Urine sediment analysis can be a time-consuming job, requiring a functional microscope, a centrifuge and staff with both the time and expertise to perform the exam. Ideally, urine samples should be analysed within 1–2 hours of collection to increase confidence in results. A delay in analysis can lead to changes in temperature or pH, which can lead to precipitation or dissolution of crystals, degradation of cells and casts, potential misdiagnosis and inappropriate decisions regarding case management (Valenciano et al, 2017). This article provides practical tips to help veterinarians become more confident in their interpretation of canine and feline urine sediment examination (including crystals, casts and bacteria) and highlight what in-house urinalysis can add to the clinical workup. In-clinic automated analysers, such as the IDEXX SediVue Dx (IDEXX Laboratories UK), can be used in lieu of a standard microscopic examination and can produce urine results using artificial intelligence in around 3 minutes.
Urinalysis
Complete urinalysis consists of three elements:
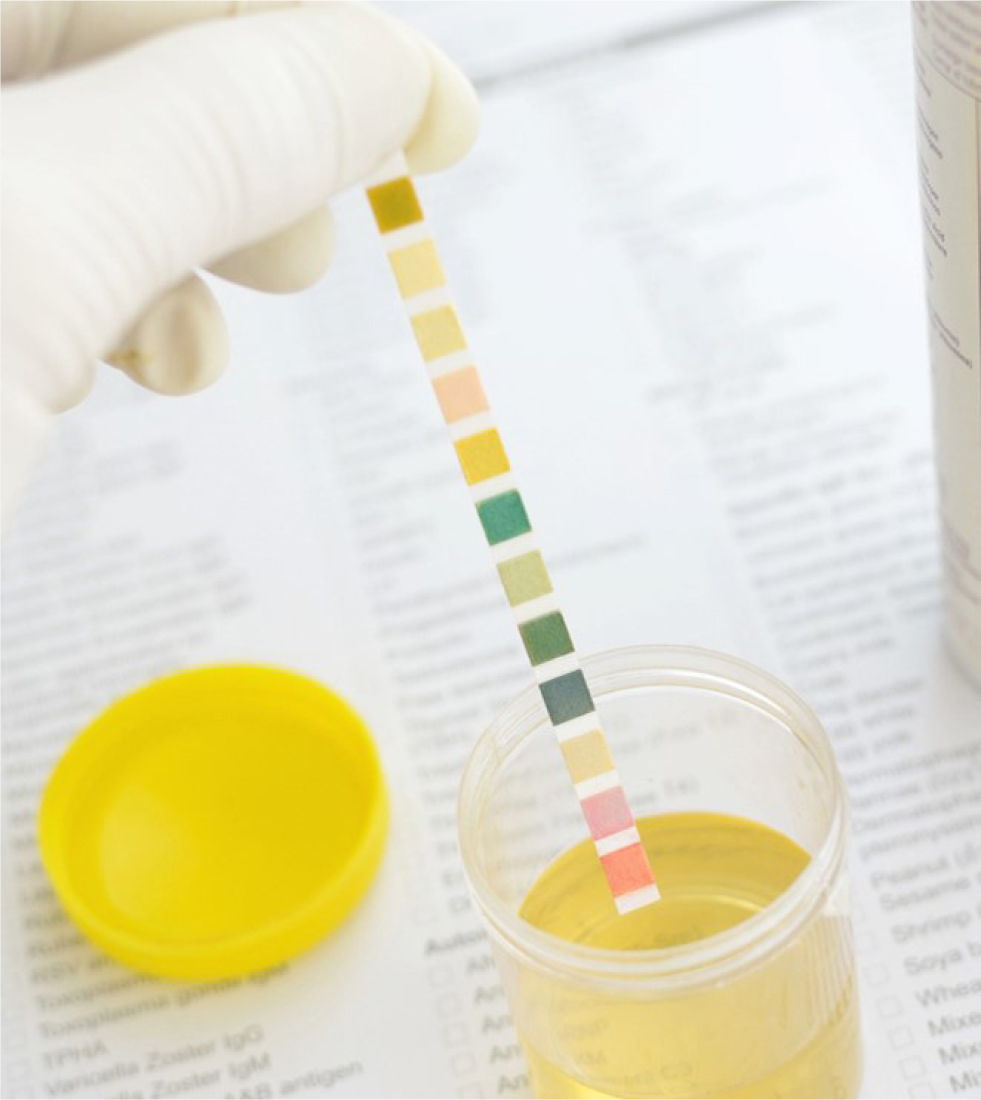
The gross exam involves the visual assessment of the urine sample with reference to colour, turbidity and perhaps even smell (sniffing urine is not recommended!). The chemical exam refers to chemical exam of urine components which is more frequently performed with urine dipstick analysis (Figure 1) and concurrent refractometer assessment of urine specific gravity. Urine dipsticks are not reliable for specific gravity assessment and a calibrated refractometer should be used instead. Dipsticks are also unreliable for white blood cells; a sediment exam should be performed instead (Holan et al, 1997; Osborne and Stevens, 1999; Reppas and Foster, 1999).
Sediment exam
Examination of urine sediment is required to identify crystals, casts, cells and bacteria. Urine samples should ideally be analysed within 30–60 minutes of collection (Reppas and Foster, 1999; Valenciano et al, 2017) and should be considered an in-clinic procedure. Cells and casts start to degrade within 1–2 hours of sample collection at room temperature, so prompt analysis is essential. Where necessary, the urine sample can be stored for up to 12 hours in the fridge (Reppas and Foster, 1999; Valenciano et al, 2017) but should be returned to room temperature before analysis is performed. Refrigeration can lead to crystal formation and an increase in urine specific gravity (Albasan et al, 2003), as chilled urine is more dense than room-temperature urine. Potential changes that can be associated with any delay in urine sediment analysis are shown in Box 1.
Changes associated with a delay in sediment analysis
Urine wet preparations can easily be performed in house on fresh urine samples (Box 2). Manual microscopy is considered the gold standard for sediment examination, but an alternative option is the IDEXX SediVue Dx analyser (Figure 2), which uses artificial intelligence to identify casts, crystals, cells and bacteria. The IDEXX SediVue Dx centrifuges urine at low speeds (2000 rpm) to preserve delicate components, then deposits formed elements into a monolayer. Multiple fields are then examined using an inverted microscope with a built-in camera and high-resolution images are obtained which can be incorporated into the patient's clinical record. Many of the images used in this article are obtained using the SediVue Dx.
How to perform a urine sediment wet prep

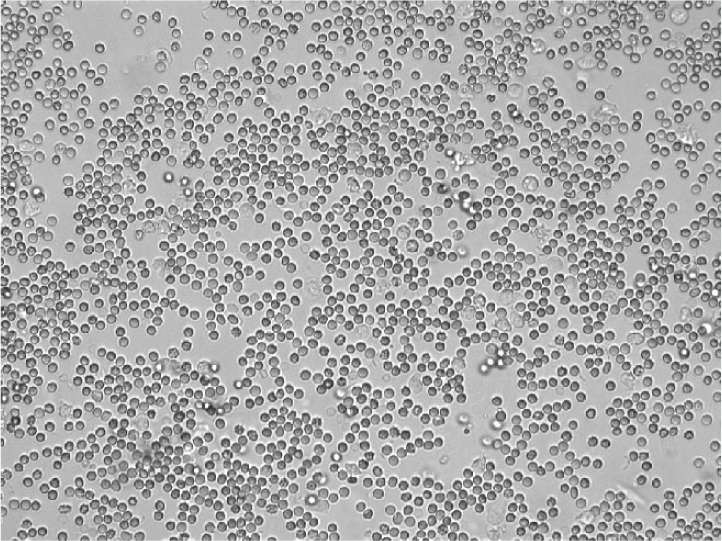
Red blood cells
Red blood cells are recognised as small biconcave structures on sediment examination (Figure 3). Delay in sediment analysis can lead to red blood cell lysis. Dilute and/or alkaline urine will increase the rate at which red blood cell lysis occurs (Yadav et al, 2020). Up to five red blood cells per high power field is considered normal for a sample collected by freecatch. Higher numbers of red blood cells may be found in samples collected traumatically (by catheter or cystocentesis).
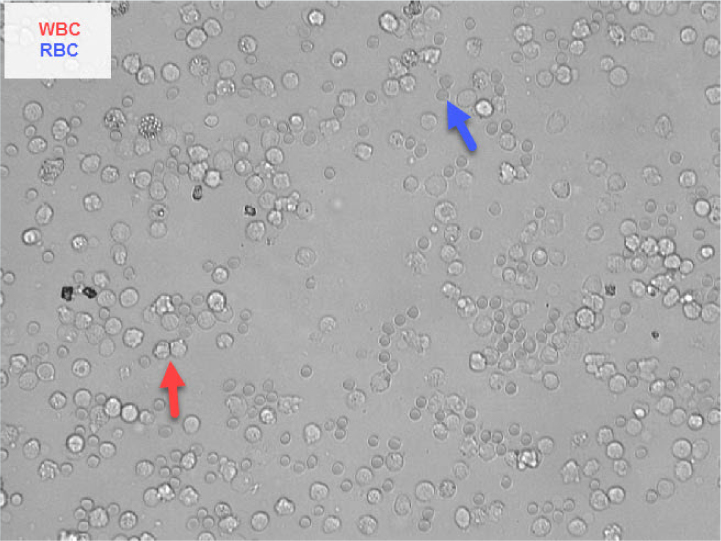
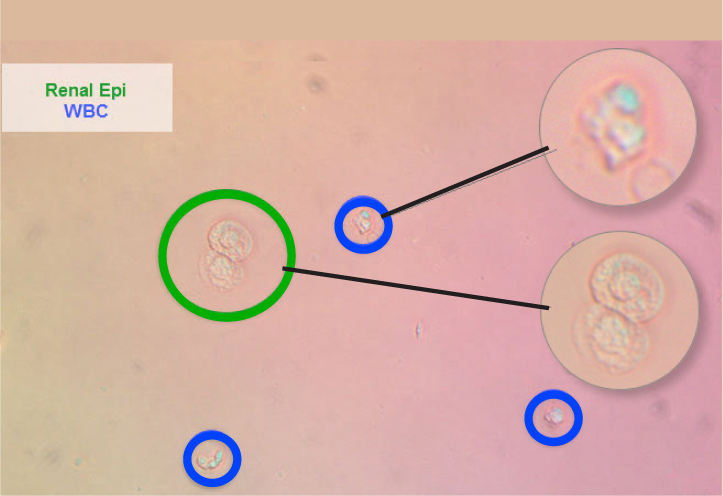
White blood cells
White blood cells are 1.5 to 2 times the size of a red blood cell and have a fine granular internal structure (Figure 4). Most white blood cells in urine will be neutrophils. Any delay in analysis will lead to white blood cell degradation making recognition more difficult. Up to five white blood cells per high power field is a normal finding (Skeldon and Ristic, 2016).
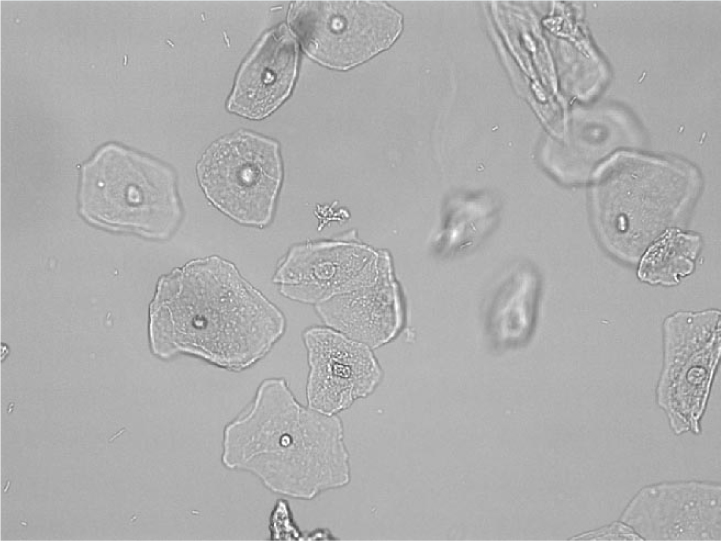
Epithelial cells
Epithelial cells are larger than any other urine constituents and vary in size depending on their site of origin (Skeldon and Ristic, 2016). Three main types of epithelial cells exist: squamous, transitional and renal. If epithelial cell numbers are increased, then urine cytology should be performed on a sample of urine preserved in EDTA.
Squamous epithelial cells
Squamous epithelial cells are the largest of the three types of epithelial cells and originate from the distal urogenital tract (eg urethra or prostatatic urethra). These cells are large, thin and angular and contain abundant cytoplasm with a small round nucleus (Figure 5). They can originate from vaginal or urethral contamination and low numbers are present in voided urine samples. Increased numbers will be present in catheterised samples from normal patients, animals with urethritis, lower urinary tract neoplasia and genital tract infections (Osborne and Stevens, 1999).
Transitional epithelial cells
Transitional epithelial cells are smaller than squamous epithelial cells and larger than renal cells. They are round to oval, with granular cytoplasm and irregular borders and a centrally-placed nucleus (Figure 6). They may have tail-like projections. It is important to differentiate transitional epithelial cells from leukocytes as they both can have a similar granular appearance, but transitional epithelial cells are around 2–4 times the size of a leukocyte. Transitional cells line the urinary tract from the renal pelvis to the urethra. These cells will naturally slough into urine in low numbers during catheterisation. Increased numbers may indicate hyperplasia associated with urinary tract inflammation (eg pyelonephritis, cystitis or prostatitis) or neoplasia; cytological evaluation of an air-dried, stained sample is warranted in such cases (Rizzi, 2014).
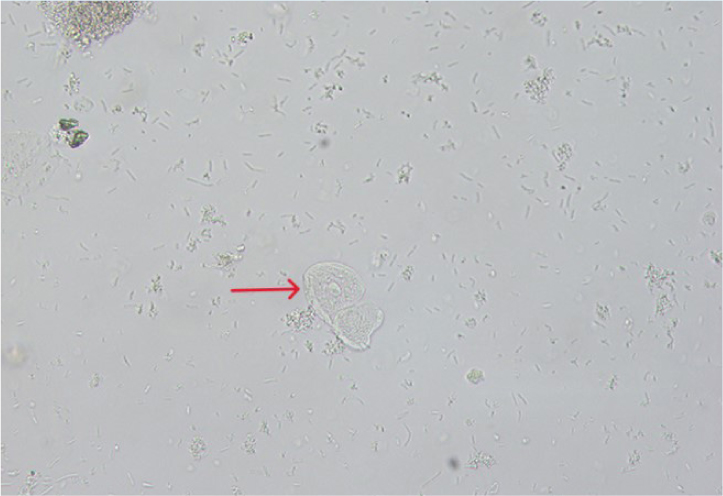
Renal tubular epithelial cells
These are the smallest of the epithelial cells, only slightly larger than a leukocyte. They are round cells with a large, round, eccentrically-placed nucleus and fine granular cytoplasm (Figure 7). Renal epithelial cells are rarely seen but increased numbers suggest renal tubular damage (Rizzi, 2014).
Casts
Casts are formed in the renal tubules (proximal, distal and convoluted) and their presence generally indicates renal tubular pathology (although small numbers of hyaline casts and granular casts can be normal in concentrated urine). Hyaline and granular casts mostly consist of a network of mucoprotein (Tamm-Horsfall protein or uromodulin) which is shed from the lining of the urinary tract into the urine. Shedding may be intermittent, and these structures are very fragile – as such, the absence of casts does not mean the absence of disease. In addition, the number of casts present does not necessarily equate to disease severity. Casts can be identified by their parallel sides, and they tend to be elongated and have rounded to tapered ends (Osborne and Stevens, 1999).
Several types of casts exist including hyaline, granular, fatty, waxy and cellular. All casts begin as hyaline casts (Osborne and Stevens, 1999) and their appearance in urine depends on the length of time they have remained in the renal tubules before being shed into the urine.
Hyaline casts
Hyaline casts are acellular and colourless with no internal structure (Figure 8). Small numbers of hyaline casts (<1 per low power field) are considered normal in concentrated urine. They can sometimes be seen in urine following strenuous exercise, but increased numbers are associated with glomerular disease or pre-renal proteinuria. Hyaline casts form the structural basis of all other casts (Osborne and Stevens, 1999; Skeldon and Ristic, 2016).
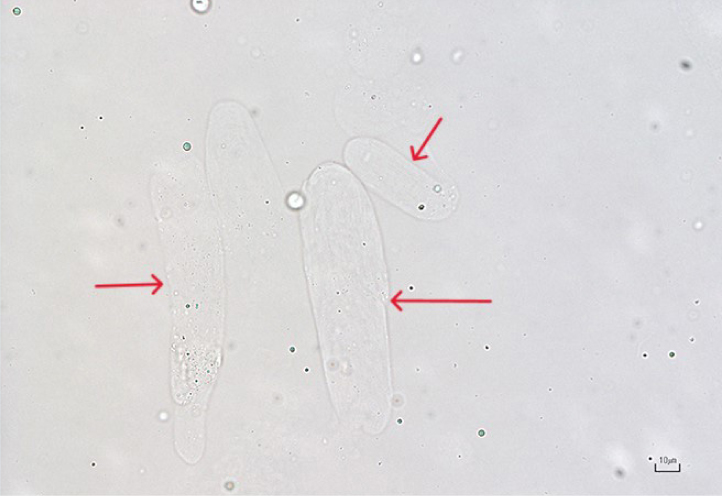
Granular casts
These are essentially hyaline casts that contain granules (Figure 9) (Skeldon and Ristic, 2016). They appear textured and, although small numbers in urine can be considered normal (<1 per low power field), increased numbers are associated with tubulointerstitial disorders, pyelonephritis and acute kidney injury. Granular casts should be interpreted in conjunction with other findings such as azotaemia and urine specific gravity.
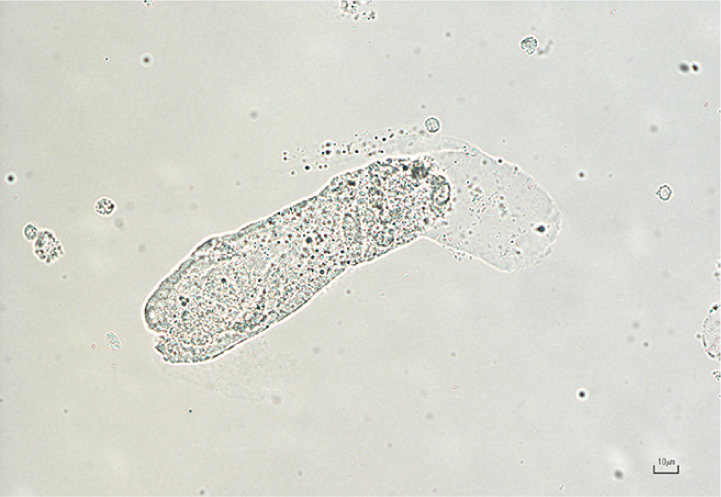
Fatty casts
These casts contain refractile droplets and represent tubular degeneration (Osborne and Stevens, 1999).
Waxy casts
Like hyaline casts, these are colourless, but are broader than hyaline casts and have broken, squared-off ends (Figure 10). These are rarely identified, and when present are always considered to be pathological (associated with chronic tubular disease), as they form when urine flow is decreased (Osborne and Stevens, 1999). These reflect the final stage of cast degeneration.
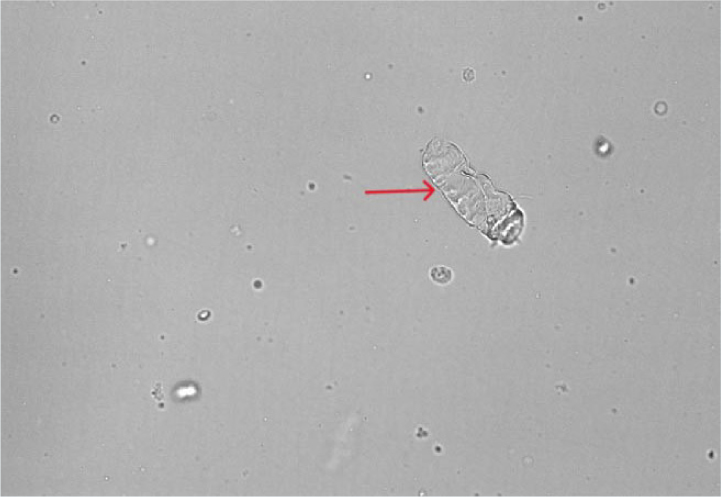
Cellular casts
As the name suggests, these casts have cells (epithelial cells, red or white blood cells) incorporated into their mucoprotein matrix (Figure 11). When present, these casts are always considered pathological (Osborne and Stevens, 1999) and should prompt investigations into an underlying cause. Epithelial cell casts can occur as a result of renal tubular necrosis and ischaemia; white cell casts are associated with renal tubuloinsterstitial inflammation, including pyelonephritis and red blood cell casts indicate renal tubular haemorrhage (Osborne and Stevens, 1999). It may be difficult to differentiate between the various types of cellular casts and cytological evaluation of the sample may be necessary in order to provide more nuclear detail.
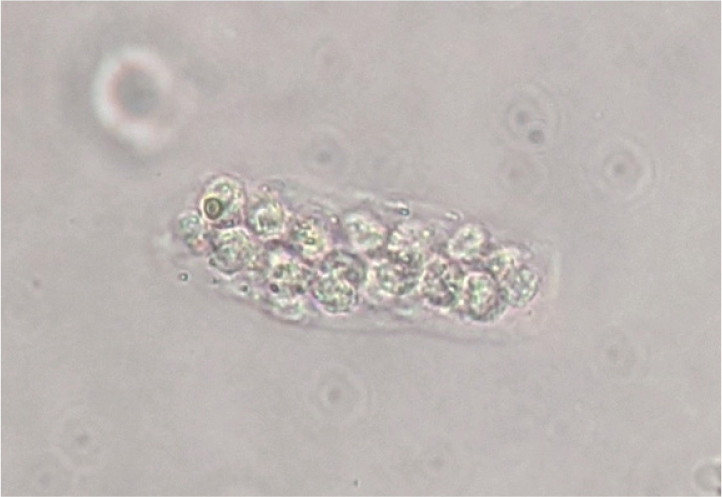
Crystals
Crystals can be identified in healthy animals and so their presence should always be interpreted with clinical context. Not every patient with crystalluria needs treatment. Crystals should be considered significant if they are identified in a very fresh urine sample, in dilute urine, in a patient with haematuria, stranguria or dysuria or when crystals are present in repeat samples. Crystals are more likely to precipitate in chilled urine, so samples should be returned to room temperature before performing a sediment exam. In contrast, keeping a urine sample at room temperature can lead to an increase in pH because of the escape of carbon dioxide and this can lead to an increased risk of struvite precipitation (Osborne and Stevens, 1999). Prompt analysis of urine is essential to avoid artefacts and misdiagnoses. Multiple crystal types are recognised and are discussed further below.
Struvite
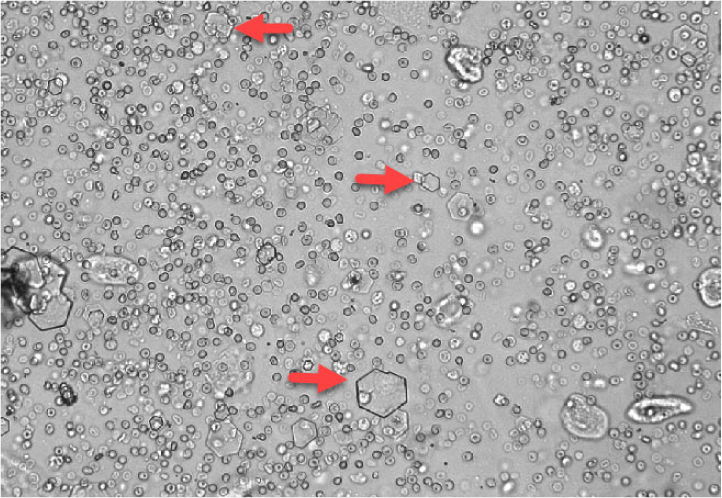
Struvite crystals are colourless with 3–6 sides and resemble coffin lids (Figure 12). They are most commonly, but not exclusively, identified in alkaline urine. Their presence does not necessarily indicate pathology as they are also seen in healthy dogs and cats. An underlying bacterial urine infection can lead to struvite crystal formation, and as such, urine culture should be considered in patients with struvite crystalluria and urinary tract signs (Osborne and Stevens, 1999).

Calcium oxalate
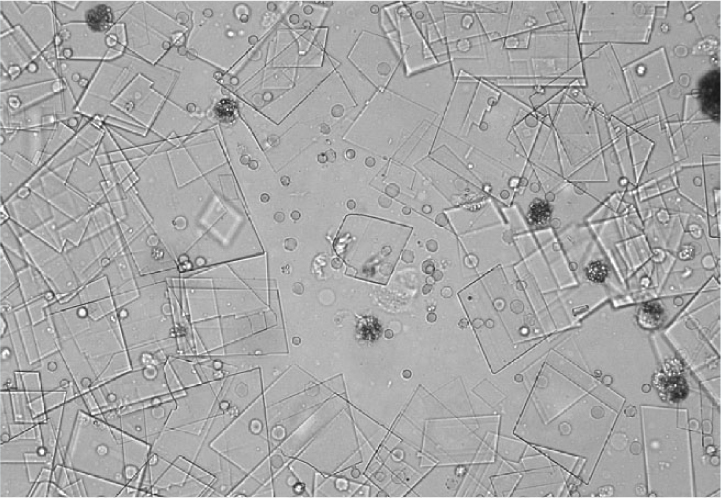
Calcium oxalate crystals are also colourless and exist in two forms. The most common form is calcium oxalate dihydrate, which has a shape resembling an envelope (Figure 13). These can be found in healthy patients as well as in patients with hypercalcaemia, other diseases or taking drugs which promote calciuresis. In contrast, calcium monohydrate crystals may be associated with recent ethylene glycol ingestion. These crystals can have shapes sometimes described as being similar to a picket fence or rubber dinghy (Figures 14 and 15). Although most commonly identified in acidic urine, calcium oxalate crystals can also be found in neutral and alkaline urine (Osborne and Stevens, 1999).

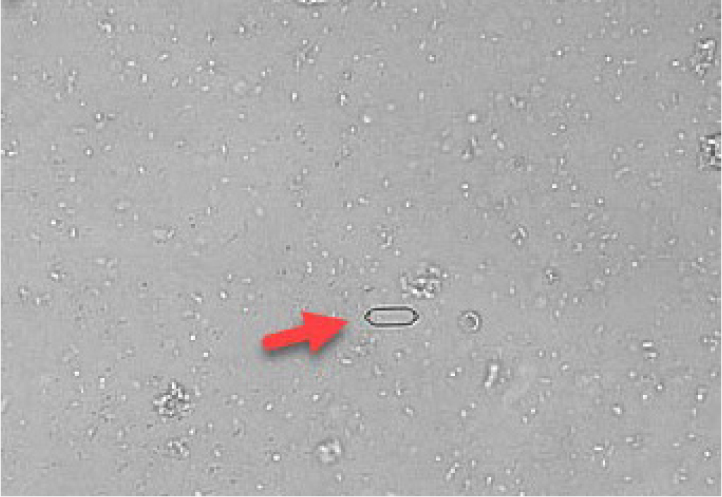
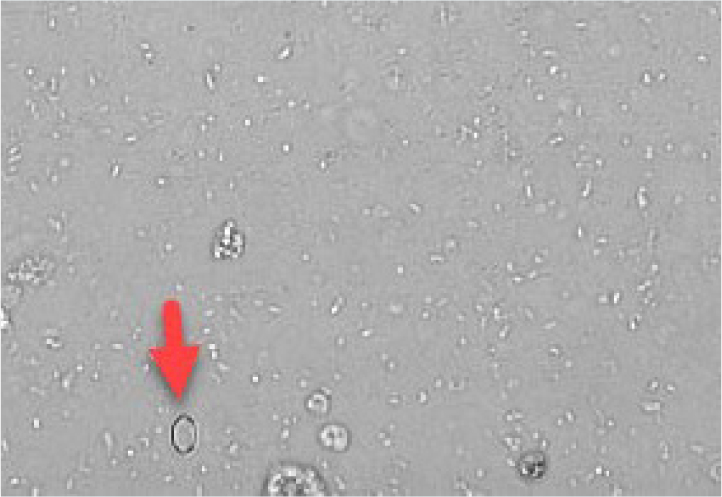
Ammonium urate
These crystals are brown–yellow on standard microscopy and have a spiky (thorn apple) appearance (Figure 16). Some breeds have a genetic predisposition to produce ammonium biurate crystals (such as the Dalmatian and the Egyptian Mau). In other breeds, identification of ammonium urate crystals should prompt testing for portal vascular abnormalities (by performing a bile acid stimulation test). High purine diets can also predispose to the formation of ammonium urate crystals. Urate crystals and xanthine crystals look very similar and a diagnosis of xanthine crystalluria is a more likely if the patient is known to be receiving allopurinol treatment (Osborne and Stevens, 1999).
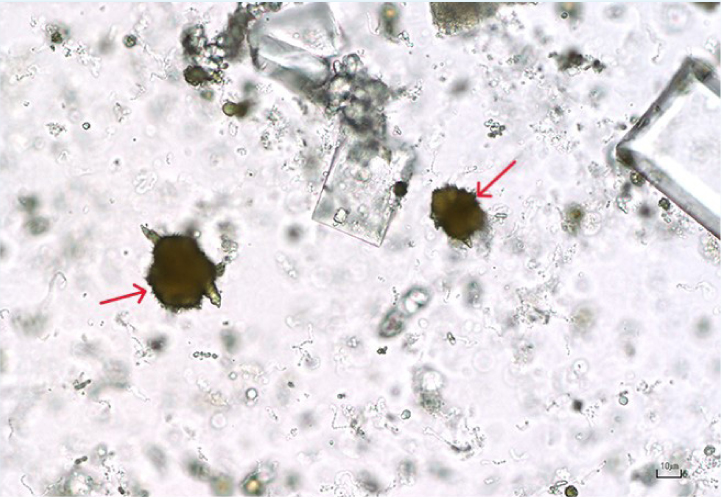
Bilirubin
These are needle-like and golden-brown–yellow in colour on microscopy (Figure 17). Bilirubin crystals can be identified in healthy dogs with concentrated urine but are always considered abnormal in the cat. Bilirubin crystalluria in the cat should prompt an investigation for cholestasis (Osborne and Stevens, 1999; Skeldon and Ristic, 2016).
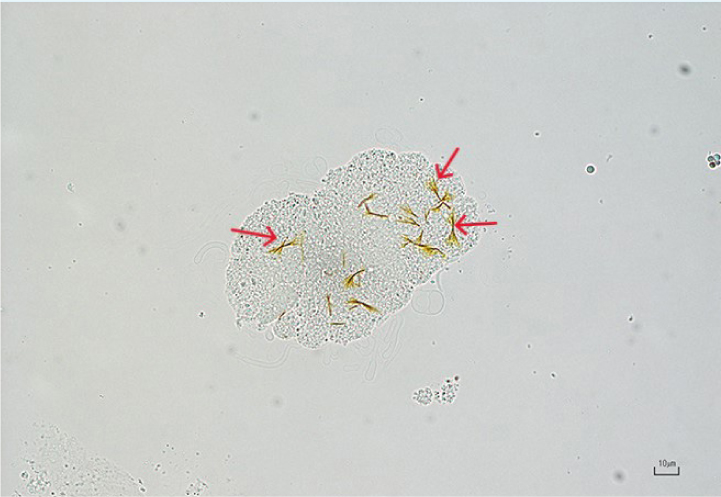
Cystine
These are flat, colourless hexagonal crystals usually found in acidic urine (Figure 18). Generally, cystinuria reflects a metabolic defect of tubular resorption of cystine. Breeds most commonly predisposed to cystinuria and subsequent formation of cystine uroliths include the Newfoundland, the English Bulldog, the French Bulldog, the Dachshund and the Mastiff. Cystine crystalluria is rare in cats (Kovaříková et al, 2021).
Cholesterol
Other crystals that are less commonly identified include cholesterol crystals (Figure 19) which are of little clinical significance (Osborne and Stevens, 1999).
Bacteriuria
Low numbers of bacteria can be present in voided urine samples from healthy patients; this should not prompt antimicrobial therapy in the absence of associated clinical signs to support bacterial cystitis (stranguria, haematuria, dysuria) (Osborne and Stevens, 1999; Rizzi, 2014). Bacterial populations can increase rapidly in urine samples, but equally, bacteria can die as a result of phagocytosis by white cells; therefore, prompt evaluation of urine is essential. It is very easy to misidentify bacteria in urine samples (because of small particles exhibiting Brownian motion) and culture should be performed where bacteriuria is suspected in clinically relevant cases, preferably on samples collected by cystocentesis (Skeldon and Ristic, 2016). Urine sediment dry preparations (Box 3) can be used to increase confidence in the identification of bacteria in urine.
How to perform a urine sediment dry preparation
Conclusions
Urine sediment analysis is an essential part of urinalysis and is best performed in-house rapidly after urine collection. The reader should be aware of the potential artefactual changes that can occur when urine sediment analysis is delayed or performed on a chilled sample. In-house examination of urine sediment is easy to perform, can increase confidence in the significance of urinary abnormalities and guide therapy.


