Septic arthritis is a relatively uncommon cause of lameness in the canine population (Bubenik and Smith, 2003); however, it is important to recognise this condition to minimise the chances of permanent damage and persistent lameness. Septic arthritis can be divided into two major aetiologies: haematogenous infections and direct inoculation of the joint via trauma or surgery.
Haematogenous arthritis can be initiated by a spread of infection from a focus elsewhere in the body, for example dermatitis or otitis, or spontaneously where a distant source of bacteria is not identified. In adult animals, haematogenous infections are often seen in a joint with pre-existing osteoarthritis, which is presumed to be related to the increased vascularisation of the affected joint aiding in bacterial invasion (Clements et al, 2005), while human healthcare literature suggests that neutrophils within osteoarthritic joints may have reduced motility and efficacy, potentially leading to reduced bacterial clearance (Dolganiuc et al, 2000). A thorough clinical examination is required to locate a potential primary focus of infection and allow differentiation from a true ‘spontaneous’ case.
Penetrating periarticular injuries are an alternative route of infection. These can result from lacerations or penetrating trauma; cat bites are a frequent aetiology. These cases are indiscriminate to age, breed or size of dog, and all periarticular injuries warrant prompt investigation to ascertain whether there is communication with the joint. Infection can be introduced into joints iatrogenically via an articular surgical approach or through periarticular implants, such as plates used in fractures or cruciate osteotomies. Postoperative joint infections can be divided into type 1 and type 2; type 1 are infections seen early in the postoperative period and type 2 can develop from 6–24 months postoperatively (Clements and Bennet, 2005). Type 1 postoperative infections may be more easily recognised, with complications developing during recuperation and wound healing. Type 2 infections are important to consider in delayed lameness and may develop later either as a result of a small inoculum of bacteria, low bacterial virulence or implants that act as a nidus in cases of future bacteraemia (Innes, 2011).
Presentation
Cases of septic arthritis commonly present with an acute, severe lameness without a known traumatic event. This often shows little improvement with rest or analgesia, unlike with lameness that occurs after a soft tissue injury or osteoarthritic flare up. Cases with pre-existing osteoarthritis may be more difficult to recognise, as they may demonstrate a more gradual increase in lameness because of pre-existing analgesic treatment.
Clinical examination is likely to demonstrate a ‘mono-arthropathy’ with heat and effusion on palpation, and pain on manipulation (Phillips and Bleyaert, 2022). While the condition has been reported in most major joints, the most commonly affected sites are the stifle and elbow (Mielke et al, 2018; Phillips and Bleyaert, 2022). Local draining lymph nodes may be enlarged, and in some cases there may be distal oedema of the affected limb. Patients are not consistently pyrexic, so normothermia should not preclude suspicion of septic arthritis. Uncommonly, patients may be systemically unwell or have multiple joints affected.
Diagnosis
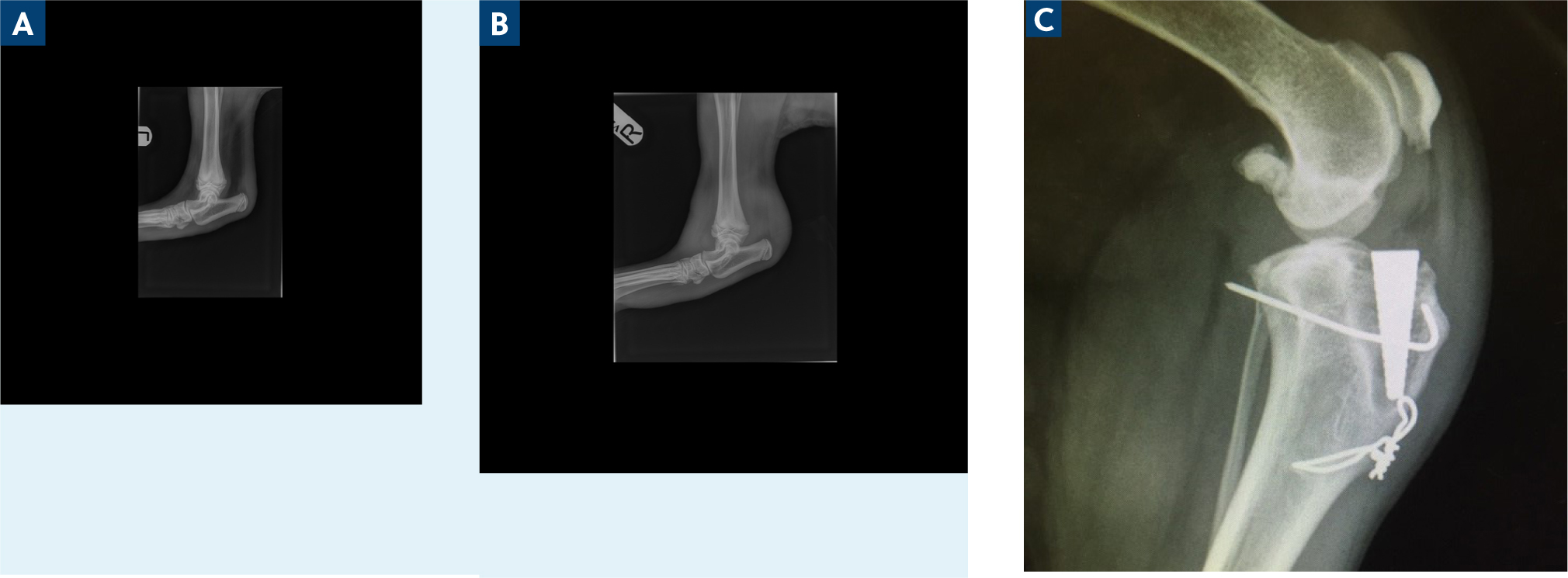
Radiographs are often the first step in the work-up of an acutely painful patient and are useful to rule out alternative pathologies including fracture. Radiographs can also be useful to assess the degree of pre-existing osteoarthritis or concurrent osteomyelitis, as well as evaluate the presence of secondary changes in more chronic cases to guide prognosis (Figure 1). However, arthrocentesis is the primary diagnostic tool in suspected septic arthritis cases.
Approach to arthrocentesis
Patients should be heavily sedated or anaesthetised for the procedure in order to reduce blood contamination and improve the likelihood of a diagnostic sample. Once prepared, it is useful to gently flex and extend the joint to familiarise oneself with the anatomy and confirm if there is effusion present which may make sampling easier. Synovial fluid is normally translucent and colourless to pale yellow, with a characteristic viscous ‘stringy’ appearance which can be used to confirm that the sample is truly synovial fluid (Clements, 2006). Access to the joint may be more difficult in older patients with pre-existing arthritis as a result of the presence of osteophytes or abnormal joint anatomy hindering needle entry to the joint. In all cases, where the needle hits bone without being able to aspirate a sample, gently draw back and redirect the needle until a sample is obtained.
The elbow
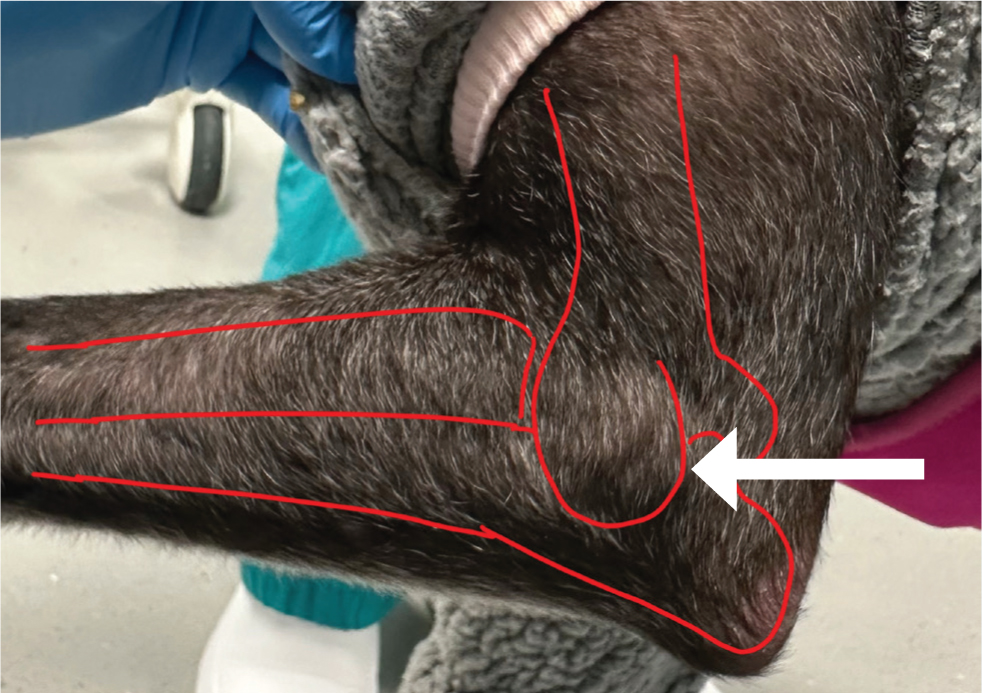
The elbow joint can be entered from the caudal aspect with the elbow in a neutral position. Palpate the olecranon and the humeral epicondyle and penetrate the skin just proximal to the olecranon with the needle aiming slightly medially and parallel to the angle of the ulna. The needle should aim to slide axially into the joint space between the ulna and the humeral condyle (Figure 2) (Scurrell and Arthurs, 2018). Medial approaches may also be used depending on patient positioning and clinician preference.
The stifle
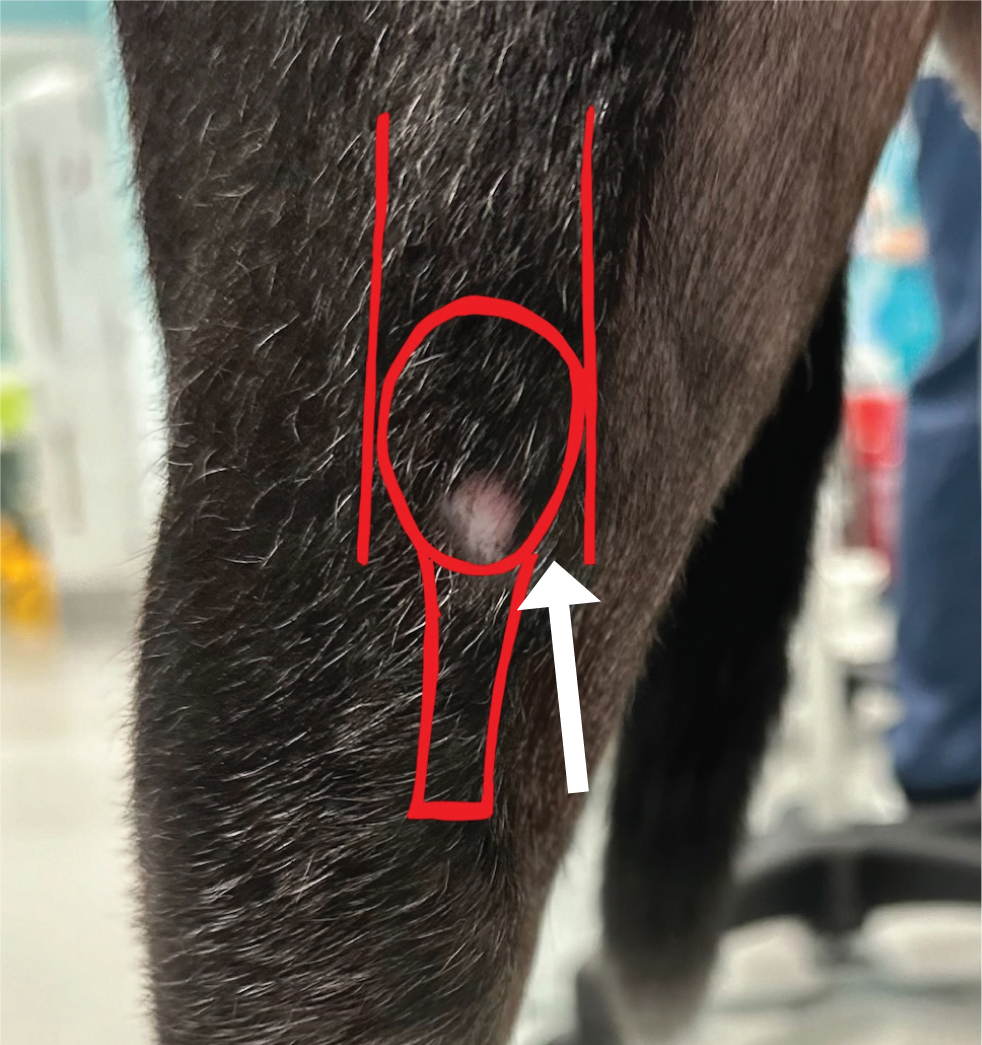
The synovial cavity can be accessed from either side of the patellar tendon depending on the clinician's preference. The approach is easiest with the patient in dorsal recumbency and the stifle partially flexed. Palpate the patellar tendon and introduce the needle just lateral to it whilst aiming proximally. Direct the needle proximally and axially to access the femoro-patellar joint space (Figure 3) (Scurrell and Arthurs, 2018).
Arthrocentesis of less commonly affected joints (such as the carpus or tarsus) is relatively straightforward and widely described (Figure 4). Clipping and aseptic preparation of the arthrocentesis site, the use of sterile gloves and meticulous technique, as demonstrated in Figure 4, is imperative to avoid introducing bacteria. Septic arthritis of the coxofemoral joint is seen less frequently (Clements et al, 2005; Phillips and Bleyaert, 2022); arthrocentesis may present more of a challenge.

Cytology and culture
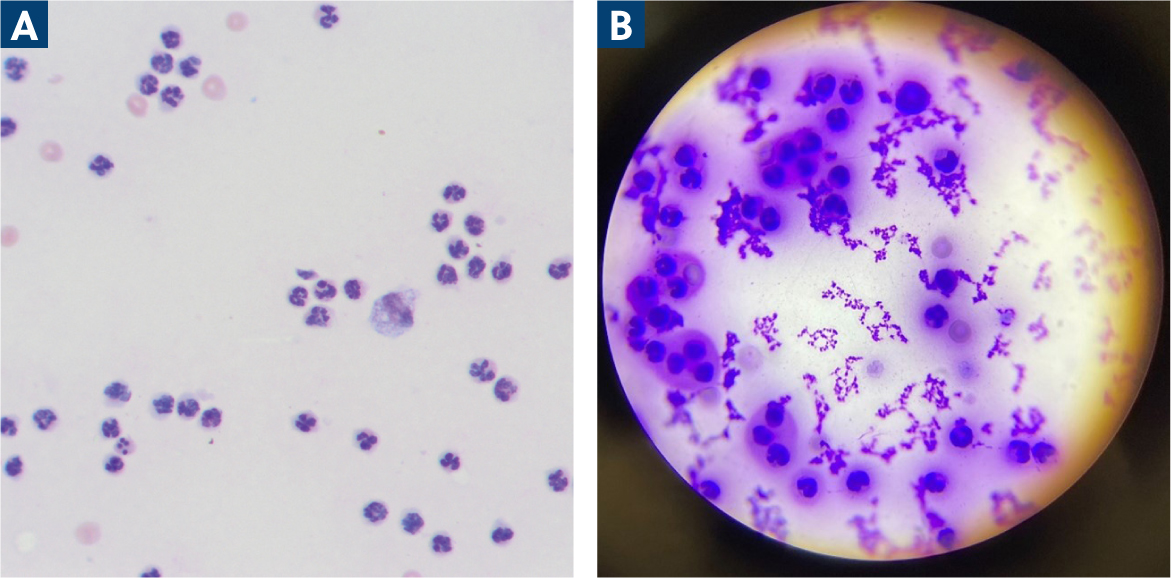
Synovial fluid samples are taken for two purposes: cytology (including total nucleated cell counts and differentiated cell counts) and culture. While gross purulent appearance of the synovial fluid sample may be characteristic for infection, in-house cytology can increase confidence in the diagnosis while culture is pending. There is no widely accepted cytological criteria and various studies apply their own cut-offs as detailed in Table 1. However, an increased nucleated cell count is anticipated with the neutrophils as the predominant type (Figure 5). Additionally, toxic neutrophils and intracellular bacteria may be seen, however their presence has been reported unreliably (Carr, 1997; Marchevsky and Read, 1999), and their absence does not preclude diagnosis of septic arthritis.
Table 1. Diagnostic criteria utilised in recent literature to categorise septic arthritis
| Source | Total nucleated cell count. (cells/uL) | Neutrophil proportion (%) | Culture result | ||
|---|---|---|---|---|---|
| Phillips and Bleyaert, 2022 | >50 000 | OR | >40 | OR | Positive |
| Lazarus et al, 2021 | >3000 | AND | >40 | - | N/A |
| Scharf et al, 2015 | >10 000 | AND | >50 | - | N/A |
| Mielke et al, 2018 | >5000 | OR | >40 | OR | Positive |
| Clements et al, 2005 | N/A | OR | N/A | OR | Positive |
Culture of synovial fluid has variable success rates reported; studies have reported 80–100% positive cultures when using blood culture medium (Marchevsky and Read, 1999; Fitch et al, 2003). Synovial membrane biopsy is an alternative source for culture; however, Marchevsky and Read (1999) reported only a 25% positive culture rate. Therefore, the increased invasiveness of surgical biopsies is perhaps only warranted in cases where explantation is performed or in persistent cases with a negative culture.
Septic arthritis characterisation
The diagnostic criteria for septic arthritis are currently ill-defined and inconsistent across current literature (Table 1). In practice, a provisional diagnosis is likely to come from a combination of clinical presentation, synovial fluid appearance and the absence of other pathology. Juvenile animals can be the more difficult age group to diagnose definitively as immune-mediated processes may have a similar acute presentation along with a similar cytological appearance, especially as bacteria are infrequently identified on smears. An improvement in response to antibiotics is reported within several days by owners (Fitch et al, 2003), so an antimicrobial trial could form part of the diagnostic plan while awaiting lab results, particularly with the risk of permanent joint damage without prompt treatment (Fischer et al, 2020).
Management
Where a high index of suspicion of septic arthritis exists, empirical antibiotics should be started while awaiting culture results. Culture results commonly include commensals frequently found on the skin, mouth or in the environment which may relate to the routes of inoculation. Several studies report that 70–90% of bacteria culture are Gram positive species, which also aids initial antibiotic selection (Phillips and Bleyaert, 2022). However, care should be taken with dosage in juvenile haematogenous septic arthritis cases as their weight is likely to change over the treatment period. It may be prudent to dispense short courses of medication and intermittently weigh these patients in order to adjust the dose accordingly. Additionally certain antibiotics may be contraindicated, for example fluoroquinolones, in growing dogs (Ramsey, 2014). While a prompt clinical improvement may be anticipated in response to antimicrobial treatment, a 6-week course of appropriate antibiotics may be required as recurrence or non-resolution is possible (Marchevsky and Read, 1999; Fitch et al, 2003; Phillips and Bleyaert, 2022). Repeat arthrocentesis to confirm effective treatment is useful before discontinuing medication.
While it would appear logical to do so, it is currently unproven whether joint lavage is beneficial in managing septic arthritis cases. Infections related to penetrating injuries will likely be undergoing lavage and debridement as part of their wound management. Additionally penetrating injuries may benefit from lavage and exploration in case of persistent foreign bodies. Postoperative infections with implants present will require explantation to resolve the infection, though antibiotic therapy can be used until there is adequate osseous healing to allow removal of the implants. Lavage of the affected joint in haematogenous cases is contentious, with poor evidence that it improves outcomes (Mielke et al, 2018). If lavage is considered necessary by the clinician because of the consistency of purulent material or other concerns, they can consider the two-needle (‘through and through’) technique, or an arthroscopic or arthrotomy approach. Whilst the two-needle lavage technique is the least invasive, it has been shown in humans to have poor ability to lavage the entire joint due to canalisation between the ingress and egress needles (Jerosch and Prymka, 1998). Therefore, if efficacy of lavage is a concern, it may be worth accepting the increased financial and surgical burden of arthroscopic lavage.
Alternative routes of antimicrobial therapy exist which may allow localised application; however, these are not commonly implemented and there are potential complications associated with each. Studies have reported chondrocyte death when exposed directly to several antibiotics, which may preclude their use via intraarticular injection (Newman et al, 2021). Additionally, the likely rapid clearance of intraarticular drugs limits their therapeutic use, leading to investigations of combining medications with dextran polymers (Lazarus et al, 2021) and gentamicin impregnated sponges to prolong their duration (Hayes et al, 2016). Sponge implantation may be more relevant in cases where explantation is already occurring, however the current evidence reported an increase in joint inflammation and impairment of renal function following this intervention (Hayes et al, 2016). Because of the lack of current evidence, studies commonly include concurrent oral antibiotic therapy (Fitch et al, 2003; Clements et al, 2005; Hayes et al, 2016; Mielke et al, 2018; Abrams et al, 2019; Lazarus et al, 2021) – this makes it challenging to draw conclusions about the efficacy of the local antimicrobials. Regional limb perfusion of antimicrobials using a tourniquet is commonly used in equine patients to treat septic arthritis (Rubio-Martinez and Cruz, 2006) and has been reported in a case of distal soft tissue infection in a dog (Abrams et al, 2019), but is rarely used in canine patients. It is commonly more proximal joints, such as the stifle or elbow, that are affected in dogs, making tourniquet application less feasible in most patients. Because of the uncertainty surrounding the ideal treatment for septic arthritis cases, and the possibility for long term sequalae, this remains an interesting area of future research.
Prognosis
Septic arthritis damages the articular surfaces through direct action of proteolytic and degradative enzymes, and via reduced nutrient exchange and tissue oxygenation from synovial vascular tamponade (Bennett and May, 1995). Thus, prognosis depends on the extent of these irreversible changes. Prompt diagnosis and treatment is imperative and has been demonstrated to improve clinical outcome (Phillips and Bleyaert, 2022). Spontaneous infections and direct penetration injuries have been reported to have more successful outcomes compared to other aetiologies. However, as a result of the retrospective nature of these studies, there are likely inaccuracies in classification and this information should be used with caution.
Pre-existing osteoarthritis is reported in up to 63% of affected joints, and whilst these patients are likely to report a poorer outcome with residual lameness as a result of that pathology, they are also associated with an increase in recurrent infection (Mielke et al, 2018; Phillips and Bleyaert, 2022). Recent studies considering all aetiologies of septic arthritis reported that patients over 20 kg were less likely to achieve a full recovery (Clements et al, 2005) and that patients over 30 kg had a higher chance of recurrence (Phillips and Bleyaert, 2022), though the exact mechanism behind these findings is unclear.
Conclusions
Septic arthritis is an important diagnosis to rule out in cases with acute lameness or deterioration in all ages of dog. Diagnosis is not always clear cut, but can be reached by piecing together history, clinical examination and diagnostic tests. Prompt recognition and treatment is necessary for improved clinical outcomes.
KEY POINTS
- Septic arthritis should be on the differentials list for any patient with an acute or acutely worsened lameness, as the speed of diagnosis and treatment has a vast impact on the long-term function and comfort of the affected joint.
- Diagnosis should be based upon piecing together the clinical picture, and cytology in-house is easily performed while awaiting external lab results.
- While culture can be unreliable, arthrocentesis samples in blood culture medium provide a realistic prospect of diagnosis.
- In older patients with pre-existing arthritis, there is up to a 63% chance of recurrence and clients should be made aware of this to encourage early presentation if there is a reappearance of symptoms.

