodilation and decreased release of inflammatory mediators (Nuñez-Borque et al, 2022).
Nitric oxide upregulation
Nitric oxide is an important gaseous signalling molecule in circulatory and immunological pathways. It is produced from the amino acid L-arginine in a reaction catalysed by the nitric oxide synthase family of enzymes. During appropriate immune responses, nitric oxide regulates microcirculatory homeostasis by protecting microvascular blood flow, regulating red cell and leukocyte deformability and assisting with leukocyte–endothelial adhesion (Bateman et al, 2003). Its role as a potent, endogenous vasodilator underlies the hallmark vasodilation and vasoplegia seen in septic shock and other forms of decompensated shock (Landry and Oliver, 2001). The inducible isoform of nitric oxide synthase is markedly upregulated in these disease states, triggered directly by stimuli including lipopolysaccharide or cytokines such as members of the interleukin family (IL-1, IL-2, IL-6), tumour necrosis factor alpha or interferon-gamma (Kotsovolis and Kallaras, 2010). Therefore, pharmacological nitric oxide synthase inhibitors are a natural target for treating septic shock, and early studies into this treatment suggested they provided an improvement in arterial pressure and systemic vascular resistance (Petros et al, 1994; Kilbourn, 1999). However, subsequent trials found an association with decreased cardiac output, organ dysfunction and increased mortality (Klabunde and Ritger, 1991; Cobb et al, 1992; Liaudet et al, 1998; López et al, 2004). Directions in human medicine include the use and development of nitric oxide synthase isoform inhibitors and nitric oxide scavengers for refractory vasodilatory shock (Jentzer et al, 2018; Ibarra-Estrada et al, 2023).
Relative vasopressin deficiency
Vasopressin, also known as antidiuretic hormone, is a peptide hormone with physiological functions through three subtypes (V1–V3) of G protein-coupled receptors. Relevant actions include water resorption through its actions on V2 receptors in the distal nephron and systemic vasoconstriction via V1 receptors in vascular smooth muscle. Its predominant function is to increase renal collecting duct permeability in the face of increasing plasma osmolality; however, a decrease in blood pressure or blood volume can also trigger its release from the neurohypophysis in an attempt to conserve free water to maintain circulating volume (Demiselle et al, 2020). In both humans and animal models of early septic shock, vasopressin levels markedly increase in an attempt to restore circulating volume and vascular tone (Wilson et al, 1981; Brackett et al, 1985; Sharshar et al, 2003). However, as the shock state progresses, the levels of produced, stored and circulating vasopressin decrease and may be associated with progression towards a vasodilatory state (Landry et al, 1997; Sharshar et al, 2002; Lin et al, 2005; Barrett et al, 2007). This decrease remains up to a week after the onset of septic shock in humans (Russell et al, 2008). This same finding is seen in canine models of prolonged haemorrhagic shock (Errington and Rocha e Silva Jr, 1972; Morales et al, 1999). The combination of progressive hypotension with inadequate vasopressin levels led to the concept of a relative vasopressin deficiency, where endogenous secretion of the hormone is inadequate in severe or decompensated shock states. The pathophysiological state of a relative vasopressin deficiency provides the rationale for administration of exogenous vasopressin or its analogues for the treatment of septic shock.
Other mechanisms of vasodilation in sepsis
There is a complex interplay of other signalling molecules, inflammatory mediators and hormones that underlies the peripheral vasodilatation seen with septic shock. Prostacyclin is a prostaglandin molecule released by vascular endothelial cells that acts in a paracrine fashion to cause local vasodilation. Its activity is upregulated and plays a role in many systemic sequelae of sepsis including vasodilatation and cardiorespiratory collapse (Russell et al, 2018). Non-steroidal anti-inflammatory drugs, a class that works by inhibiting cyclo-oxygenase thus preventing prostaglandin production, were evaluated as a treatment for sepsis after animal models suggested a potential benefit on both morbidity and mortality. However, a clinical trial in human patients with sepsis did not find any benefits of this class of medication, and non-steroidal antiinflammatory drugs are not recommended for small animals with sepsis because of potential renal and gastrointestinal side effects (Bernard et al, 1997).
Adrenomedullin is an endogenous peptide hormone that has been associated with several aspects of sepsis pathophysiology including immunological, endocrine and cardiovascular effects. Adrenomedullin has vasodilatory effects on the peripheral vasculature, and it also has been shown to play a role on endothelial stabilisation and maintenance of normal vascular permeability (Kato and Kitamura, 2015; Geven et al, 2018a). Levels of adrenomedullin have been associated with disease severity in patients with sepsis, and it has been investigated as a specific biomarker of sepsis in critically ill human patients (Marino et al, 2014; Lundberg et al, 2020). Preclinical inquiry into exogenous adrenomedullin administration has found benefits on endothelial barrier function, markers of haemodynamics and outcomes in animal models of sepsis (Geven et al, 2018b; van Lier et al, 2021). This molecule remains a promising target for future investigation for understanding and treating sepsis and septic shock.
Lastly, the adenosine triphosphate-sensitive potassium channel is present in vascular smooth muscle and mediates systemic vasodilation in states of low oxygen tension by allowing potassium efflux, leading to hyperpolarisation and muscular relaxation (Buckley et al, 2006). Neurohormonal activators of these channels are increased in several types of vasodilatory shock (Landry and Oliver, 2001).
These examples are just a few possible avenues of future exploration for pharmacological treatment of septic shock. Given the multitude of conditions that can lead to vasodilatory shock, a single therapeutic is unlikely to benefit all patients and disease phenotypes. Nevertheless, animal models (both pre-clinical and veterinary) will play an integral role in future translational research directions (Marshall and Leligdowicz, 2022).
Vasopressor therapy for vasodilatory shock
Normalisation of tissue perfusion is the central goal of treating vasodilatory shock. In most cases, the mean arterial pressure is the best surrogate marker of adequate oxygen delivery to the tissues. After adequate fluid resuscitation, persistent hypotension is best addressed by administering vasoactive agents in an attempt to correct the pathologic vasodilation. Selection of these agents should be made on a case-by-case basis, and eventual discontinuation performed gradually to prevent systemic vascular collapse. Different strategies and rationale of vasopressor selection are reviewed here. A summary of receptor activities, cardiopressor effects and recommended dosing ranges for each vasopressor can be found in Table 1.
| Receptor activity | Contractility | Heart rate | Cardiac output | Vasomotor tone | Continuous infusion dose | |
|---|---|---|---|---|---|---|
| Noradrenaline | Alpha: +++ |
↑ | ↑ | ↑ | ↑↑↑ | 0.05–1 mcg/kg/min |
| Dopamine | Alpha: ++ |
↑↑ | ↑↑ | Variable based on dose | ↑↑| | 5–20 mcg/kg/min |
| Adrenaline | Alpha: +++ |
↑↑↑ | ↑↑↑ | ↑↑ | ↑↑↑ | 0.05–1 mcg/kg/min |
| Vasopressin | Alpha: - |
0 | 0 | 0 | ↑↑| | 0.5–5 mU/kg/min |
Table adapted with permission from Silverstein and Hopper, 2022. Receptor activity ranges from no activity (−) to maximal activity (+++). Degree of cardiovascular effects ranges from none (0) to marked increase (↑↑↑). Micrograms: mcg; milliunits: mU; kilograms: kg; minute: min; vasopressin-1 receptor: V1; dopamine D1-5 receptors: D1-5. Alpha and Beta refer to types of adrenergic receptors.
Noradrenaline
Noradrenaline, a catecholamine molecule, primarily acts on the alpha-1 and alpha-2 adrenergic receptors with additional activity at the beta-1 adrenergic receptor. It causes both arterial and venous constriction, leading to increased systemic vascular resistance with lesser increases in inotropy and chronotropy. Patients in vasodilatory shock generally experience an increase in cardiac output with noradrenaline administration as the pathological venodilation of the venous capacitance system is reversed and preload is increased. Patients with concurrent myocardial dysfunction (eg from primary cardiac disease or systemic diseases such as sepsis) may have additional benefits from the beta-1 effects of noradrenaline (Hamzaoui et al, 2010; 2018). One study in anesthetised beagle dogs receiving inhaled isoflurane and acepromazine (an alpha-1 receptor antagonist) found that a continuous infusion of noradrenaline increased mean arterial pressure through beta-1 effects, leading to increased stroke volume and cardiac output (Cannarozzo et al, 2023). In clinical practice, individual patient variations in shock-related vasomotor and myocardial dysfunction often necessitate both alpha- and beta-mediated effects to increase mean arterial pressure.
The most recent Surviving Sepsis Campaign guidelines make a strong recommendation for noradrenaline as the first-choice vasopressor for adults with septic shock based on high-quality evidence (Evans et al, 2021). In human paediatric patients, noradrenaline or adrenaline are suggested as first-line agents, albeit with less data available in this patient population (Weiss et al, 2020). Noradrenaline is also a viable first-line choice for most other forms of vasodilatory shock. The adult recommendations are largely based on analysis of several randomised controlled trials that suggest a lower mortality and lower risk of tachyarrhythmias compared to dopamine, which was previously considered a first-line option for septic shock (Avni et al, 2015). Limited evidence in experimental animals has found that dopamine can cause tachyarrhythmias at often therapeutic doses, but there is no clinical evidence demonstrating a higher rate of tachyarrhythmias, morbidity or mortality with its use in small animal patients (Tisdale et al, 1995; Furukawa et al, 2002; Rosati et al, 2007). The adverse event rate of noradrenaline is similar to vasopressin and adrenaline (Russell et al, 2008; Gordon et al, 2016). The consequences of escalating doses of this potent vasoconstrictor include ischaemic injury to the digits and splanchnic circulation and increased frequency of dysrhythmias; however, the effect of high dose noradrenaline on mortality is less clear (Jenkins et al, 2009; Martin et al, 2015; Yamamura et al, 2018; Wieruszewski et al, 2021).
There are no data available in the veterinary literature comparing outcomes or adverse effects between vasopressor options for the treatment of naturally occurring vasodilatory shock. Minneci (2004) evaluated the effects of noradrenaline, vasopressin and adrenaline on haemodynamics and mortality and in a canine model of septic shock. In this study, 78 purpose-bred beagles were infected with escalating doses of a serum-resistant strain of Escherichia coli via intraperitoneal injection. The dogs were given antibiotics and fluid resuscitation alongside infusions of a vasopressor. Noradrenline (0.2–2.0 mcg/kg/min) significantly increased mean arterial pressure and led to a survival benefit. An earlier study using a canine model of endotoxaemia found similar benefits on mean arterial pressure, cardiac index and oxygen delivery (Hayes et al, 1998).
Another study evaluated the clinical decision-making of Diplomates of the American College of Veterinary Emergency and Critical Care (DACVECCs) for treating vasodilatory shock (Murphy et al, 2022). A survey of 219 DACVECCs found that 87.5% and 83.1% of those polled would use noradrenaline as the first-line vasopressor for vasodilatory shock for dogs and cats, respectively. The authors conducted a similar survey in 2014 where the firstchoice vasopressor was almost equally divided between dopamine and noradrenaline; this was consistent with the then-contempo-rary Surviving Sepsis Campaign guidelines. It appears that, in the absence of veterinary-specific data, DACVECCs follow human guidelines for their small animal patients.
Vasopressin
Exogenous vasopressin can be administered parenterally to cause vasoconstriction via its actions on the V1 receptors in peripheral vasculature. As previously discussed, patients with septic shock may have a relative vasopressin deficiency. Therefore, it follows that the addition of exogenous hormone may counteract some of the vasodilation seen with long-standing shock. In addition, reducing the requirement for additional catecholamines can avoid the adverse effects seen with progressively higher doses of noradrenaline. This includes a lower risk of atrial fibrillation, presumably by avoiding additional direct beta-1 adrenergic myocardial stimulation, and a resulting increase in myocardial oxygen demand (McIntyre et al, 2018). There may also be decreases in mortality, acute kidney injury and the need for renal replacement therapy with the use of vasopressin (Serpa Neto et al, 2012; Nedel et al, 2019). As with other vasopressors, high doses of vasopressin can lead to cardiac, splanchnic and digital ischaemia (Dünser et al, 2003).
The Surviving Sepsis Campaign recommends vasopressin as the second-line vasopressor for persistent hypotension despite treatment with noradrenaline; this is a weak recommendation based on moderate-quality evidence. For patients that are successfully weaned from both vasopressin and noradrenaline, recent data suggest that the best outcomes occur when noradrenaline is discontinued first. This practice leads to a decreased incidence in hypotension and may also lead to decreased in-hospital and 28day mortality (Hammond et al, 2019; Wu et al, 2020; Song et al, 2021).
There is one publication investigating the use of vasopressin for vasodilatory shock in small animals (Silverstein et al, 2007). This proof-of-concept case series found that vasopressin was able to increase mean arterial pressure in five dogs with vasodilatory shock that was refractory to fluid resuscitation and high dose dopamine (>10 mcg/kg/min). These dogs had a small, clinically insignificant increase in central venous pressure and did not experience any tachyarrhythmias. Studies using canine models also demonstrate the efficacy of vasopressin in treating experimentally-induced haemorrhagic shock (which can progress to a vasodilatory phenotype if left untreated) (Cowley Jr et al, 1974; Yoo et al, 2006; 2007a; 2007b). Minneci et al (2004) also showed that vasopressin led to an increase in ejection fraction and cardiac index and provided a survival in a canine model of septic shock that received antibiotics and fluid therapy. Similar to noradrenaline, the prescribing behaviours of DACVECCs also follows the Surviving Sepsis Campaign guidelines. The majority of DACVECCs surveyed by Murphy et al (2022) selected vasopressin as the second-line agent for vasodilatory shock in dogs and cats. Almost a third of those polled chose dopamine as a the second-choice vasopressor. The authors speculated that this is likely because of the high cost of vasopressin as a barrier for many small animal patients.
Adrenaline
Adrenaline is another catecholamine molecule with potent, balanced effects on beta- and alpha-adrenergic receptors. Moderate–high doses cause both venous and arterial constriction, while the drug's effects on the myocardium lead to positive inotropy and chronotropy. At lower doses, the beta-adrenergic effects predominate and can lead to vasodilation. The Surviving Sepsis Campaign guidelines offer two clinical scenarios for using adrenaline in critically ill patients with septic shock:
Clinical evidence in humans with septic shock found no difference in mortality when comparing adrenaline to noradrenaline or vasopressin (Myburgh et al, 2008; Belletti et al, 2017). However, the side effect profile of these agents plays a large part in the Surviving Sepsis Campaign recommendations. The adverse effects of adrenaline include tachyarrhythmias and impaired perfusion to the splanchnic circulation (De Backer et al, 2003). The direct effects of adrenaline on beta-2 receptors in skeletal muscle also increase aerobic lactate production, a change that can affect resuscitation efforts by complicating interpretations of blood lactate levels (Levy et al, 2008).
There is one study investigating the use of intravenous adrenaline in a canine model of anaphylaxis. Mink et al (2004) conducted a randomised, controlled, crossover study comparing various routes of bolus dosing to continuous infusions of adrenaline in anesthetised dogs sensitised to ragweed. After inducing anaphylaxis, the dogs either received a continuous infusion of the drug titrated to maintain a mean arterial pressure of 70% of pre-shock levels or received a 0.01 mg/kg bolus adrenaline via intravenous, subcutaneous or intramuscular routes. These dogs were compared to a group that did not receive any treatment. The researchers found that only a continuous infusion of adreanline provided a rapid, sustained improvement in haemodynamics (as measured by mean arterial pressure, cardiac output, stroke volume and stroke work) compared to both the bolus and non-treatment groups. The continuous infusion group also required a lower total volume of the drug compared to the bolus groups. A study on experimentally induced endotoxaemia in a canine model also found benefits to mean arterial pressure, cardiac index and oxygen delivery (Hayes et al, 1998). These dogs experienced a 2.7-fold increase in their serum lactate levels. Another study in healthy, anesthetised cats found that continuous infusions of adrenaline increased heart rate, cardiac index and mean arterial pressure; these cats also had a corresponding increase in serum lactate (Pascoe et al, 2006). Interestingly, Minneci et al (2004) found a dose-related harmful effect of adrenaline. Compared with controls, dogs that received adrenaline with concurrent fluid therapy and antibiotics had greater decreases in cardiac index, ejection fraction, serum pH and creatinine and survival than with other vasopressors.
Dopamine
Dopamine acts as a neurotransmitter in the central nervous system and has cardiovascular effects on the myocardium and vascular smooth muscle. Exogenous dopamine does not cross the blood–brain barrier and instead, acts peripherally in a dose-de-pendent manner on dopaminergic as well as alpha- and beta-adrenergic receptors. It also releases noradrenaline from sympathetic nerve endings. Low-dose effects (1–4 mcg/kg/min) are primarily dopaminergic and vasodilatory in nature. At moderate doses (5–10 mcg/kg/min), beta effects predominate and result in increased heart rate and cardiac contractility. At higher doses (>10 mcg/kg/min), the alpha-adrenergic effects predominate and lead to an increase in vasomotor tone.
Since the 2016 version of the Surviving Sepsis Campaign guidelines, dopamine has been supplanted by noradreanline and is no longer the vasopressor of choice for adults with septic shock because of its side effect profile (Rhodes et al, 2017). Noradrenaline is recommended as the first-line option as it is associated with lower mortality and a lower risk of tachyarrhythmias in humans when compared with dopamine (Avni et al, 2015). However, the guidelines support dopamine as an alternative if noradrenaline is unavailable.
There are no clinical trials investigating the use of dopamine in naturally occurring vasodilatory shock in small animals. Vincent et al (1987) investigated its use in canine subjects with experimental septic shock induced by administration of endotoxin. In these dogs, dopamine increased systemic vascular resistance and mean arterial pressure. Similar effects on mean arterial pressure and systemic vascular resistance have been seen in anaesthetised cats and normo- and hypotensive anaesthetised dogs (Abdul-Rasool et al, 1987; Pascoe et al, 2006; Rosati et al, 2007). Unpublished research in clinically hypotensive dogs did not find an increase in adverse effects in dogs that received dopamine versus noradrenaline, and both drugs increased mean arterial blood pressure from baseline values (personal communication, 2023).
Among DACVECCs surveyed by Murphy et al (2022), dopamine was the second most-common choice for initial vasopressor therapy of vasodilatory shock in dogs (11% of those surveyed) and cats (14.4% of those surveyed). It was also the third most commonly chosen second-line vasopressor for both species (30.7% of those polled for dogs and 34.5% of those polled for cats) behind vasopressin.
Other vasoactive agents
Several other vasoactive agents have undergone preliminary trials in the treatment of septic shock, but there is no convincing evidence as to their superiority over the above vasopressors.
Selepressin is a selective V1a agonist (specific to vascular smooth muscle) that was once touted as a potential alternative for vasopressin. Theoretically, this mechanism could avoid unintended effects mediated by the V1b and V2 receptors including those on the hypothalamic–pituitary–adrenal axes and on sodium/water handling in the kidneys. A small pilot trial found that selepressin was able to main a target mean arterial pressure (without the need for noradrenaline) and resulted in a lower net fluid balance as compared to a placebo group (Russell et al, 2017). However, a larger trial that followed was discontinued early as it did not reveal any differences as compared to placebo in any major endpoints (Laterre et al, 2019). For this reason, the Surviving Sepsis Campaign makes a weak recommendation against the use of selepressin as a first-line therapy.
Terlipressin is a long-acting, synthetic vasopressin analogue that also has increased specificity for V1 receptors as compared to vasopressin. Similarly, no benefits have been shown in its use as compared to noradrenaline; in fact, because of an increased adverse effect profile, the Surviving Sepsis Campaign recommends against its use for the treatment of septic shock (Liu et al, 2018). Nevertheless, terlipressin gained Food and Drug Administration approval with an indication for improving kidney function in adults with hepatorenal syndrome (Thangaraj et al, 2023).
Lastly, angiotensin II – the endogenous hormone whose vasoconstrictor effects are mediated by the renin-angiotensin-aldoster-one system – has been investigated in both a small pilot and larger clinical trial of patients with septic shock. The Surviving Sepsis Campaign meta-analysis of these data did not find any differences in outcomes or adverse effects as compared to noradrenaline (Evans et al, 2021). Given the paucity of data in combination with a clear endogenous role, the Surviving Sepsis Campaign guidelines suggest that angiotensin II ‘may have a role as an adjunctive vasopressor therapy’ (Evans et al, 2021).
Refractory vasodilatory shock
Persistent vasodilation and hypotension despite the administration of catecholamines is considered to be refractory shock and necessitates the use of rescue agents. Human patients requiring high dose vasopressor therapy (often defined as those patients requiring doses >0.5 mcg/kg/min of noradrenaline or equivalent dosing with other agents) have a poor prognosis for survival to discharge (Jenkins et al, 2009; Benbenishty et al, 2011; Brown et al, 2013). These patients require additional investigation to determine whether they have impaired vascular responsiveness to catecholamines or whether other pathologies (eg decreased cardiac output) may be responsible. Refractory vasodilation or vasoplegia can be caused by metabolic derangements such as hypocalcaemia, severe acidaemia or a relative or absolute depletion of cortisol (ie critical illness-related corticosteroid insufficiency), among many other causes (Jentzer et al, 2018). This latter category of patients may benefit from the addition of physiologic doses of hydrocortisone (Evans et al, 2021). The Surviving Sepsis Campaign's recommendation of 200 mg per day of hydrocortisone for adult humans has been extrapolated to 2.5–3 milligrams per kilogram per day for small animals with suspected critical illness-related corticosteroid insufficiency (Silverstein and Hopper, 2022). There is mounting evidence in humans that the addition of the mineralocorticoid fludrocortisone may lead to improved outcomes rather than using hydrocortisone alone (Bosch et al, 2023). Other rescue agents and extracorporeal modalities such as renal replacement therapy or extracorporeal membrane oxygenation are beyond the scope of this review but can be found elsewhere (Jentzer et al, 2018; Vallabhajosyula et al, 2018; Lahiry et al, 2019).
Conclusions
Given the ongoing push for large clinical trials for the treatment of vasodilatory and/or septic shock in humans and the lack of conclusive clinical data in small animal patients, it is reasonable to follow the recommendations published for human physicians, like the Surviving Sepsis Campaign guidelines. With this in mind, noradrenaline is a rational first-line vasopressor selection. Because there is no evidence that dopamine is inferior in the small animal patient population, it also acceptable as a first-line agent based on clinician preference and/or availability. Vasopressin may be used as a second-line agent for patients with normal cardiac function and hypotension refractory to escalating noradrenaline doses, again with the understanding that cost may limit its use for many patients. Adrenaline may be considered as a third-line agent, if indicated, and should be chosen earlier if cardiac dysfunction is contributing to the shock state. However, hypotension refractory to a single vasopressor should prompt consideration for causes of refractory shock as discussed previously. Other human-based recommendations, including the timing and dosing of vasopressors as well as blood pressure targets, are beyond the scope of this review but provide additional meaningful context for tailoring vasopressor therapies. Adjunctive treatments including nitric oxide scavengers, nitric oxide synthase isoform inhibitors and novel vasoactive agents are emerging areas of research in combination with traditional vasopressor therapy. Continued investigation into the effectiveness and safety of vasopressors for the treatment of vasodilatory shock in small animal patients will provide valuable data for veterinary specific guidelines in the future.


