Hyperthyroidism is the most common endocrinopathy diagnosed in senior and geriatric cats. The prevalence in English cats >10 years of age has been reported to be 8.7% (Stephens et al, 2014). Hyperthyroidism is most commonly caused by hyperplastic or adenomatous thyroid goitre development, resulting in high levels of circulating thyroid hormones (T3 and T4). Thyroid carcinomas are rare, accounting for <1–3% of all cases of hyperthyroidism (Naan et al, 2006).
Hyperthyroidism can be managed long-term with either anti-thyroid medications (methimazole or carbimazole) or dietary restriction of iodine, with the aim of controlling excessive thyroid hormone concentrations.
Alternatively, thyroidectomy or radioactive iodine (radioiodine, RAI) treatment can be used to remove the hyperfunctional thyroid tissue and source of excessive thyroid hormone production, curing the cat of hyperthyroidism.
Radioactive iodine has been used to treat hyperthyroidism for over 30 years. It is widely considered to be the gold standard treatment for feline hyperthyroidism and is becoming increasingly available across the UK, as more centres are now able to provide this option.
General FAQs about radioactive iodine
Why is radioactive iodine considered the gold-standard treatment?
The advantages of RAI include:
- Potential to be curative
- Lack of damage to parathyroid glands, local nerves (sympathetic and recurrent laryngeal) and normal thyroid tissue
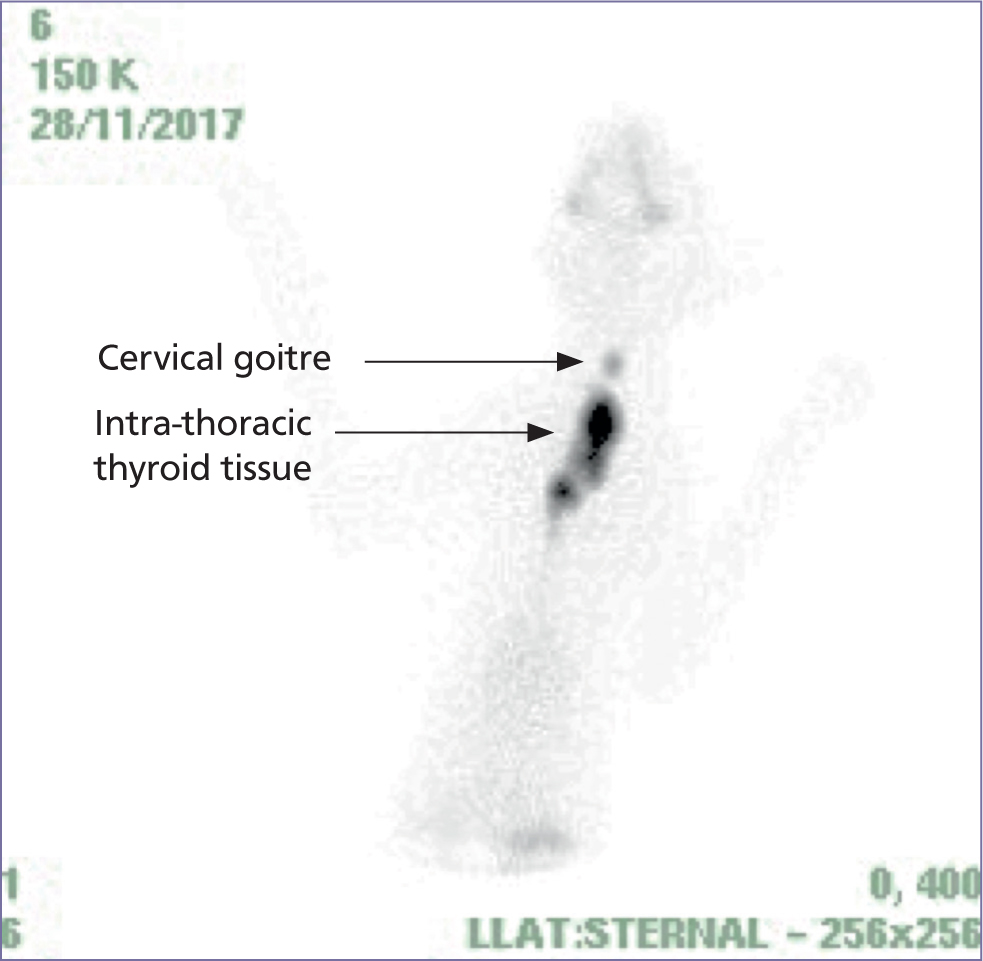
- The ability to treat all hyperfunctional thyroid tissue regardless of location, i.e. ectopic and intrathoracic tissue will also be targeted
- Benign and malignant (thyroid carcinoma) hyperthyroidism can be treated
- Well tolerated
- No requirement for anaesthesia
- Single treatment required in majority of cases
- Treatment can be repeated if required, for example if inadequate response or redevelopment of hyperthyroidism at a later stage in contralateral gland or ectopic tissue
- Lack of requirement for daily anti-thyroid medication administration
- Lack of requirement for a restricted diet.
How successful is radioactive iodine treatment?
Radioactive iodine treatment can be expected to successfully cure hyperthyroidism with a single dose in ≥95% of cases (Lucy et al, 2017). This will reverse the clinical signs and secondary effects of hyperthyroidism, such as thyrotoxic cardiomyopathy. The small percentage of cats not cured by their first RAI treatment may experience either a partial response (meaning thyroxine levels are reduced but euthyroidism is not reached) or no response; this may be because of severe and/or chronic hyperthyroidism associated with a large volume of thyroid tissue and/or carcinomatous tissue. A second treatment using a higher dose of radioiodine may be given. A requirement for a second treatment may potentially occur in those cats living many years after their first treatment, likely reflecting a younger age of first diagnosis of hyperthyroidism and development of thyroid hyperplasia or adenoma in a contralateral gland or ectopic tissue at a later stage. Repeat treatment is, however, rarely needed, but is well tolerated if required.
What are the disadvantages of radioactive iodine treatment?
The main drawbacks are:
- High upfront cost for the owner
- Requirement for the cat to spend time away from home for at least the initial period post treatment, with hospitalisation (‘isolation’) in a dedicated RAI ward with minimal handling (4–14 days)
- Period of post-treatment handling and waste restrictions typically lasting 4–5 weeks
- Early hospital discharge (i.e. after 4–14 days) requires owner compliance with specific handling and waste restrictions
- Irreversible treatment (compared to being able to titrate the dose of anti-thyroid medications) and potential development of permanent iatrogenic hypothyroidism (this risk also occurs with thyroidectomy)
- Not all cats are good candidates, because of the requirement for hospitalisation and limited handling, particularly in the first few days after RAI treatment when the cat is most radioactive; for example a clinic would need to carefully consider management of insulin-dependent diabetic hyperthyroid cats and apathetic hyperthyroid cats, to balance patient care with limitation of staff radiation exposures
It is worth bearing in mind, however, that the cost of 18 months to 2 years of oral anti-thyroid medication and monitoring can be equivalent to the cost of RAI treatment. Also, hospitalisation times have reduced over the last 10–15 years and the average post-treatment stay is now 4–14 days in the UK, provided owners can comply with handling and waste restrictions at home. Waiting lists were also previously a barrier to treatment, because of the limited numbers of treatment centres, but there are now many more RAI clinics (17 at the time of writing), bringing waiting times down.
How does radioactive iodine work?
The thyroid gland uses iodine to synthesise the thyroid hormones triiodothyronine (T3) and thyroxine (T4). Thyroid tissue does not differentiate between radioisotopes of iodine (e.g. I131) and dietary sources of iodine. Administered RAI is absorbed into the circulation then actively concentrated in hyperfunctional thyroid tissue (hyperplastic and neoplastic), resulting in high tissue concentrations regardless of location. The radioiodine isotope sodium iodide I131 is used, which emits gamma and beta radiation. Irradiation from beta particles within thyrocytes causes cellular necrosis or apoptosis (because of sublethal DNA damage) (Peterson et al, 2019). The majority of the radiation effect (80%) is via beta radiation particles, which travel a maximum of 2 mm in the tissue, hence local tissues, including the parathyroid glands, local nerves and atrophied thyrocytes, are unaffected by the radiation.
How is radioactive iodine given?
Radioactive iodine can be given subcutaneously (SC), intravenously and orally; most UK clinics use the SC route. Treatment is delivered as a one-off injection. Patient sedation for the RAI injection is used variably between centres. Protective equipment is used to minimise radiation exposure to personnel when preparing and administering an injection (e.g. lead lined syringe guards and tungsten vial shields for the isotope vial, as shown in Figure 1).
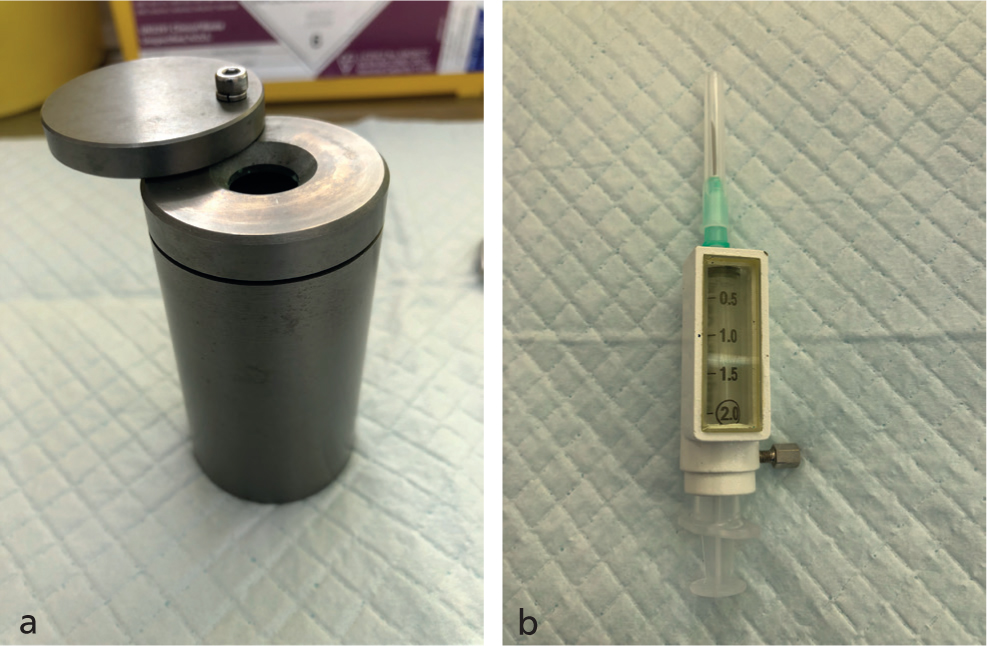
How is the dose of radioactive iodine determined?
This will vary between clinics. Two types of protocols are typically used, either fixed or variable dosing. With fixed dosing the clinic uses a single set dose of I131, meaning inevitably that some cats will be overdosed while some will be underdosed. Variable dosing enables the treatment to be titrated much more accurately to the cat's individual requirements to reduce the risk of under- or over-dosing and iatrogenic hypothyroidism. Scoring protocols have been published to aid the choice of dose, based on thyroid volume, T4 concentration and severity of signs (Mooney, 1994; Peterson and Becker, 1995). Limitations of these protocols exist, however, including the subjective aspects of assigning scores for example when evaluating the severity of clinical signs and estimating tumour volume without scintigraphy. Furthermore, the spectrum of disease treated currently is wider, with very mild hyperthyroidism and severe chronic hyperthyroidism seen more frequently and requiring doses outside of the original scoring systems. Some US centres titrate doses further using scintigraphy to measure the volume of hyperfunctional thyroid tissue and help predict RAI uptake (Peterson et al, 2019).
Mild degrees of hyperthyroidism can usually be treated with low doses of RAI (<111 megabequerels (MBq)). Cats with severe and chronic hyperthyroidism typically have large volume tumours and need much higher doses (e.g. 185–400 MBq). Thyroid tumours usually require significantly higher doses (900–1110 MBq), since the tumour cells are thought to concentrate RAI less effectively and large volumes of tissue may be present (Hibbert et al, 2009; Peterson et al, 2019). Not all centres are able to administer high-dose treatments.
How quickly does radioactive iodine treatment work?
The effects of RAI are rapid, with reduction in T4 often observed within 10 days (unpublished data); published studies report 80% and 95% of cats to be euthyroid or hypothyroid by 1 and 6 months respectively (Peterson and Becker, 1995). The initial peak effect is attributed to acute necrosis of thyrocytes and the more delayed effect because of apoptosis of cells that sustain sublethal DNA damage (Mooney, 1994).
What is involved for the clinic offering a radioactive iodine service?
Establishing an RAI service requires a clinic to obtain an Environment Agency Permit to hold open sources of radiation and to comply with the Health and Safety Executive Ionising Radiation Regulations 2017. The clinic has to appoint a radiation protection advisor and radiation waste advisor to help establish clinic protocols including patient dosing schedules; patient holding times; handling and waste restrictions; and waste management. Local radiation rules, systems of work, spillage contingency plans and spill kits are set up to minimise occupational exposure. Regular audits and staff radiation refresher training are expected by the Radiation Protection Advisor and the Environment Agency.
Are there any side effects of radioactive iodine treatment?
Radioactive iodine treatment is typically very well tolerated; acute radiation side effects are only rarely seen in cats treated with very high doses required for thyroid carcinoma or large volume adenomas. Radiation side-effects in this scenario may manifest as dysphagia, dysphonia, inappetence, dyspnoea and coughing. Longer-term iatrogenic hypothyroidism (transient or permanent) is a potential side effect, similar to thyroidectomy. The risk can be reduced by using the minimal effective RAI dose and by discontinuing all anti-thyroid medication or dietary therapy before administering RAI (Peterson, 2019).
Patient considerations
At what stage should radioactive iodine treatment be considered for a hyperthyroid cat?
Curative treatment options (RAI and surgery) should be considered at the time of diagnosis in all hyperthyroid cats, although it is appreciated that there are many cat and owner-based factors that need to be balanced to determine the most appropriate long-term treatment strategy for each individual cat (Boland et al, 2014; Higgs et al, 2014). The potential consequences of long-term treatment should be discussed at the outset. Long-term medical (and potentially dietary) management controls the excessive levels of circulating thyroid hormone but fails to prevent a thyroid tumour from growing. In chronic disease (e.g. >2 years), significantly greater numbers of cats have large to huge tumours, ectopic tissue, bilateral disease and suspected carcinomas, shown in Figures 2–4 (Peterson and Broome, 2015; Peterson et al, 2016). Malignant transformation from thyroid adenoma to thyroid carcinoma has been postulated and is a concern in cats with severe and chronic hyperthyroidism (Hibbert et al, 2009). Radioactive iodine treatment is therefore best considered early in the course of the disease, if viable for the owner and cat, when the tumour is smaller; at this stage, the cat will require less RAI and is less likely to have comorbidities. In particular, severe cardiomyopathy because of thyrotoxicity can develop with chronic and/or severe hyperthyroidism and may result in the cat not being suitable for RAI treatment (see case example in Figure 2).


Which cats are good candidates to refer for radioactive iodine?
The cat needs to be systemically reasonably well to receive RAI, as handling is limited immediately afterwards. Certain comorbidities might exclude the cat before referral, for example insulin-dependent diabetes, or congestive heart failure, which may be clinic dependent. Additionally, some centres may not take on cases with features that could suggest thyroid carcinoma (e.g. very high T4 levels (>300 nmol/L), recurrence after surgery, large goitres), due to their dosing capabilities. Specific indications where RAI should be considered are detailed in Table 1. Pre-treatment assessments are made to minimise the risk of potential problems occurring during the period when the cat cannot be handled a lot (or returned to a main hospital ward) and confirm patient suitability; the level of assessment varies between clinics from assessing renal biochemical parameters alone through to more thorough evaluation including evaluation of serum biochemistry, haematology, urinalysis, blood pressure measurement with cardiac, thoracic and abdominal imaging. A requirement to administer medication for other stable conditions e.g. amlodipine for hypertension, is not necessarily a reason to exclude a cat from RAI, provided a method of administering the medication with minimal handling can be established e.g. in food or treats, for when the cat is in the RAI treatment ward.
Table 1. Indications for radioactive iodine treatment in hyperthyroid cats
| Unilateral or bilateral thyroid disease | Owner desire for a curative treatment |
| Ectopic or intra-thoracic thyroid tissue | Owner desire to avoid daily medication or dietary restrictions |
| Poor compliance with anti-thyroid medication or dietary therapy | Preference to avoid anaesthesia |
| Adverse reaction to anti thyroid drugs | Middle aged-senior cats expected to live >1–2 years |
| Recurrence of hyperthyroidism post thyroidectomy | |
| Resistance to anti-thyroid medication or dietary therapy | |
| Severe and/or chronic hyperthyroidism | |
| Thyroid carcinoma |
The cat also needs to be able to cope well with hospitalisation, maintaining a good appetite voluntarily and using a litter tray away from home.
Does the cat need to be stabilised before I refer for radioactive iodine?
Not necessarily, this should be discussed with your local clinic. If there is a waiting time before the cat can be treated, medical or dietary management is advisable to address thyrotoxicity (and comorbidities should be stabilised if possible too). In some cases, it may not be possible to medically stabilise the cat's hyperthyroidism because of an adverse drug reaction; feeding Hill's y/d maybe an alternative interim strategy if an urgent appointment for RAI is unavailable. Withdrawal of medical or dietary therapy may be required 1–2 weeks before referral, if the cat has been stabilised, but timings vary between clinics.
Is concurrent chronic kidney disease an exclusion for treatment?
Again, this needs to be discussed with your local clinic and depends upon the severity of chronic kidney disease (CKD). Cats with advanced CKD (late International Renal Interest Society (IRIS) stage 3 or 4) are not usually treated with RAI, as a reversible treatment may be preferable to control the reduction in thyroid levels, specifically aiming to avoid the development of iatrogenic hypothyroidism and the risk of decompensating renal function. Concurrent CKD (typically IRIS Stage 1 or 2) may, however, be masked by hyperthyroidism at the time of diagnosis. Hyperthyroidism causes an increased glomerular filtration rate (GFR) by increasing renal blood flow; following treatment the GFR reduces and CKD may become apparent (by assessment of creatinine, urea ± symmetric dimethylarginine (SDMA) concentrations ± clinical signs). Unfortunately, currently there are no reliable predictors for this occurring. Unmasking of CKD (both non-azotaemic and azotaemic) is common post treatment of hyperthyroidism regardless of treatment type, occurring in 15–51% of hyperthyroid cats (Riensche et al, 2008; Williams et al, 2010a). Owners need to be counselled before RAI to understand the possibility of this happening and be clear that this is not a side-effect of the RAI acting directly on the kidney. Most cats, however, tend to progress by only one IRIS stage after receiving RAI (Harley et al, 2011) and can be managed successfully in the longer term, for example with renal prescription diets.
Specific treatment FAQs
What happens after the radioactive iodine treatment is given?
The cat will be housed (‘isolated’) in a designated radiation ward (‘controlled area’). Handling is limited to reduce staff exposure to radiation. Staff are required to wear protective clothing (gloves, gowns and boots) to minimise the risk of contamination from radioactive residues excreted in urine, faeces and saliva. Clinical waste has to be stored by the hospital and can only be sent for incineration after a set period of time has elapsed, to allow decay of the radioactivity. Release of the cat from the radioiodine ward may be determined by an established hospital holding period or based on the cat reaching a set level of radioactivity (when measured by a Geiger counter shown in Figure 5); the cat becomes less radioactive as it excretes the I131 and the isotope decays.

Why do holding times vary between centres?
There is no UK regulatory body dictating patient post-treatment holding periods. While centres must adhere to the Environment Agency and the Health and Safety Executive regulations, individual holding times depend on the clinic's radiation protection advisors' recommendations (and interpretation of risk based on available evidence), the clinic's dosing schedules and the dose of RAI administered.
What happens after the cat is discharged?
Handling and waste management restrictions must be followed by owners after discharge of the cat from a radioiodine unit, and these vary between centres; at the Feline Centre, handling restrictions are applied for 4 weeks post injection and waste management restrictions apply for 5 weeks post injection. Some cats therefore board for the full period of restrictions if owners are unable to comply with restrictions, for example because of young children being at home, owner pregnancy, or inability to keep the cat indoors, and owners may incur additional boarding charges.
Post treatment re-examination and reassessment of thyroid levels, renal biochemistry and blood pressure is recommended at 1, 3, 6 and 12 months, with most of the monitoring carried out by the referring vet. Monitoring is important to identify development of hypertension, iatrogenic hypothyroidism and unmasking of CKD. Improvement in thyrotoxic cardiomyopathy is expected within 6–12 months (see Figure 6a and b)

Post-treatment related FAQs
How worried do I need to be about iatrogenic hypothyroidism?
It is not uncommon for T4 concentrations to measure low at 4 weeks after RAI treatment, but most cats become euthyroid within 6 months, as residual thyrocytes start to produce T4 again. Post RAI monitoring is important to pick up overt hypothyroidism (defined as low T4 and increased TSH) (Peterson et al, 2017), particularly in cats with CKD, as it has been found that iatrogenic hypothyroidism affects the prognosis of cats with concurrent azotaemia because of CKD (Williams et al, 2010b).
The current recommendation is to measure T4 in conjunction with thyroid stimulating hormone (TSH), at a reference laboratory, to diagnose hypothyroidism; a feline TSH assay is not available, however the canine assay can be used. The combination of a persistently low T4 with a significantly elevated TSH (>0.9 ng/mL; Peterson et al, 2017) is consistent with iatrogenic hypothyroidism and allows sick euthyroid disease to be excluded. It is difficult to determine the true prevalence of iatrogenic hypothyroidism associated with RAI, however, because of variable dosing protocols and methods used to diagnose hypothyroidism in studies. Also, the thyroid hormones may continue to change over a prolonged period; changes in T4 and TSH concentrations have been reported to occur for up to 22 months post RAI (Peterson and Rishniw, 2017), while most studies have only followed cats post radioiodine for 6–12 months.
When is iatrogenic hypothyroidism treated?
Treatment may be advised if iatrogenic hypothyroidism develops in a cat with significant deterioration in renal function (development of or progression of azotaemia) or clinical signs of hypothyroidism (lethargy, weight gain, dermatological changes e.g. development of a greasy coat). In the absence of these problems some cats may only be monitored to determine whether they can spontaneously recover thyroid function.
Clinics offering RAI aim to minimise the number of cats developing iatrogenic hypothyroidism; however, the development of this should not be necessarily be considered a ‘treatment failure’, since the thyroid tumour will have been treated and the risk of large volume disease and thyroid carcinoma addressed. Management of iatrogenic hypothyroidism is considerably easier than treatment of chronic and severe hyperthyroidism.
How is iatrogenic hypothyroidism treated?
L-thyroxine is used (liquid formula or tablets; 50–75 μg PO BID) and is tolerated well. Ideally the medication is dosed on an empty stomach (or dose adjusted accordingly) and measurement of T4 and TSH repeated 3–4 hours post-pill 4–6 weeks later, aiming to normalise T4 and TSH concentrations.
Why is the cat still hyperthyroid after returning from radioactive iodine treatment?
This may be either a treatment failure (e.g. because of an inadequate dose for the volume of thyroid tissue or resistant thyroid carcinoma) or reflect a delayed response. In slow-responding cats (reported to be around 15–18% of treated cats (Finch et al, 2019; Peterson and Becker, 1995), a partial reduction in T4 will usually be seen by 1 month, with euthyroidism reached over the following weeks to 12 months. Depending on the level of T4, anti-thyroid medication or an iodine-restricted diet maybe restarted (if appropriate) or the patient may simply be monitored at 3-month intervals.
The cat is hyperthyroid again, has radioactive iodine treatment failed?
This does not indicate a failure of RAI if the cat became euthyroid or even hypothyroid after RAI. Feline RAI treatment does not aim to ablate all thyroid tissue, therefore if the cat has residual thyroid tissue post RAI, it has the potential to develop a new tumour in time and become hyperthyroid again. For example, if unilateral disease was present at the time of RAI treatment, it is possible for the cat to develop a new thyroid tumour in the contralateral gland at a later date or even theoretically in residual tissue in the first gland; bilateral disease is thought to occur in almost two-thirds of cases (Peterson and Broome, 2015). The same possibility would be associated with unilateral thyroidectomy; recently, recurrence of hyperthyroidism was described in 44% of cats even following bilateral thyroidectomy (Covey et al, 2019). Note that the time before recurrence can be protracted (e.g. >2 years) and may now reflect identification of hyperthyroidism at a younger age, meaning the cats live longer after the first RAI treatment. Repeat RAI treatment is well tolerated, however it is very rarely required.
Conclusions
Radioactive iodine treatment has the potential to cure a cat's hyperthyroidism. It is well tolerated with a high success rate and is becoming increasingly available for UK feline patients.

