Feline gastrointestinal eosinophilic sclerosing fibroplasia is a recently described disease in cats that presents as eosinophilic mass(es) that are associated with the gastrointestinal tract and associated abdominal lymph nodes, often localised near the pylorus or ileocaecocolic junction (Craig et al, 2009; Linton et al, 2015). There have also been two case reports where feline gastrointestinal eosinophilic sclerosing fibroplasia was localised to the mesentery or retroperitoneum, and even presented as a cavitated mass (Thieme et al, 2019; Kambe et al, 2020). A case report also described similar histopathologic changes outside of the abdominal cavity; a feline eosinophilic sclerosing lymphadenitis in medial retropharyngeal lymph node of a cat associated with Pseudomonas aeruginosa infection. A possible change of the name ‘feline gastrointestinal eosinophilic sclerosing fibroplasia’ has been suggested as it might not only affect the gastrointestinal tract (Zampieri et al, 2022). Feline gastrointestinal eosinophilic sclerosing fibroplasia is likely underdiagnosed because these mass lesions can be misinterpreted as lymphoma, granuloma, fibrosarcoma, adenocarcinoma and mast cell tumour, as histopathological diagnosis can be challenging (Gamble, 2010; Schulman and Lipscomb, 2010; Munday et al, 2014; Linton et al, 2015).
The pathogenesis of feline gastrointestinal eosinophilic sclerosing fibroplasia is still poorly understood; however, an aberrant response to antigens from bacteria or parasites has been considered, and a case of feline gastrointestinal eosinophilic sclerosing fibroplasia associated with fungal organisms (Phycomycetes) has also been reported (Craig et al, 2009; Eckstrand et al, 2013; Grau-Roma et al, 2014).
Clinical presentation
Signalment and history
This disease is most commonly reported in middle aged and male cats (male cats were overrepresented in some studies) of all breeds, with Ragdolls being overrepresented in previous studies, and Maine Coons, Persians, Exotic Shorthairs, Bengal and Scottish Fold cats also being reported, although less frequently (Craig et al, 2009; Suzuki et al, 2013; Linton et al, 2015; Cho et al, 2017; Montenegro et al, 2022). With some breeds, such as the Ragdoll, being overrepresented, a genetic predisposition could be considered. The median age in a recent study with 60 feline gastrointestinal eosinophilic sclerosing fibroplasia cases was 5.4 years (interquartile range: 3.3–8.9) (Černá, author's data, 2023). Of these 60 cats, less than half were domestic shorthair or domestic longhair cats and 58% were pedigree breeds: Ragdolls(25%), Exotic Shorthair (10%), Persian (8%), Maine Coon (5%), Sacred Birman (3%), American Shorthair (2%), Bengal (2%), Bobtail (2%) and British Shorthair (2%) cats.
Clinical signs are typically chronic, and a recent study reported median duration of clinical signs was 90 days (interquartile range: 17.5–247) with most cats showing a median of three clinical signs (interquartile range: 2–4) (Černá, 2023). The most common presenting signs of cats with feline gastrointestinal eosinophilic sclerosing fibroplasia (Table 1) are weight loss, hyporexia, chronic vomiting and/or diarrhoea and lethargy; less frequently an acute onset of vomiting and/or diarrhoea has been reported (Craig et al, 2009; Linton et al, 2015; Černá, 2023). A palpable intestinal mass was identified in 85–100% of cases, with abdominal pain and pyrexia being less common (Craig et al, 2009; Linton et al, 2015).
Table 1. Presenting clinical signs of cats with feline gastrointestinal eosinophilic sclerosing fibroplasia
| Clinical sign | % of cats |
|---|---|
| Weight loss | 60 |
| Hyporexia/anorexia | 55 |
| Chronic vomiting (>2 weeks) | 37 |
| Lethargy | 35 |
| Chronic diarrhoea | 27 |
| Acute vomiting (<2 weeks) | 13 |
| Acute diarrhoea | 10 |
| Constipation | 10 |
| Tenesmus | 8 |
| Polyphagia | 7 |
| Haematochezia | 7 |
| Decreased grooming | 5 |
| Melena | 2 |
| Excessive grooming | 2 |
Diagnostic examination
The most common bloodwork abnormality in cats with feline gastrointestinal eosinophilic sclerosing fibroplasia is usually peripheral eosinophilia, which is present in about 50% of cats (Craig et al, 2009; Linton et al, 2015; Černá, 2023). Anaemia was present in almost one-third of the cats in a recent study (Černá, 2023), and less common findings include neutrophilia, monocytosis, lymphocytosis, basophilia and neutropenia. On serum biochemistry, hypoalbuminemia and hyperglobulinemia were the most common abnormalities, occurring in 27–45% and 14–67% of cats (Munday et al, 2014; Linton et al, 2015). Hypocholesterolaemia and total hypocalcaemia have also been reported (Černá, 2023). Serum hypocobalaminemia is not commonly reported in these cases; however, it has not been routinely measured in the previous studies.
Large studies evaluating abdominal ultrasonography findings of cats with feline gastrointestinal eosinophilic sclerosing fibroplasia are lacking to date; however, one study reported 5 cats that had a solitary mass with mural thickening and loss of layering in the stomach, duodenum, jejunum and colon (Weissman et al, 2013). Most of the gastrointestinal masses are associated with loss of the intestinal layering (Figure 2) but some masses (20%) can be associated with altered – rather than lost – layering, symmetrical or asymmetrical circumferential thickening, eccentric growth and a heterogeneously mixed wall echogenicity which had hyperechoic areas. Peritoneal changes were reported in 73% of the cats, with one-third of the cats having peritoneal effusion, although gastrointestinal perforation was not identified (Černá, 2023). Enlarged lymph nodes were also often reported (90%) with the ileocolic, pancreaticoduodenal and mesenteric lymph nodes often being affected (Černá, 2023).
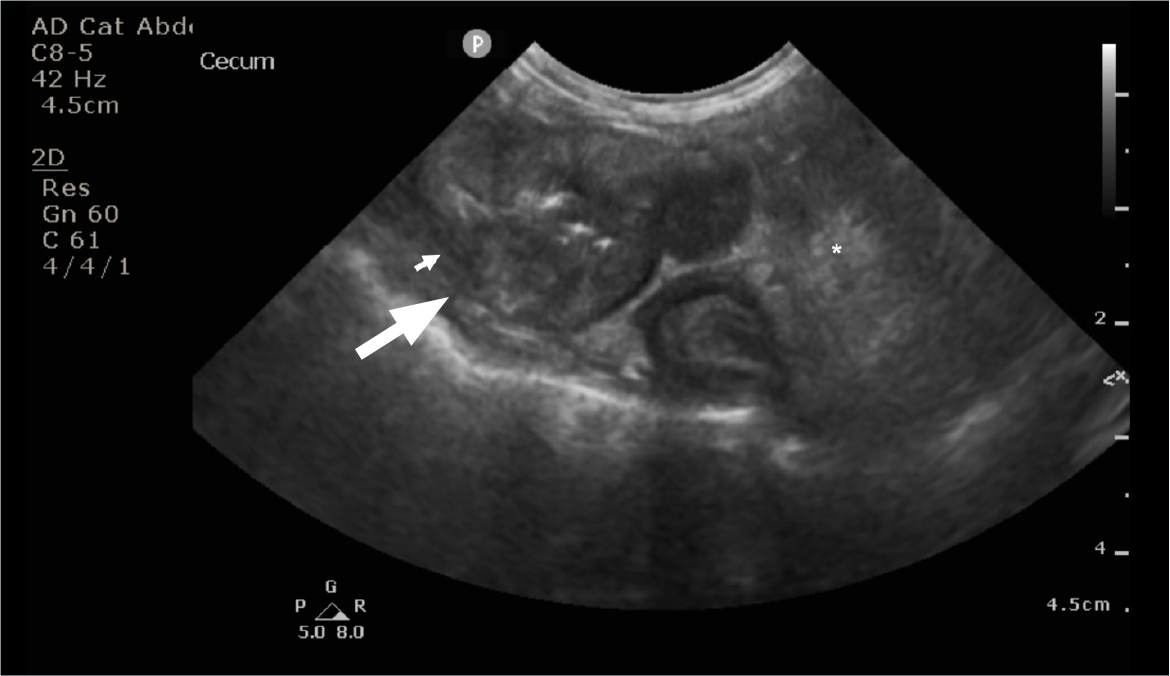
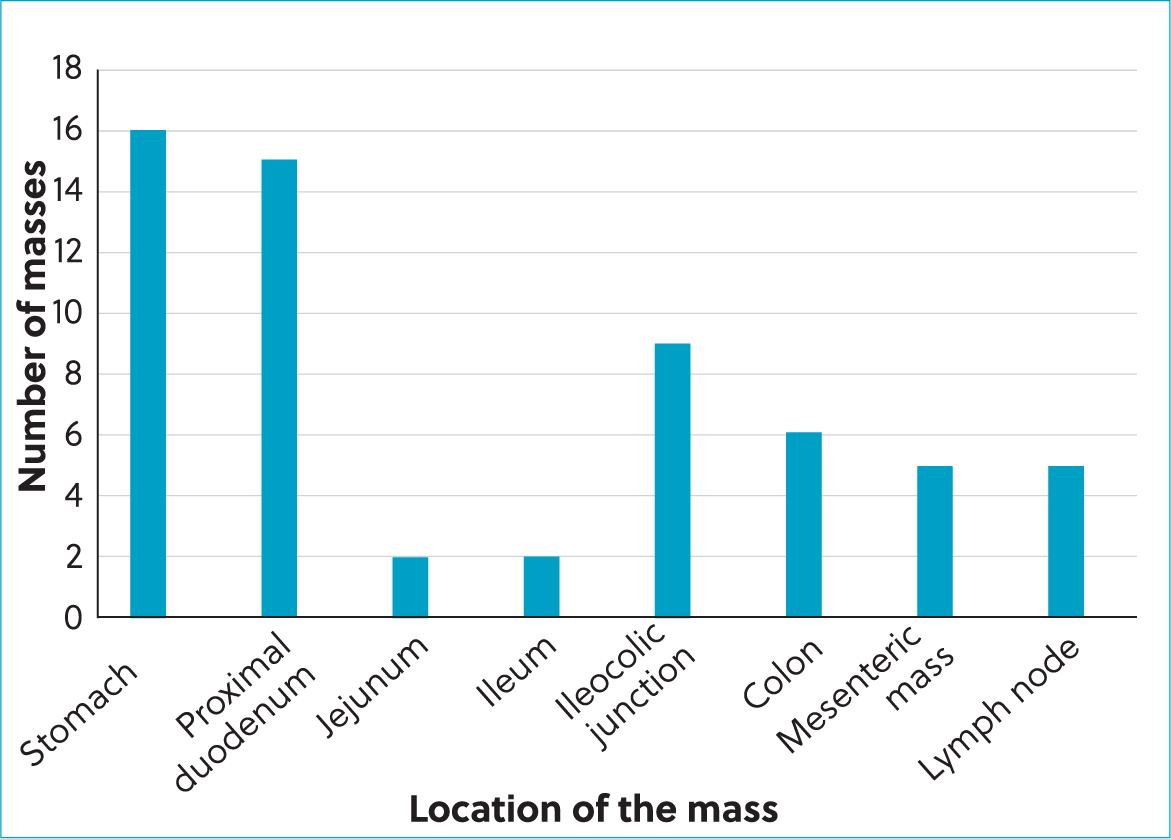
The most common location of the masses (Figure 2) was the small intestine (32%), including (Černá, 2023):
- The proximal duodenum: 25%; Figure 3
- The jejunum: 3%
- The ileum: 3%
- The stomach: 27%
- The ileocaecocolic junction: 15%
- The colon: 10%
- The lymph nodes: 8%
- The mesentery: 8%.

Most cats only had one mass; however, in a recent study, in about 15% of cats a mass was present in more than one location (Černá, 2023). The additional masses most commonly involved the mesentery and surrounding lymph nodes (44%) or the stomach and proximal duodenum. It is also possible that a second mass can appear later (even several years later) in a different location.
Histopathology and infectious disease testing
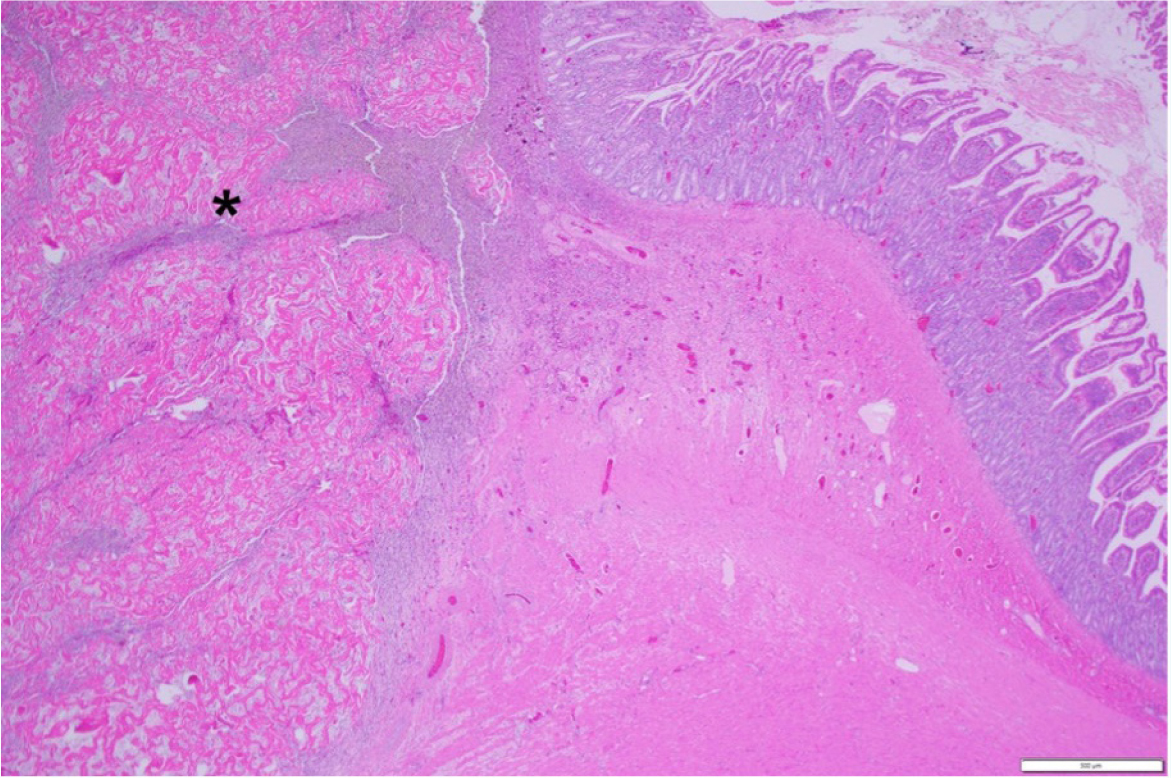
The diagnosis of feline gastrointestinal eosinophilic sclerosing fibroplasia is most commonly confirmed with histopathology (Figure 4). Intralesional bacteria were identified in about half of the cases overall (in all of the ileocaecocolic junction and colon lesions) in one study (Craig et al, 2009) and in 69% of cats in another study using either culture or conventional light microscopy, special stains and fluorescence hybridisation (Linton et al, 2015). In a recent study, only 32% of the cases had bacteria present on histopathology, and fungal organisms were detected in 1% of the cases; however, one limitation of this retrospective study is that infectious organisms might have been missed in some cats because fluorescence in situ hybridisation was only performed in 3 cats and bacterial culture was only performed in 30% of the cats in this study (Černá, 2023). Even though bacteria are commonly associated with feline gastrointestinal eosinophilic sclerosing fibroplasia, fungal organisms have only been reported once previously, in a case report of feline gastrointestinal eosinophilic sclerosing fibroplasia associated with phycomycetes (Grau-Roma et al, 2014). Bacterial culture was performed in 30% of cases in a recent study and 22% of these were negative, while in the others the most common bacteria were E. coli (50%), Staphylococcus species (50%), Enterococcus species (33%), Streptococcus species (8%) and Bacteroides fragilis (8%) (Černá, 2023).
Management
Surgical removal of the mass has been performed in most cases with feline gastrointestinal eosinophilic sclerosing fibroplasia. However, several studies have also reported medical management with corticosteroids, and/or cyclosporine or mycophenolate alone or in combination with surgery (Linton et al, 2015; Takao et al, 2015; Agulla et al, 2021; Kim et al, 2021). A mass in a second location has been reported to develop in some of the cases, several months or even years after surgical removal of the initial mass (Weissman et al, 2013; Munday et al, 2014; Brloznik et al, 2017). In a recent study, 98% of the cats were started on corticosteroid therapy, so it was not possible to assess the survival time of the cats treated with surgery alone. However, there was no statistical difference between the survival of the cats that had their masses surgically resected and cats where the mass was only biopsied, including cats with complete resection with clear margins confirmed by histopathology (Černá, 2023). This supports the hypothesis that complete surgical resection may not be needed in these cats and corticosteroid therapy is needed.
Corticosteroids appear to be important in the treatment of cats with feline gastrointestinal eosinophilic sclerosing fibroplasia. Cats being treated with surgery alone had a significantly shorter survival time than those being treated with surgery and corticosteroids in one study (Craig et al, 2009) and improved survival time was reported when prednisolone was included in the therapeutic regimen in another study, regardless of whether or not they also had surgery (Linton et al, 2015). Recurrence of masses has been previously reported when surgery was not followed by corticosteroids (Weissman et al, 2013; Brloznik et al, 2017; Černá, 2023). In a recent study, one cat had a mass resected from the ileocaecocolic junction but was not started on corticosteroid therapy, and represented 7 months later with a mesenteric mass. Another cat in this study was diagnosed with feline gastrointestinal eosinophilic sclerosing fibroplasia in the colon, which was resected, then with a pyloric mass 2 years later, which was also resected, and another pyloric mass 3 years after that; while all of these masses were surgically resected, corticosteroid therapy was not started until after the resection of the third mass. There was no recurrence of abdominal masses in either of these cats after starting corticosteroid therapy for over 1.5 years (Černá, 2023).
The indication to follow surgery with corticosteroid therapy is further supported by the same study where treatment with prednisolone was discontinued in 13 cats; 85% of these had to have prednisolone restarted a median of 114 days after discontinuation because of recurrence of clinical signs. In the study, the median time to the lowest dose of prednisolone was 369 days, with a median lowest maintenance dose of 0.65 mg/kg/day to control the clinical signs; however, as this is a retrospective study, some patients were lost to follow up and it is therefore unclear if prednisolone was tapered further in these cats (Černá, 2023).
The use of secondary immunosuppressive agents and/or antibiotics in cats with feline gastrointestinal eosinophilic sclerosing fibroplasia has been reported previously (Craig et al, 2009; Linton et al, 2015). In one study, there was no statistical difference between the survival of cats that were treated only with corticosteroids versus including secondary immunosuppressive agents, regardless of whether or not antibiotics were given (Munday et al, 2014). Larger prospective studies are needed to evaluate the effectiveness of secondary immunosuppressive agents and/or antibiotics in the treatment of feline gastrointestinal eosinophilic sclerosing fibroplasia.
The role of dietary management in control of feline gastrointestinal eosinophilic sclerosing fibroplasia is currently unclear. In a recent study, hydrolysed or selected protein diets were trialled in 37% of the cats and, in 41% of these, the owners reported an improvement of clinical signs on these diets. This suggests diet modification as a possible treatment of cats with feline gastrointestinal eosinophilic sclerosing fibroplasia (Černá, 2023). However, these cats were all already being treated with corticosteroids and further studies are needed to evaluate the use of hydrolysed diets in the cats with feline gastrointestinal eosinophilic sclerosing fibroplasia. A large prospective randomised study is needed to determine the most appropriate therapy for cats with feline gastrointestinal eosinophilic sclerosing fibroplasia.
Prognosis
The prognosis for cats with feline gastrointestinal eosinophilic sclerosing fibroplasia has been reported as variable in previous publications, and may be guarded to surviving for several years (Craig et al, 2009; Weissman et al, 2013; Linton et al, 2015). Linton et al (2015) reported that most cats surviving the perioperative period remained well for several years – the mean survival in a retrospective study on 60 cats with feline gastrointestinal eosinophilic sclerosing fibroplasia was 937 days (range: 44–2689), with 88% of the cats still alive at the time of the publication (Černá, 2023). This shows the importance of reaching the correct diagnosis for cats with feline gastrointestinal eosinophilic sclerosing fibroplasia – many of these masses can be misdiagnosed as neoplasia, which usually carries a poor prognosis.
Conclusions
Feline gastrointestinal eosinophilic sclerosing fibroplasia is an important differential diagnosis for abdominal masses in cats, and it has a much better prognosis than previously reported, regardless of whether the mass is removed surgically or is treated with medical management alone. Corticosteroid therapy is needed for these cases.
KEY POINTS
- Feline gastrointestinal eosinophilic sclerosing fibroplasia is most commonly reported in middle aged cats, with Ragdoll cats being overrepresented.
- Feline gastrointestinal eosinophilic sclerosing fibroplasia presents as eosinophilic mass(es) that are associated with the gastrointestinal tract and associated abdominal lymph nodes, often localised near the pylorus or ileoceacocolic junction.
- Clinical signs are typically chronic with weight loss, hyporexia, diarrhoea, lethargy and vomiting being most common.
- Common observed bloodwork abnormalities include eosinophilia, anaemia and hypoalbuminaemia.
- Corticosteroids appear to be important in the treatment of cats with feline gastrointestinal eosinophilic sclerosing fibroplasia, and cats being treated with surgery alone had a significantly shorter survival time than those cats treated with surgery and corticosteroids.


