Cranial cruciate ligament (CrCL) and meniscal diseases are common causes of stifle joint pain, impaired athletic performance, osteoarthritis and pelvic limb lameness in dogs (Vasseur et al, 1985; Hayashi et al, 2003; Comerford et al, 2006; Witsberger et al, 2008; Kowaleski et al, 2017). With many proposed causal factors for cruciate pathology, including limb conformation, breed predisposition, obesity and age-related ligamentous degeneration (Vasseur et al, 1985; Hayashi et al, 2003; Comerford et al, 2006; Witsberger et al, 2008; Kowaleski et al, 2017), the majority appear to result from progressive pathological ligamentous failure of unknown origin (Vasseur et al, 1985; Hayashi et al, 2003; Comerford et al, 2006; Witsberger et al, 2008; Kowaleski et al, 2017). Such pathology results in stifle instability and subsequent increased tibial translation, internal rotation and stifle joint hyperextension. Clinically apparent joint instability following CrCL pathology further results in the development of synovitis and joint degradation, increasing the susceptibility of mechanical damage to the medial meniscus.
Radiography is warranted in all cases of suspected CrCL disease to assess stifle pathology, with radiographic signs consistent with CrCL including intra-articular effusion, cranial displacement of the tibia with tarsal flexion applied and osteoarthritic changes. Magnetic resonance imaging and ultrasonography are alternative diagnostic imaging techniques to conventional radiology for the study of the stifle joint in small animals (Gnudi and Bertoni, 2001; Arnault et al, 2009; Böttcher et al, 2010; Marino and Loughin, 2010; Przeworski et al, 2016; Franklin et al, 2017; Sample et al, 2017). However, diagnostic accuracy of sonographic evaluation has since been deemed unreliable, with availability and feasibility of magnetic resonance imaging limited despite superior sensitivity and specificity to conventional radiography (Gnudi and Bertoni, 2001; Marino and Loughin, 2010; Franklin et al, 2017; Ramírez-Flores et al, 2017). Radiographic effacement of the infrapatellar fat pad, as identifiable on medio-lateral stifle radiography, is one of the earliest and most consistent findings in dogs with CrCL rupture and remains the most frequently used diagnostic tool for assessing cruciate pathology before confirmation of diagnosis by stifle arthrotomy or arthroscopy (Innes et al, 2004; Chuang et al, 2014; Fuller et al, 2014; Sample et al, 2017). Radiographic degenerative findings are likely correlated with chronicity of pathology.
The value of conventional radiography for the assessment of meniscal injury is limited given the inability to accurately visualise these structures radiographically (Marino and Loughin, 2010; Dillon et al, 2014; Ramírez-Flores et al, 2017). Accurate identification of meniscal pathology remains important for the prevention of ongoing lameness and osteoarthritic changes (Marino and Loughin, 2010; Dillon et al, 2014; Ramírez-Flores et al, 2017). The displacement of the torn portion of the menisci may create a palpable or audible meniscal click during manual manipulation and weight-bearing, with Gleason et al (2020) reporting that palpation of such a click strongly correlates with the presence of a meniscal tear and, more specifically, bucket handle tears. However, these authors also noted that absence of a click does not reliably exclude meniscal pathology. The clinical applicability of advanced diagnostic modalities, including computed tomography and magnetic resonance imaging, have also been described in both human and veterinary fields and may be of ongoing clinical interest despite limited availability and increased cost of their use (Przeworski et al, 2016; Franklin et al, 2017; Muir et al, 2018).
Joint effusion and synovial membrane thickening are often colloquially termed as effusion-synovitis when measured together as a surrogate for synovial inflammation (Wang et al, 2019). In human health literature, applying a continuous measure of effusion-synovitis to examine its structural relationship with the knee has been described (Wang et al, 2019). However, assessments of such veterinary-based studies are non-continuous, non-quantitative and rely heavily on clinician-subjective classification systems to measure synovial capsular distension (Bleedorn et al, 2011; Sample et al, 2017). Extension of the soft tissue opacity beyond the cranial margin of the medial tibial condyle and the cranial margin of the femoral condyle perpendicular to the tibial plateau angle is often considered abnormal and consistent with an increased synovial fluid volume (Bleedorn et al, 2011; Fuller et al, 2014; Kowaleski et al, 2017; Sample et al, 2017). Further, caudal displacement of the fat density located caudal to the joint capsule of the stifle is thought to be similarly consistent with synovial distension (Bleedorn et al, 2011; Fuller et al, 2014; Kowaleski et al, 2017; Sample et al, 2017).
Sample et al (2017) suggested that clinically relevant diagnostic markers that compare the severity of cruciate ligament pathology and stifle effusion-synovitis would be valuable tools for clinical decision-making in affected dogs. However, veterinary studies describing such methods are currently limited. The present study aimed to objectively quantify stifle effusion-synovitis in dogs through radiographic assessment, to investigate an association between the severity of effusion-synovitis and cruciate and meniscal disease, and to establish a standardised quantitative radiographic measurement screening tool to predict severity of cruciate and meniscal injury. The authors hypothesised that stifles with complete tears would have significantly greater effusion-synovitis than partial tears, and that stifles with concurrent meniscal injuries would have significantly greater effusion-synovitis than those without meniscal pathology.
Materials and methods
Study population
The medical records of patients that presented to a veterinary referral hospital between March 2018 and December 2019 for unilateral pelvic limb lameness that had subsequent pelvic limb radiography performed were reviewed. Patients with a presumptive diagnosis of CrCL pathology made by clinical assessment and conventional radiography, confirmed via stifle arthroscopy or arthrotomy, were included in the study. A total of 100 stifles with CrCL disease, with or without meniscal pathology, met the inclusion criteria. Diseased stifles with concurrent pathology, previous surgical intervention, or significant cranial tibial subluxation (Plesman et al, 2012) were excluded from the study (n=17). Patients deemed inappropriately positioned on stifle radiography were also excluded from the study. Bilateral orthogonal stifle radiographs were obtained in all cases, and patients without contralateral stifle pathology or visible pathology in either stifle on radiography were used as control patients (n=30).
Following evaluation by stifle arthroscopy or arthrotomy, patients with confirmed disease were assigned to one of the following groups:
Diagnostic imaging and quantitative measurement
Digital radiographic assessment with mediolateral and craniocaudal projections of the stifles was performed, with dogs deeply sedated (butorphanol 0.1–0.2 mg/kg intravenously and medetomidine hydrochloride 0.01 mg/kg intravenously) or anaesthetised. Images were assessed by a single clinician to evaluate the percentage of effusion-synovitis in two regions of interest (cranial and caudal joint space) (Figure 1a), the degree of cranial fat pad compression (Figure 1a) and radiographic osteoarthritis score (Marino and Loughin, 2010).
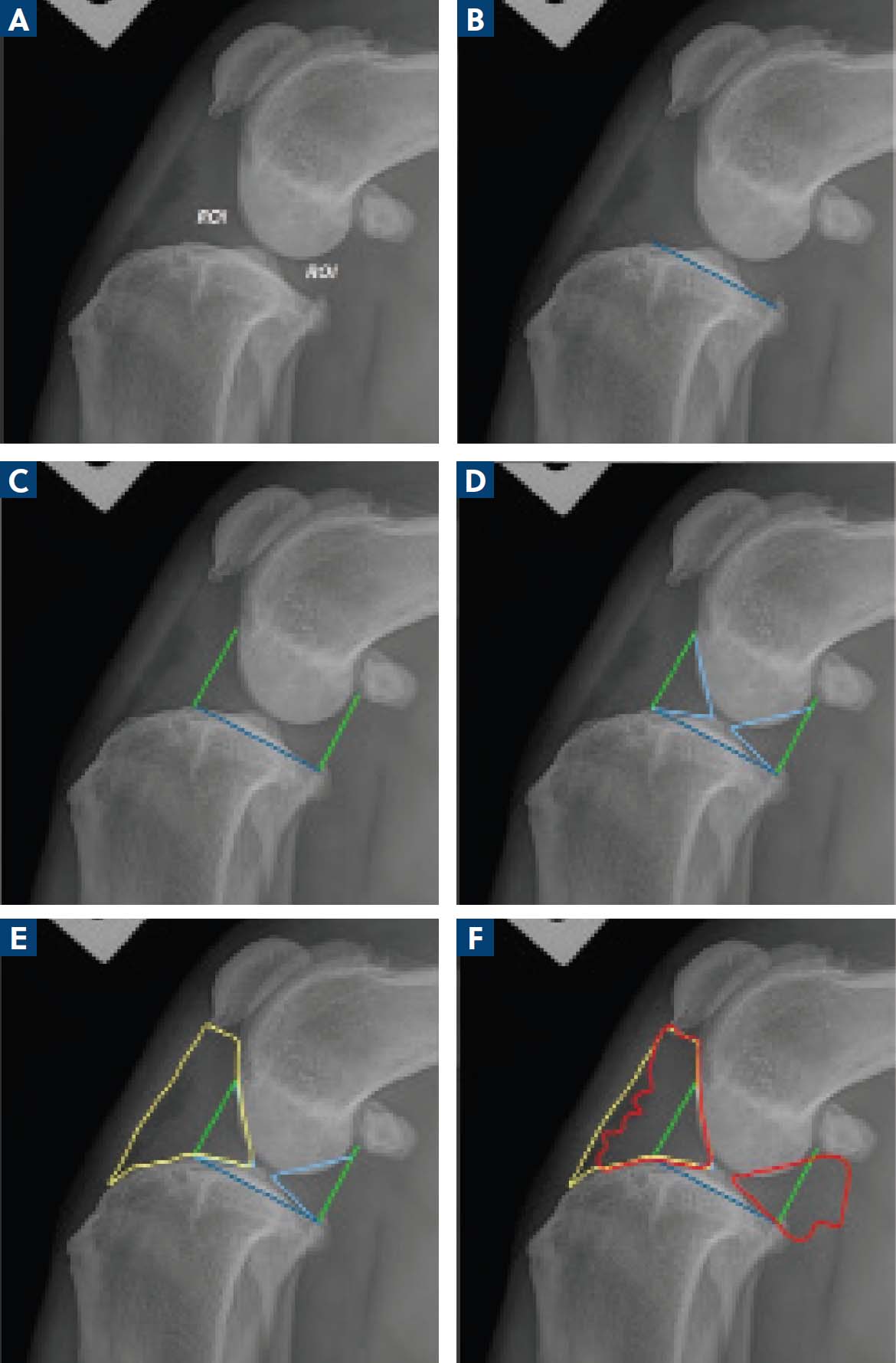
All mediolateral radiographic projections were required to include the stifle and the tarsus with adequate superimposition of both the femoral and tibial condyles for measurement, with the tibiotarsal and femorotibial angles at approximately 90°. All radiographic projections were calibrated to the L–R marker. All radiographic measurements were performed by a single author using an image processing application capable of pixelometry (Horos Viewer, Horos Project Pty Ltd). The tibial plateau angle was first calculated using previously described techniques (Kowaleski et al, 2017) (Figure 1b). A line was drawn at the cranial margin of the medial tibial condyle to the cranial margin of the femoral condyle, perpendicular to the tibial plateau angle, as per Fuller et al (2014) (Figure 1c). Extension of the soft tissue opacity beyond this was considered excessive. Similarly, a line estimating the caudal margin of the caudal poles of the menisci was drawn perpendicular to the tibial plateau angle towards the cranial aspect of the fabella to estimate the caudal joint space (Figure 1c). The area (cm2) of normal synovia both cranially (Cr) and caudally (Cd) was obtained (Figure 1d). The total area (cm2) of the cranial fat pad (CFP), including normal synovia, was calculated by measuring the space between the distal pole of the patella, the straight patellar ligament, cranioproximal tibia and the cranial aspect of the intercondylar eminence (Figure 1e). Lastly, the total area (cm2) of soft tissue opacity from the intercondylar eminences cranially (cranial effusion-synovitis; CrES) and from the intercondylar eminences caudally (caudal effusion-synovitis; CdES) were calculated (Figure 1f). The authors refer to this method as quantitative measurement assessment. All obtained values, in cm2, were then applied to the equations demonstrated below to calculate these values as a percentage:
Blinded review
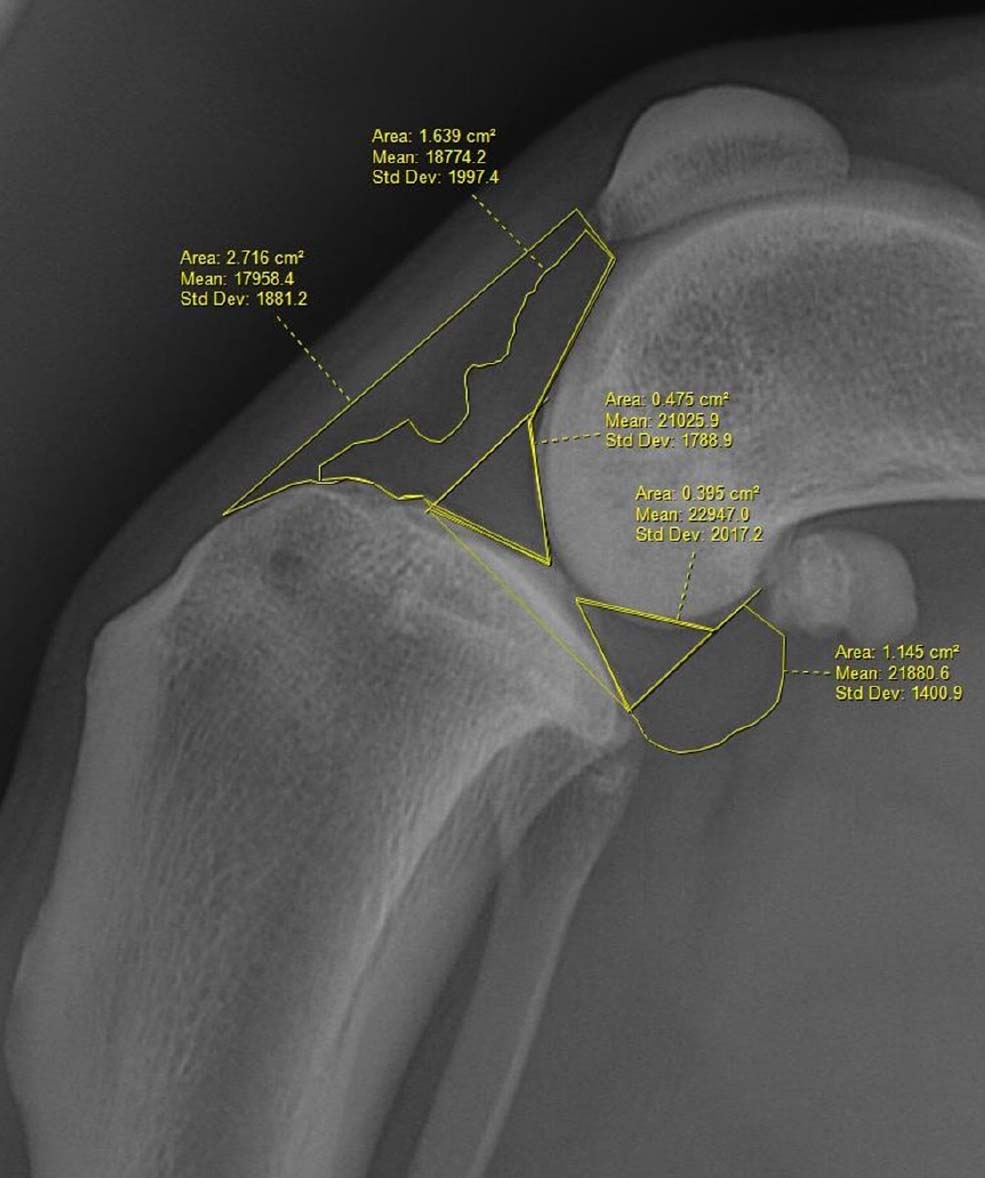
A total of 15 medio-lateral stifle radiographs of dogs with confirmed diseased stifles were selected at random from the entire data population. Two board-certified surgeons (European College of Veterinary Surgeons, American College of Veterinary Surgeons) and an American College of Veterinary Surgeons resident received a table of probability (Table 1) and were asked to conduct a blinded review of each radiograph to subjectively predict the likely degree of cruciate and meniscal pathology in terms of severity (ie PT, CT, PTM or CTM) and to grade the extent of effusion-synovitis numerically (1: mild; 2: moderate; 3: severe). This was referred to as the subjective numerical assessment. A single case demonstration of the quantitative measurement assessment was provided to each surgeon (Figure 2) who was then asked to perform the quantitative measurement on each medio-lateral stifle radiograph provided and allocate each stifle to a group based on these measurements. A comparison between time to completion, accuracy and inter-rater reliability was recorded.
| % fat pad compression | Cranial | Complete tear | 100% when FPC >75% |
| 91.67% when FPC >70% | |||
| 87.1% when FPC >65% | |||
| Partial tear | 100% when FPC <50% | ||
| % effusion-synovitis | Caudal | Meniscal tear | 86% when CdES >70% |
| 71% when CdES >65% |
CdES=caudal effusion-synovitis; FPC=fat pad compression.
Statistical analysis
Data were analysed using Prism for Windows (GraphPad software, San Diego, CA, USA) and significance level was set at P<0.05. Descriptive analysis was performed for gender, demographics (age, weight), breed distribution, duration of lameness before presentation, radiographic osteoarthritis score and degree of pathology. Degree of cruciate and/or meniscal pathology was compared using one-way analysis of variance. The Pearson correlation coefficient was used to assess for an association between amount of effusion-synovitis and age, weight, duration of clinical signs, amount of effusion compared to time to radiography, and radiographic osteoarthritis score. Cohen's Kappa coefficient was used to assess inter-rater reliability, with values below 0.20 considered as poor agreement, 0.21–0.40 as fair agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as good agreement and >0.81 as excellent agreement. Both positive and negative likelihood ratios and predictive values were also calculated for quantitative measurement assessment and subjective numerical assessment.
Results
Study population
A total of 100 stifles underwent stifle arthroscopy or arthrotomy for CrCL disease during the study period. Among these stifles, 26% had partial CrCL tears, 28% had complete CrCL tears, 2% had partial CrCL tears with concurrent meniscal injury and 44% had complete CrCL tears with concurrent medial meniscal injury. Diagnosis was achieved by arthroscopic evaluation in 48% of stifles and arthrotomy in 52% of stifles.
The mean age at time of presentation was 78.48 months (standard deviation 38.05, range 8–146), with a mean weight of 30.43 kg (standard deviation 11.96, range 6.6–63.0). Labrador and Labrador cross dogs were the most common breed, accounting for 25% of the population, followed by Staffordshire Bull Terriers (10%) and Golden Retrievers (8%). A total of 54% of dogs were spayed females and 32% of dogs were neutered males; 66% were left stifles and 34% were right stifles. The mean duration of clinical signs was 33.76 days (standard deviation 59.35, range 1–360) and the average radiographic osteoarthritis score was 1.340 (standard deviation 0.770, range 0–3). There was no significant difference in the tibial plateau angle between the CT/CTM group compared to the PT/PTM group.
Quantitative measurement assessment
A significant difference was identified regarding the percentage of CrES between control and CT groups (P=0.014), control and CTM groups (p=0.015), CT and PT groups (P=0.0173), and CTM and PT groups (P=0.012) (Table 2). Regarding CdES, a significant difference was identified between control and CT groups (P=0.011), control and CTM groups (P=0.0034), PT and CT groups (P=0.0015), and PT and CTM groups (P=0.0041) (Table 2).
| PT | PTM | CT | CTM | |||||
|---|---|---|---|---|---|---|---|---|
| SNA | QMA | SNA | QMA | SNA | QMA | SNA | QMA | |
| Accuracy (%) | 26.67 | 56.67 | 0 | 33.3 | 46.67 | 77.76 | 20 | 64.1 |
| Sensitivity | 0.39 | 0.66 | 0 | 0 | 0.61 | 0.78 | 0.33 | 0.54 |
| Specificity | 0.78 | 0.83 | 0.98 | 0.98 | 0.45 | 0.66 | 0.97 | 0.97 |
| PPV | 0.43 | 0.62 | 0 | 0 | 0.32 | 0.5 | 0.85 | 0.91 |
| NPV | 0.75 | 0.85 | 0.95 | 0.95 | 0.73 | 0.88 | 0.73 | 0.8 |
| LR+ | 1.77 | 3.8 | 0 | 0 | 1.1 | 2.29 | 11 | 18 |
| LR- | 0.78 | 0.41 | 1 | 1 | 0.86 | 0.33 | 0.69 | 0.47 |
| Time to completion | SNA | 9.3 minutes (mean) | (SD 1.52, range 8–11) | |||||
| QMA | 23.67 minutes (mean) | (SD 1.52, range 22–25) | ||||||
CT=complete tear group; CTM=complete tear with meniscal pathology group; LR+=positive likelihood ratio; LR-=negative likelihood ratio; NPV=negative predictive value; PPV=positive predictive value; PT=partial tear group; PTM=partial tear with meniscal pathology group; QMA=quantitative measurement assessment; SD=standard devation; SNA=subjective numerical assessment.
CTM stifles had significantly greater CdES (P=0.0005) than CT only. There was a significant difference of fat pad compression (CFP–CrES) observed between PT vs CT groups (P=0.0111), as well as between PT vs CTM groups (P=0.0003). Every stifle with fat pad compression >75% had CT, 91.67% when fat pad compression was >70%, 87.1% when fat pad compression was >65% and 85.7% when fat pad compression was >60%. All stifles with fat pad compression <50% had PT. A total of 86% of stifles with a caudal effusion-synovitis >70% had a meniscal injury, with a 71% probability of meniscal injury with caudal effusion-synovitis >65%.
Pearson correlation coefficient demonstrated no significant association between amount of effusion-synovitis and age, weight, duration of clinical signs, amount of effusion and time to radiography, or radiographic osteoarthritis score.
Blinded review
Subjective numerical assessment
Mean time to completion was 9.3 minutes (standard deviation 1.52, range 8–11). The PT group had an accuracy of 26.67%, and a mean sensitivity and specificity of 0.39 and 0.78 (positive preditive value (PPV) 0.43, negative predictive value (NPV) 0.75, positive likelihood ratio (LR+) 1.77, negative likelihood ratio (LR−) 0.78), respectively, with the PTM group's accuracy 0%, with sensitivity and specificity of 0 and 0.98 (PPV 0, NPV 0.95, LR+ 0, LR− 1) (Table 3). The CT group had an accuracy of 46.67%, and a mean sensitivity and specificity of 0.61 and 0.45 (PPV 0.32, NPV 0.73, LR+ 1.1, LR− 0.86), respectively, while the CTM group's accuracy was 20%, with a sensitivity and specificity of 0.33 and 0.97 (PPV 0.85, NPV 0.73, LR+ 11, LR− 0.69) (Table 3). Cohen's Kappa coefficient for correctly predicting cruciate and/or meniscal pathology using subjective numerical assessment was 0.16 (poor agreement), with an agreement of effusion severity grade of 0.65 (substantial agreement).
| C | PT | PTM | CT | CTM | |
|---|---|---|---|---|---|
| C | – | cr0.581, cd0.694 | cr0.55, cd0.62 | cr0.014*, cd0.001* | cr0.015*, cd0.0034* |
| PT | cr0.581, cd0.694 | – | cr0.766, cd0.712 | cr0.0173*, cd0.0015* | cr0.0169*, cd0.0041* |
| PTM | cr0.55, cd0.62 | cr0.766, cd0.712 | – | cr0.063, cd0.061 | cr0.056, cd0.054 |
| CT | cr0.014*, cd0.011* | cr0.0173*, cd0.0015* | cr0.063, cd0.061 | – | cr0.423, cd0.0005* |
| CTM | cr0.015*, cd0.0034* | cr0.0169*, cd0.0041* | cr0.056, cd0.054 | cr0.423, cd0.0005* | – |
C=control group; CT=complete tear group; CTM=complete tear with meniscal pathology group; cd=caudal effusion-synovitis; cr=cranial effusion-synovitis; PT=partial tear group; PTM=partial tear with meniscal pathology group.
Denotes statistical significance (p=<0.05).
Quantitative measurement assessment
Mean time to completion was 23.67 minutes (standard deviation 1.52, range 22–25). The PT group had an accuracy of 56.67%, and a mean sensitivity and specificity of 0.66 and 0.83 (PPV 0.62, NPV 0.85, LR+ 3.8, LR-0.41), respectively, with the PTM group's accuracy 33.3%, and sensitivity and specificity of 0 and 0.98 (PPV 0, NPV 0.95, LR+ 0, LR-1) (Table 3). The CT group had an accuracy of 77.76%, and a mean sensitivity and specificity of 0.78 and 0.66 (PPV 0.5, NPV 0.88, LR+2.29, LR-0.33), respectively, while the CTM group's accuracy was 64.1%, with a sensitivity and specificity of 0.54 and 0.97 (PPV 0.91, NPV 0.8, LR+18, LR-0.47) (Table 3). Cohen's Kappa coefficient for correctly predicting cruciate and/or meniscal pathology using quantitative measurement assessment was 0.23 (fair agreement).
Effect of radiographic positioning
Tibiotarsal and femorotibial angles were positioned at an approximate 90° angle to ensure radiographic accuracy and repeatability. No significant change in cranial or caudal joint spaces was identified when the femoral tibial angle was between 75° and 105° degrees. However, when reviewing the excluded population, a 19.4% (range: 11.3–22.4%) mean decrease in caudal joint space was apparent every 5° change thereafter, with a 10.2% (range: 8.4–13.3%) mean decrease in cranial joint space.
Discussion
Diagnosis of CrCL and meniscal pathology continues to pose unique diagnostic challenges to clinicians despite being the most common orthopaedic condition affecting the pelvic limb in the dog (Bleedorn et al, 2011; Dillon et al, 2014; Ashour et al, 2019). Although the diagnostic use of various imaging modalities for imaging the canine stifle, including radiography, computed tomography, magnetic resonance imaging and ultrasonography, has been well described, further investigation towards a more accurate, non-invasive imaging modality remains desirable (Gnudi and Bertoni, 2001; Arnault et al, 2009; Böttcher et al, 2010; Marino and Loughin, 2010; Przeworski et al, 2016; Franklin et al, 2017; Sample et al, 2017). Thus, the present study aimed to establish and propose a standardised quantitative radiographic measurement screening tool to predict severity of cruciate and meniscal injury with improved accuracy compared to methods previously described. Concurrently, the authors attempted to objectively quantify stifle effusion-synovitis in dogs through radiographic assessment, and to investigate an association between the severity of effusion-synovitis and cruciate and meniscal disease.
Immediate clinical diagnosis of cruciate and meniscal pathology is centred around appropriate physical examination of the stifle; however, both CrCL and meniscal pathology can sometimes be difficult to diagnose by classical tests of cranial drawer and cranial tibial thrust (Dillon et al, 2014; Sample et al, 2017; Ashour et al, 2019). Factors including phase of the disease process, when physical examination findings may be subtle, as well as osteoarthritis and periarticular fibrosis may all contribute to differences in stifle stability through examination (Ashour et al, 2019). Cranial tibial translation assessed by tibial compression test in extension appears to display significant correlation with arthroscopically-confirmed CrCL damage, whereas severity of cranial drawer does not (Ashour et al, 2019). However, Ashour et al (2019) also noted that tibial compression laxity was not apparent in some stifles with confirmed CrCL rupture, suggesting that the test may not be very sensitive. Further, severity of palpable stifle effusion was correlated with radiographic effusion (Ashour et al, 2019). In dogs, more recent studies have identified the presence of a meniscal click throughout examination to have a sensitivity and specificity between 31.0–58.3% and 94.4–96.0%, respectively, for meniscal pathology (Dillon et al, 2014; Gleason et al, 2020). Incorrect diagnosis of meniscal disease may result in increased patient morbidity, prolonged recovery and increased financial commitments for the owner (Dillon et al, 2014).
Many changes within the osteoarthritic joint occur before radiographic bony changes, with stifle synovitis developing initially as a response to cruciate degeneration and often being associated with some degree of fibre rupture in the cruciate ligament complex (Scanzello and Goldring, 2012). Identification of stifle effusionsynovitis, a common radiographic feature in veterinary patients with cruciate pathology, is often associated with high inter- and intra-rater agreement when subjective measures are used (Chuang et al, 2014; Fuller et al, 2014; Sample et al, 2017; Muir et al, 2018). Based on a trend towards increasing agreement and reproducibility for more experienced observers, subjective measurement of stifle effusion-synovitis remains the preferred methodology for selection in studies assessing cruciate integrity (Fuller et al, 2014; Sample et al, 2017). An agreement also exists regarding stifles with mild synovitis-effusion evident, with such cases rendering radiographic detection difficult. However, previous studies support the development of synovitis-effusion with macroscopically intact CrCLs, and as such, radiographic findings must be considered with an appropriate orthopaedic examination to provide a relatively reliable indicator of cruciate integrity (Bleedorn et al, 2011; Döring et al, 2018).
In humans, radiography for the diagnosis of anterior cruciate ligament pathology has reportedly limited value as findings are often indirect and limited to osseous abnormalities (Tai et al, 2009; Ng et al, 2011). The radiographic presence of an effusion in the knee following trauma is suggestive of an acute tear of the anterior cruciate ligament in the absence of an intra-articular fracture or underlying arthritis (Tai et al, 2009; Ng et al, 2011). When assessing lateral radiographs, anteriorisation of the tibia results in significant translation of the medial and lateral compartments, regularly seen with complete anterior cruciate ligament tears, while partial tears demonstrate minimal translation relative to the normal contralateral limb (Tai et al, 2009). Qualitative visual grading of such radiographs has a high sensitivity and low–moderate specificity among clinicians, leading to further investigation via additional imaging modalities (Tai et al, 2009; Ng et al, 2011). Multiple veterinary studies have identified that, subjectively, the amount of radiographic effusion and severity of cruciate pathology correlate linearly (Fuller et al, 2014; Sample et al, 2017). However, to the author's knowledge a comprehensive quantitative assessment of relationships between specific radiographic and arthroscopic findings is lacking.
The results in the present study demonstrate that the use of a standardised quantitative radiographic measurement screening tool to predict severity of cruciate and meniscal injury may improve diagnostic accuracy of cruciate and meniscal pathology in a clinical setting. In this study, quantitative measurement assessment demonstrated overall improved sensitivity, specificity and agreement when compared to subjective numerical assessment. However, no significant difference in these measures was appreciated. This is most likely a result of a low population of blindly reviewed radiographs by each clinician. Mean LR+ was 1.73 times more likely to positively predict the likelihood of disease using quantitative measurement assessment than subjective numerical assessment, corroborated by improved PPV observed with quantitative measurement assessment. Further, the authors were able to quantify that severity of cruciate and meniscal injury correlates linearly with degree of synovitis-effusion found radiographically. This has been reported previously with subjective radiographic assessment but was yet to be confirmed quantitatively in dogs (Fuller et al, 2014; Sample et al, 2017). Studies using a subjective measurement grading system have demonstrated high inter- and intra-rater agreement (Fuller et al, 2014; Sample et al, 2017). Inter-rater agreement in this study was considered unsatisfactory for both subjective numerical assessment (poor agreement) and quantitative measurement assessment (fair agreement). However, clinicians were required to predict the specific severity of cruciate and meniscal disease based on degree of effusion-synovitis evident radiographically rather than solely assign a grade to the effusion, and this likely resulted in the reduced agreement. Agreement using subjective numerical assessment for effusion severity grade was similar to that previously reported.
Despite repeatably acceptable agreement between clinicians when predicting grade of effusion severity, this study demonstrates agreement lower than ideal for predicting the severity of cruciate and meniscal pathology. Multiple authors have recommended ongoing research in order to establish better non-invasive methodologies for diagnosis cruciate and meniscal disease. Magnetic resonance imaging has become an increasingly popular tool to assess soft tissue damage of the knee, with reportedly high diagnostic accuracy (Yoon et al, 2010; Guenoun et al, 2012). Studies have shown magnetic resonance imaging to be quite accurate in diagnosing meniscal and ligamentous injury in humans, with 92.86% and 94.74%, accuracy in the diagnosis of complete and partial tears respectively (Yoon et al, 2010; Guenoun et al, 2012). Additionally, diagnostic evaluation of meniscal disease is associated with high sensitivity and specificity. Lefevre et al (2016) noted that magnetic resonance imaging, being more precise than a clinical examination, has influenced clinical practice and the treatment of human patients by eliminating unnecessary diagnostic arthroscopies. However, magnetic resonance imaging studies in canines have focused predominantly on diagnosis of meniscal pathology as a result of the difficulty in accurate diagnosis through physical examination. Despite some conflicting evidence as to its overall diagnostic accuracy regarding meniscal pathology (Böttcher et al, 2010), most reports have identified acceptable diagnostic sensitivity and specificity above 90% (Przeworski et al, 2016). Galindo-Zamora et al (2013) stated that diagnosis of CrCL tear is difficult, with pathology often characterised by increased signal intensity of a hypointense ligament on magnetic resonance imaging. The diagnostic use of magnetic resonance imaging for cruciate pathology has been debated in the literature despite the use of differing magnetic fields. The disparity between accurate meniscal and cruciate disease diagnosis may be a reflection of technical limitations associated with the use of magnetic resonance imaging in canine models. Given these limitations, some authors have questioned the routine use of magnetic resonance imaging for stifle pathology when physical examination combined with radiography have reportedly similar accuracy, are more readily available, affordable and require less technical expertise. The feasibility of further cross-sectional imaging, such as computed tomography, has also been studied for assessing stifle pathology (Muir et al, 2018).
Limited research regarding the use of computed tomography is available, with studies appearing to demonstrate satisfactory accuracy of computed tomography in the diagnosis of CrCL avulsions in young dogs; however, plain computed tomography images were less useful in evaluating the integrity of the cruciate ligaments and the menisci (Muir et al, 2018). Further, the use of combined contrast arthrography demonstrated no improved benefit for imaging of the affected stifle as the medium is readily diluted and absorbed following administration (Muir et al, 2018). The diagnostic use of ultrasonography has also been proposed to diagnose meniscal pathology, with a correct classification rate, sensitivity and specificity of 90%, 95% and 82% respectively when compared to arthroscopic examination (Franklin et al, 2017). These results are supported by Arnault et al (2009) who found that a diagnosis of CrCL rupture could only be made sonographically in 15.4% of cases.
In humans, meniscal pathology without concomitant cruciate injury can result in the development of joint effusion as a response to synovial activation (Roemer et al, 2009; 2013). Synovial activation of a joint is reflected as synovitis and joint effusion, which may be triggered by meniscal pathology alone. Studies have also reported ipsi-compartmental perimeniscal synovitis as a response to meniscal injury (Roemer et al, 2009; 2013). Marked synovial stimulation is thought to be apparent during the healing phase of meniscal tears or enhancement of repair through synovial activation (Roemer et al, 2009; 2013), but the authors were unable to comment on the order of events or if effusion may even exhibit a detrimental effect on meniscal composition that ultimately results in tears. Isolated meniscal tears in dogs are rare, thought to occur most likely secondary to trauma, and similarly demonstrate effacement of the infrapatellar fat pad radiographically (Roemer et al, 2009; 2013). With isolated meniscal disease infrequently reported in canines, veterinary understanding of the effect of meniscal injury on the development of synovitis-effusion is limited. The findings in the present study indicating that CTM stifles had significantly greater CdES (P=0.0005) than CT only may suggest perimensical synovitis-effusion development within the caudal joint space secondary to meniscal pathology. However, this finding was not replicated statistically significantly in the cranial joint space despite showing greater CrES in the CMT group than CT alone.
In combination with current literature, it may be appropriate to consider concurrent meniscal pathology when synovitiseffusion is deemed significant. It is also possible to hypothesise that in combination with stifle biomechanical alterations, synovial activation as a response to cruciate pathology may predispose patients to meniscal injury and further synovitis-effusion, which may also corroborate the aforementioned findings. Further studies are required to quantify the effect of isolated meniscal injury on synovial activation and its detrimental intra-articular effects. Human studies have identified that knees with acute pathology had 125% greater joint effusion than those in the subacute group, which then diminished and remained at a relatively low level after 6 weeks (Yoon et al, 2010). It was hypothesised that this indicates joint effusion as a sensitive sign of intra-articular pathology in the acute setting, with a rapid decrease in volume observed over time. These findings are dissimilar to those identified in this study. This may be related to the chronic nature of cruciate pathology in the dog compared to humans, in combination with likely earlier diagnostic identification of disease.
Limitations
There were some limitations to the present study. The retrospective nature of this study poses several limitations, including an inferior level of evidence compared with prospective studies. The intra-observer repeatability using quantitative measurement agreement was not assessed, and the authors are therefore unable to comment on observer consistency when using quantitative measurement agreement. Although the authors’ assessed inter-observer agreement of both subjective and quantitative measurement tools, intra-observer assessment is an important means to provide more evidence for the clinical use of quantitative measurement agreement. Additionally, a low number of radiographs were blindly reviewed by each clinician. This manuscript would have benefitted from a larger cohort of blindly reviewed images. Further, the present study's specific radiographic inclusion criteria limited acceptable radiographs to those with femorotibial angles between 75° and 105°. This range was selected based on clinician preference in pre-operative imaging, suited for tibial plateau leveling osteotomy measurements. Radiographs performed at other angles, such as those for tibial tuberosity advancement procedures, may provide varying results than those identified in this study. This requires further exploration.
Conclusions
The results of this study propose that a quantitative radiographic measuring tool for predicting severity of cruciate and meniscal disease may improve diagnosis of stifle pathology in a clinical setting and aid in pre-operative clinician decision making compared to subjective classification schemes. Several classifications for measuring stifle effusion-synovitis exist, but these are highly subjective and lack the ability to accurately predict severity of disease. Although further research is warranted to assess the feasibility of this proposed quantitative tool, the results identified here are encouraging. Further, the authors were able to identify that cruciate and meniscal injury correlates linearly with degree of synovitiseffusion found radiographically. The authors recommend ongoing investigation into various diagnostic methodologies for accurate diagnosis of cruciate and meniscal disease in dogs.


