In the ever-evolving global landscape, boundaries of various kinds are shifting or even dissolving altogether. This transformation finds its roots in a nexus of environmental, climatic, socioeconomic, geopolitical and behavioural changes. A striking illustration of this occurrence lies in the propagation and exchange of pathogens across diverse regions, ecosystems and countries. This phenomenon finds application in the importation of pet animal parasites, some of zoonotic importance, from enzootic areas to areas free of these infections among European countries. Parasites considered ‘exotic’ enter non-enzootic European countries via pet animals that serve as a Trojan horse, occasionally find suitable conditions to thrive locally and eventually become enzootic (Trees and Ridge, 2016).
Indeed, the number of travelling dogs and cats is steadily increasing. Pets travel with owners for holidays and tourism, when adopted and rehomed in a different country and in the form of trade from breeding facilities located in a different country than that of purchase. Pet movements have been facilitated because of discontinuation of quarantine restrictions and regulations that were previously in force – primarily for rabies control – in many European countries including Norway, Sweden, Finland, Ireland, the UK, Cyprus and Malta (Hunter, 2016). Over the years, quarantine was replaced in many European countries by the ‘Pet Travel Scheme’, which allows dogs and other pets to move between European Union countries and certain non-European Union countries with specific health checks, microchipping, vaccinations and documentation. However, the documentation that is needed in most countries (eg administration of praziquantel for Echinococcus spp. control or negative serological examination for Leishmania spp.) clearly does not cover all possible parasitic infections and leave room for the import of new parasites (Diakou, 2017).
Furthermore, veterinarians find themselves confused and unsure of their role and possible contribution in advising owners of adopted animals, the examinations they should perform on imported animals and the prevention they should advise for pets visiting enzootic areas with their owners (Wright et al, 2020). Likely because of their way of transmission, pathogenicity and/or relatively simple ways of detection and diagnosis, some parasitic infections are more frequently diagnosed than others when transported via pets in non-enzootic counties. Table 1 provides information on the common clinical manifestations, diagnostic tests and preventative measures for parasites which are commonly imported from one European country to another. These parasites include the protozoa Leishmania spp., Hepatozoon spp., the metazoa Dirofilaria spp., Thelazia spp., Linguatula spp. and Strongyloides spp. and ticks as vectors of important pathogens. Furthermore, there are parasites (such as feline lungworms) that, at the time of writing, are quite prevalent in some areas of the continent (especially southern Europe) but have not received analogous attention as imported parasites in non-enzootic regions. However, in order to prevent a rapid expansion of their distribution areas, there is merit in considering preventive examinations for their detection.
Table 1. Parasites most prevalently reported as imported from one European country to another
| Pathogen | Common clinical manifestations | Diagnostic tests | Preventive measures |
|---|---|---|---|
| Leishmania infantum | Skin and ocular lesions, lymphadenomegaly, splenomegaly, weight loss, muscular atrophy, onychogryphosis, epistaxis, anaemia, renal dysfunction | Serological tests, polymerase chain reaction | Repellents, immunostimulants, vaccination |
| Dirofilaria immitis | Cough, exercise intolerance, pulmonary hypertension, right heart failure, thromboembolism, syncope | Knott's test, antigen detection | Macrocyclic lactones, repellents |
| Hepatozoon spp. | Elevated temperature, lethargy, weight loss, anaemia, hyperglobulinaemia | Blood smear examination, polymerase chain reaction | Acaricides, repellents |
| Linguatula serrata | Rhinitis, cough, epistaxis, sneezing, olfaction impairment | Nasal excretion or faecal examination | Exclusion of raw viscera from the dog's diet |
| Thelazia callipaeda | Conjunctivitis, ocular discharge, keratitis, corneal opacity and ulceration, photophobia | Ocular examination | Moxidectin, milbemycin oxime |
| Ticks | Irritation, anaemia, skin inflammation and eosinophilic aggregation | Skin inspection | Acaricides, repellents |
| Strongyloides stercoralis | Diarrhoea, vomiting, anorexia, dehydration weight loss, cough, tachypnoea | Faecal examination (Baermann) | Avoidance of infected areas |
| Feline cardio-pulmonary nematodes | Respiratory distress, nasal discharge, weight loss | Faecal examination (Baermann) | Avoidance of free-roaming and preying in enzootic areas |
Leishmania infantum
Leishmania infantum is a protozoan parasite transmitted in Europe by sand flies of the genus Phlebotomus, causing canine leishmaniosis. Infection in dogs can remain subclinical, or develop into a severe, usually chronic and occasionally fatal disease (Maia and Cardoso, 2015). The clinical signs may develop a few months up to several years after infection (Koutinas et al, 1999; Solano-Gallego et al, 2011). The most common clinical signs are:
- Skin and ocular lesions
- Lymphadenomegaly
- Splenomegaly
- Weight loss
- Decreased appetite
- Muscular atrophy
- Onychogryphosis
- Epistaxis
- Anaemia
- Renal dysfunction.
Dogs are considered the main reservoir of the parasite, but humans and other mammals can also be infected. The disease is enzootic in the Mediterranean European countries (ie Portugal, Spain, France, Malta, Italy, Croatia, Albania, Greece and Cyprus) where the average seroprevalence in dogs reaches up to 25%, with peak seroprevalences that exceed 80% in some locations of Portugal, Spain and Italy (Maia and Cardoso, 2015).
Canine leishmaniosis is one of the most prevalently reported imported parasitic infections in European countries. This is likely a result of the factual high prevalence of the causative agent in the enzootic areas of Europe, rendering the infection of visiting or imported dogs particularly probable. In addition to this, its pathogenicity to pet animals and its zoonotic potential triggers the interest and concern of the scientific community, leading to screening examinations and reports. The UK, the Netherlands and Germany have all reported a high number of imported cases of canine leishmaniosis, while several cases have also been reported in Denmark, Sweden, Hungary and Serbia (Maia and Cardoso, 2015). An intriguing study was carried out in Switzerland, in which 291 dogs imported or returning from southern Europe underwent serological examination. Of these, 111 dogs (38%) tested positive for L. infantum (Mettler et al, 2005). Similarly, in Austria, leishmaniosis was the most commonly diagnosed parasitic infection in dogs originating from southern European countries (Leschnik et al, 2008). In some of these non-enzootic areas, sporadic cases in dogs with no travel history have also been reported, but it is unclear how the infection was acquired (Maia and Cardoso, 2015). It is worth noting that the number of diagnostic tests for Leishmania infections has increased in the recent years, even in non-enzootic areas, in an effort to improve screening and prompt diagnosis (Maia and Cardoso, 2015; Miró et al, 2022). In northern European countries, the sand fly vector is either absent or rare, resulting in limited local transmission of the disease. However, the increasing impact of climate change and the expanding distribution of the vector heighten the risk of disease establishment in these regions.
Taking the above into account, it becomes evident that implementing measures to proactively hinder the introduction of the infection into non-enzootic regions is of utmost importance. When a trip to an enzootic area is planned for a pet (eg for holidays), the owner should be given instructions for specific preventive measures that include the use of repellents (eg deltamethrin, permethrin, flumethrin), immunostimulants (domperidone), or even vaccination where such products are available (Maia and Cardoso, 2015). Vaccination combined with repellents and immunostimulants is especially advisable for extended stays in the enzootic area. Preventive examinations on returning home could be advised by the veterinarian, as none of these measures can guarantee that the animal will avoid infection. The type of test to be selected in this case depends, among other factors, on the time that lapsed between the day of return and the day of test, as seroconversion usually requires several months (4–22 months as evidenced in a survey that followed up naïve dogs exposed to transmission conditions; Oliva et al, 2006) to be detected with the common serological tests (ELISA, IFAT), while DNA detection in selected biological material may provide earlier results (Oliva et al, 2006). Detection of the parasite in selected biological material (lymph node aspirates, bone marrow) by cytology can directly demonstrate infection (Figure 1), but a level of examiner experience in recognising the parasite is needed. In many cases, cytology may show a negative result despite infection (Solano-Gallego et al, 2011).
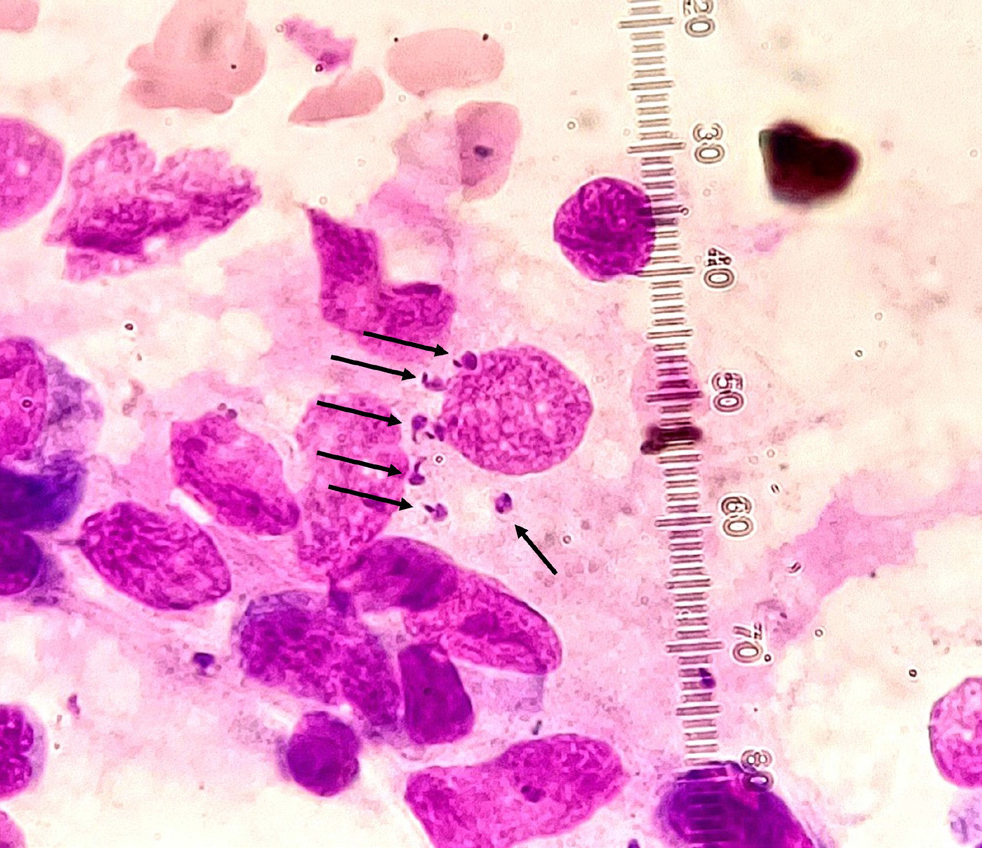
For dogs imported from enzootic areas, it is important that tests are performed before travel (ie in the country of origin) with the most sensitive method available, so that only non-infected dogs may enter non-enzootic areas. After import, repeating the test annually for at least 2 years and in any event of relevant clinical signs is also advisable (Mettler et al, 2005; Paltrinieri et al, 2010; Maia and Cardoso, 2015).
Hepatozoon spp.
Hepatozoon spp. are protozoan parasites which are transmitted to dogs and cats by the ingestion of an infected tick of various species (Baneth, 2011; Morelli et al, 2021a). Less common ways of transmission are through predation and vertically (transplacentally) (Baneth, 2011; Schäfer et al, 2022a). Following infection, the parasites spread through the bloodstream or lymphatic system, targeting organs such as the lymph nodes, spleen, bone marrow, lungs, kidneys and liver (Baneth, 2011). Eventually, the parasites invade white blood cells (neutrophils and monocytes) and develop into gamonts, the form that must be taken by the blood-sucking arthropods for the life cycle to be completed. Hepatozoonosis is often asymptomatic, but may also develop into a clinical, occasionally life-threatening, disease. The clinical signs and laboratory findings in hepatozoonosis include elevated body temperature, lethargy, weight loss, anaemia and hyperglobulinaemia. In case of high parasitaemia, leucocytosis (neutrophilia) is also observed (Baneth, 2011).
Hepatozoon spp. are enzootic in areas characterised by temperate, subtropical and tropical climates, where there is a high prevalence of tick species acting as vectors. In Europe, such areas are primarily adjacent to the Mediterranean basin (Baneth, 2011). For example, Hepatozoon spp. infection in cats (Schäfer et al, 2022b) was documented at a prevalence of 37.9% in Cyprus (Attipa et al, 2017) and 25.5% in Greece (Morelli et al, 2021a). Nevertheless, imported cases have been reported several times in northern European countries such as the UK and Germany (Attipa et al, 2018; Schäfer et al, 2022a; 2022b).
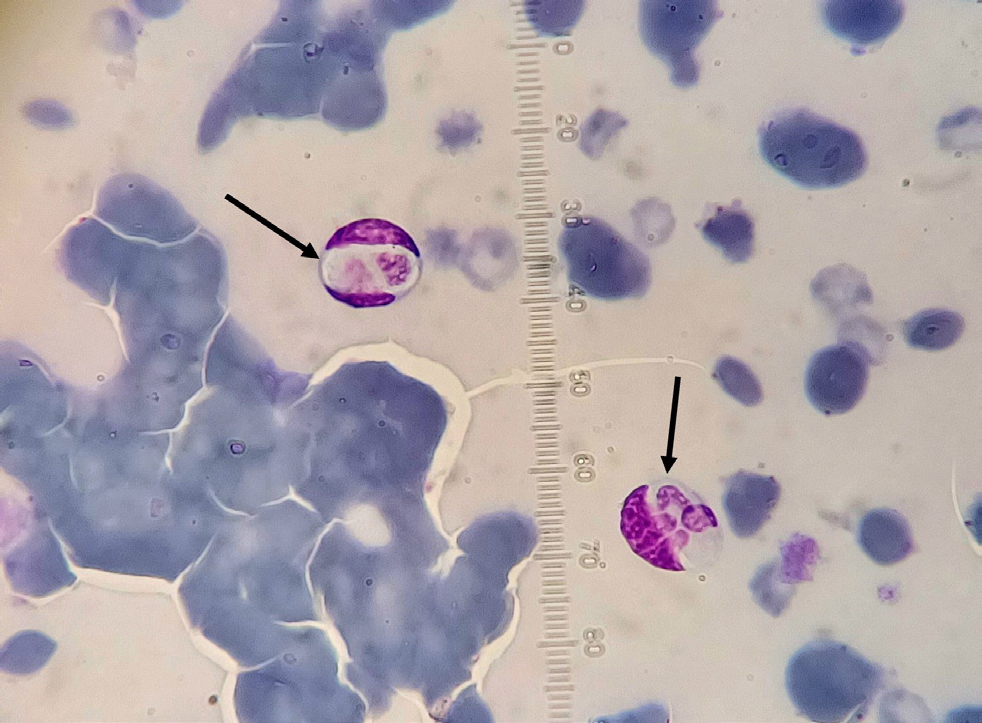
As many different species of ticks have a vectorial role for Hepatozoon spp. (including the common dog tick Rhipicephalus sanguineus, which has a wide distribution in Europe; European Centre for Disease Prevention and Control and European Food Security Authority, 2021), it is important to monitor any import of this pathogen in central and northern European countries that are considered free of infection. Accordingly, it is advisable that all imported dogs and cats are examined, regardless of the presence of clinical signs. The infection can be diagnosed by blood smear microscopic examination (detection of the characteristic gamonts in white blood cells; Figure 3), but polymerase chain reaction in peripheral blood and buffy coat is the most sensitive method for Hepatozoon spp. detection (Schäfer et al, 2022a). At the same time, the application of antiparasitic agents against ticks alongside examination for ticks and mechanical removal after outdoor activity is an additional prevention of parasite transmission and establishment in a new area.
Dirofilaria immitis and Dirofilaria repens
The filarial nematodes Dirofilaria immitis and Dirofilaria repens are transmitted by the bite of an infected culicid mosquito. Dogs are the main host of these parasites which can also infect wild canids and other carnivores including cats (Simón et al, 2012). Adult D. immitis inhabit the pulmonary artery clades and the right chambers of the heart, causing heartworm disease, a severe, potentially fatal condition. The infection is associated with pulmonary hypertension, heart failure, thromboembolism, syncope and sudden death. Adult D. repens live in the subcutaneous tissues, commonly causing nodules associated with local hypotrichosis, alopecia and pruritus (Albanese et al, 2013). Both parasites are zoonotic, with most human cases in Europe being caused by D. repens (Simón et al, 2022).
Dirofilaria spp. are enzootic in many European countries but are more prevalent in the south, where the prevalence of infection in dogs is particularly high in some areas (eg northern Italy, northern Greece, Canary Islands) (Montoya et al, 1998; Genchi et al, 2011; Diakou et al, 2016a). Imported filarial infections, caused by D. immitis and D. repens, have attracted the attention of scientists as these parasites show a clear distribution expansion tendency in Europe (Alsarraf et al, 2021; Morchón et al, 2022). Accordingly, many European countries report imported cases; in some of them, the infection eventually became enzootic (Pantchev et al, 2011; Fuehrer et al, 2021). For example, imported cases of both D. immitis and D. repens infections in Germany first appeared in the literature in the 1990s and the first autochthonous cases of D. repens infection were reported in the 2000s (Fuehrer et al, 2021). Furthermore, D. immitis and D. repens DNA was detected in mosquitoes collected in Germany, and as the climate of the country permits the life cycle to be completed, the scenario of the parasites becoming enzootic is realistic (Fuehrer et al, 2021). Similarly, descriptions of multiple imported cases, both in dogs and in humans have been reported from Austria, followed in a few years by infections with local transmission characteristics (Fuehrer et al, 2021). Several countries followed the same pattern (Fuehrer et al, 2021) and areas which were considered free of infection a few decades ago, are now characterised as enzootic, either for only D. repens or for both D. repens and D. immitis (Morchón et al, 2022).
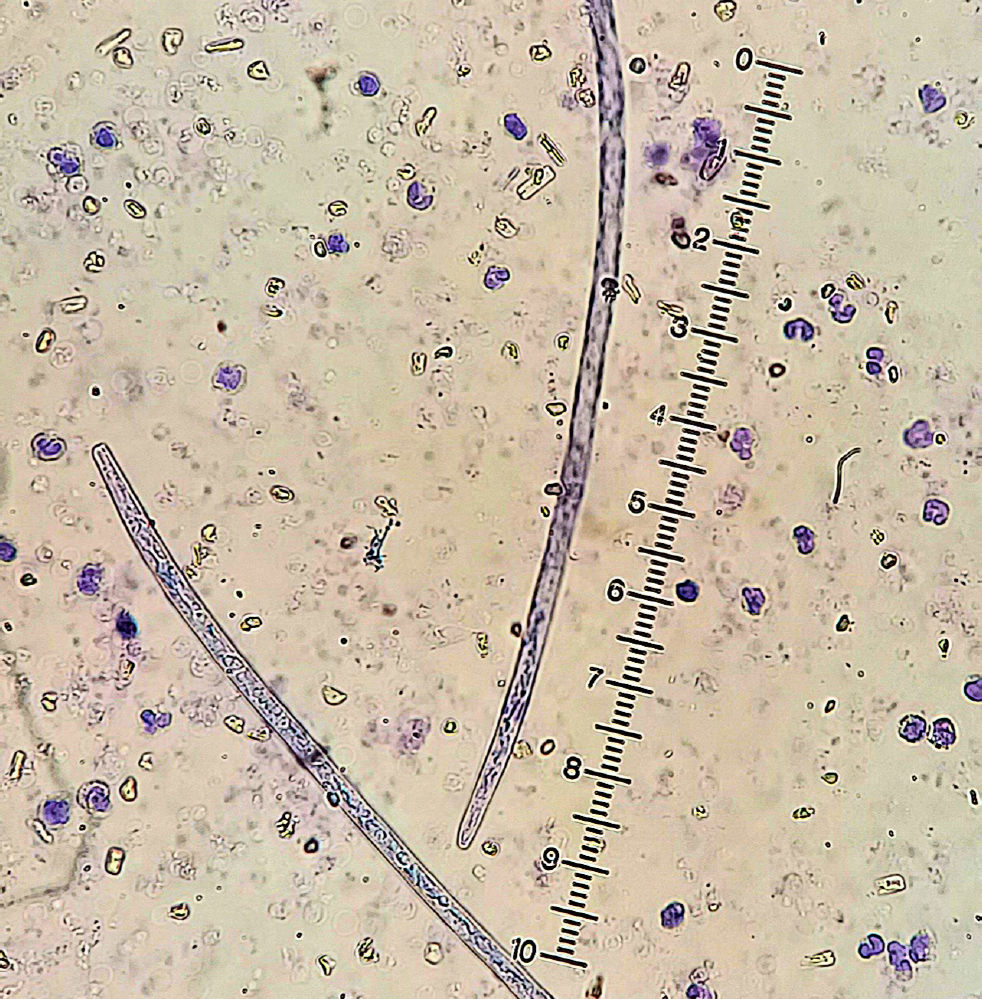
Prevention of Dirofilaria spp. infection is relatively simple and straightforward with the administration of macrocyclic lactones. Macrocyclic lactones are highly effective against the L3 and L4 stages of Dirofilaria spp., and they have a ‘reach-back’ efficacy, killing the parasite 30–60 days post-infection (Nolan and Lok, 2012; Diakou and Prichard, 2021). As such, dogs that visit an enzootic area should receive a preventive dosage of a macrocyclic lactone at the latest 30 days after their arrival to that area and continue monthly dosing until 30 days after their return home. Dogs that are imported from an enzootic area should be tested for heartworm, both with the modified Knott's test for the detection and identification of circulating microfilariae (Figure 2) and by serology for antigen detection (Diakou and Prichard, 2021). These tests should be repeated at least once, 6–9 months later, given that D. immitis has a long prepatent period and a dog may test negative early after its import despite being infected. In this case, and until the dog is confirmed negative, administration of repellents is also recommended, as circulation of microfilariae may start any time in between. Interruption of the life cycle by avoiding mosquito bites is crucial in an effort to prevent the establishment of the parasite in a new area. The American Heartworm Society has published a practical algorithm indicating the needed testing and treatment that aims to diminish any risk of heartworm transmission as a result of pet importation and travel (American Heartworm Society, 2018).
Linguatula serrata
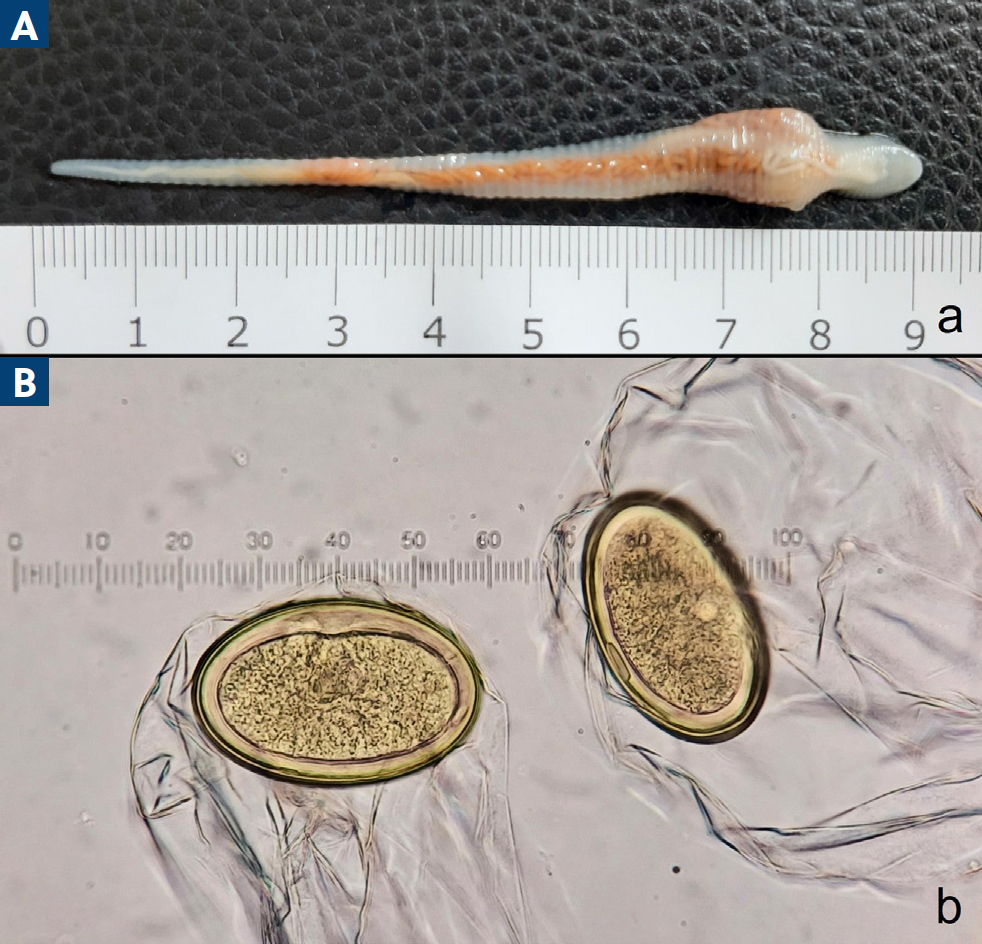
Linguatula serrata (the tongue-worm; Figure 4a) is a parasite of the order Pentastomida. Carnivores, especially dogs, are definitive hosts, and the adult parasites reside in the upper respiratory system, nasal cavities and paranasal sinuses. Female L. serrata produce eggs that are expelled via the oral, nasal or faecal route to the environment. Various herbivores serve as intermediate hosts, where the larvae and nymphs of the parasite develop in the internal organs (eg liver, lymph nodes, lung, spleen, heart). Dogs, cats and other definitive hosts get infected by ingesting raw, infected viscera (Tabaripour et al, 2019). L. serrata can also infect humans (Pan American Health Organization, 2003; Hajipour and Tavassoli, 2019); infection may develop into i) nasopharyngeal linguatulosis after consuming raw viscera and ii) visceral or ocular linguatulosis when parasite eggs are accidentally ingested (Pan American Health Organization, 2003; Koehsler et al, 2011; Sarmadian et al, 2021).
Linguatulosis in dogs may be subclinical, but usually is accompanied by rhinitis, nasopharyngitis, cough, epistaxis, sneezing and reverse sneezing, itching and olfaction impairment. Importantly, it has been suggested that this inflammation may trigger neoplasia development in adjacent tissues (Bordicchia et al, 2014). L. serrata is widely distributed in Asia, the Middle East and Africa, while its presence in many European countries has been documented in wildlife and dogs (Springer et al, 2018; Hajipour and Tavassoli, 2019). There have been several reports of canine linguatulosis cases, mainly in the UK but also in Germany and Finland, imported from Romania (most cases), Spain and Italy (Globokar Vrhovec et al, 2005; Mitchell et al, 2016; Villedieu et al, 2017; Springer et al, 2018; Thomas, 2018; Sievänen et al, 2021; Berberich et al, 2022; Macrelli and Mackintosh, 2022).
Because of its pathogenicity to pet animals and its zoonotic potential, linguatulosis should be promptly diagnosed. Diagnosis may be achieved by detecting the parasite eggs (Figure 4b) in routine, parasitological faecal examination. Furthermore, in case of compatible clinical signs, classical and reverse rhinoscopy may reveal the presence of the parasites in the nasal cavities. Often, diagnosis is reached by examination of an expelled parasite from the infected dog through sneezing or coughing. Canine linguatulosis treatment consist of nasal cavities flushing with saline solution and mechanical removal of parasites at rhinoscopy, when they can be reached (Ioniţă and Mitrea, 2016; Villedieu et al, 2017). Although currently there is no antiparasitic product registered against L. serrata, ivermectin (0.2 mg/kg) and milbemycin oxime (0.6 mg/kg orally) have proved successful (Ioniţă and Mitrea, 2016; Mitchell et al, 2016). Exclusion of raw meat, particularly viscera, from the dog's diet would prevent infection of dog's travelling in enzootic areas and of course, proper disposal of dog's faeces would prevent further spread of the parasite in new areas.
Thelazia callipaeda
Thelazia callipaeda is a nematode that follows an indirect life cycle, and lives on the eyes (under the lids and the nictitating membrane) of dogs, cats, wildlife and humans. Female parasites produce first-stage larvae (L1) which are discharged within lachrymal secretions. The zoophilic fruit fly Phortica variegata, which feeds on plant juices and lachrymal secretions of animals and humans, serves as the intermediate host in Europe. The fly ingests the L1 found in lachrymal secretions and allow them to develop into second (L2) and third-stage larvae (L3). Ultimately, infected flies transmit L3 to the eyes of the definitive host, where they grow into adults (Otranto and Traversa, 2005; Otranto et al, 2006). Thelaziosis may remain asymptomatic, but usually induces a range of clinical signs, such as conjunctivitis, epiphora, ocular discharge, keratitis, corneal opacity and/or ulceration and photophobia as a result of the mechanical irritation caused by the parasites and secondary bacterial infections (Otranto and Traversa, 2005).
Many southern and central European countries report autochthonous cases, while the UK and Belgium have reported only imported cases so far (do Vale et al, 2019). Because thelaziosis displays a seasonal pattern attributed to the intermediate host's activity (Otranto et al, 2004), dogs that travel in enzootic areas during the warm months of the year (eg summer vacation with owners) are expected to be at higher risk of infection.
Thelaziosis should be considered as a potential diagnosis for any case of eye-related clinical signs, particularly in animals that have visited areas with a known presence of the disease or were imported from such regions. Furthermore, it is crucial to examine the eyes for the presence of T. callipaeda as a standard part of clinical assessments for these animals. This examination should be conducted even in the absence of obvious eye signs to detect asymptomatic infections. Diagnosis is made by inspection (topical anaesthesia is required for some of the manipulations) of the cornea, the conjunctiva under the lids and the nictating membrane (Figure 5). The parasites appear as white nematodes 7.5–18.5 mm in length, with waive motility that increases with light intensity (Diakou et al, 2015; do Vale et al, 2019). Thus, diagnosis is often straightforward; however, when the parasites are not yet fully developed or if there are few of them, they may be difficult to observe (Pimenta et al, 2013). Flushing of the eye with saline and collecting the effluent to be examined under the stereoscope or microscope helps to overcome this problem.
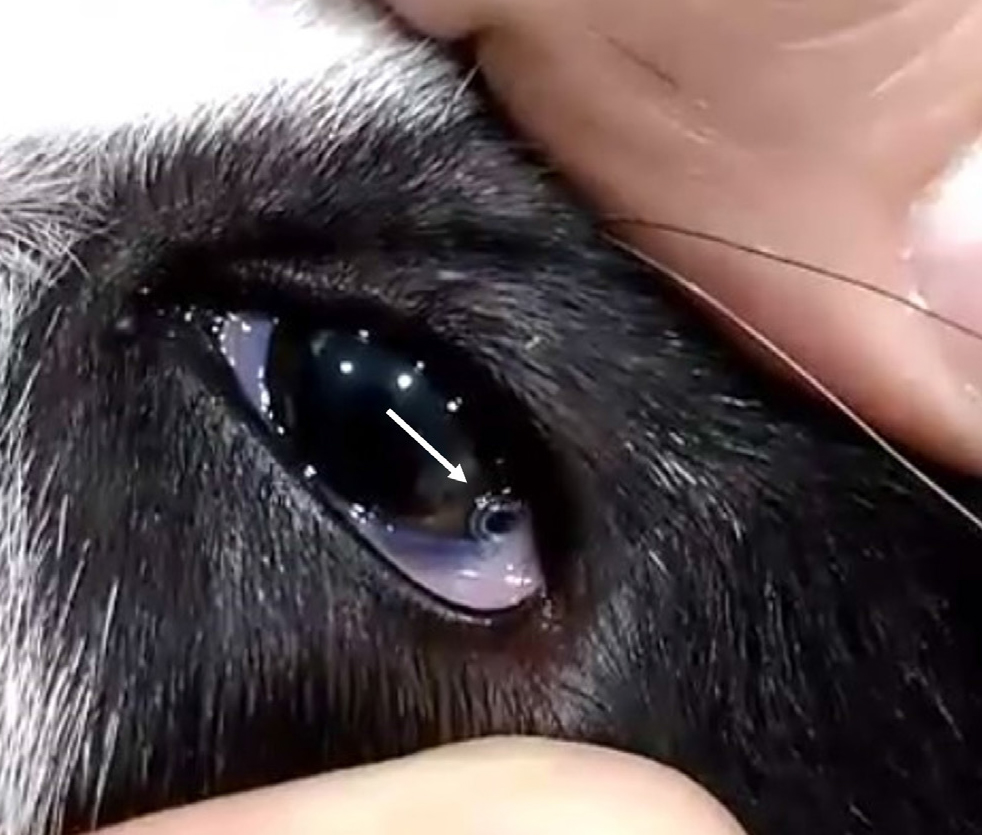
Treatment of thelaziosis is achieved by removal of all visible nematodes with atraumatic forceps under local anaesthesia. Moxidectin and milbemycin oxime are effective against T. callipaeda and should be administered after mechanical removal of the nematodes to eliminate any remaining parasites. Topical antibiotics may also be required in case of secondary bacterial infections (Diakou et al, 2015; do Vale et al, 2019; Marino et al, 2021).
Monthly administration of moxidectin or milbemycin oxime would prevent the infection (Diakou, 2017; Marino et al, 2021). This kind of prophylaxis would simultaneously prevent Dirofilaria spp. infection, thus representing a practical approach for covering traveling dogs that may be exposed to mosquitos and fruit flies in enzootic areas (Diakou et al, 2015).
Ticks and tick-borne pathogens
The factors at the core of parasite importation and expansion mechanism (ie increase of pet animal movements and climate change) find perfect application in forming the conditions for ticks and tick-borne diseases to transfer to and become established in new areas. Importantly, most tick-transmitted pathogens (eg Borrelia burgdorferi, Anaplasma phagocytophilum, Rickettsia spp., the agents of Lyme disease, human granulocytic anaplasmosis and spotted fever respectively) are a threat not only to companion animals health but also human health, causing clinical conditions that vary from a mild, influenza-like disease to severe illness with multiple organ involvement and death (Morelli et al, 2021b).
There are several descriptions of ticks and accompanying tick-borne pathogens being imported from one European country to another. It is worth noting that ticks, and consequently the pathogens they carry, are prevalent in the countryside and suburban areas commonly visited by pet animals (especially dogs) and their owners during holidays. Thus, the risk of importation of these agents after returning home is significant (Buczek and Buczek, 2020). Several investigations both in cats and dogs in touristic areas of Europe have shown that this is a realistic scenario that should be considered when owners travel with their pets, and when pets are rehomed in a different country (Diakou et al, 2017; 2019; Schäfer et al, 2019a, b; Diakou et al, 2022).
Accordingly, there are reports of non-endemic ticks entering countries; for example, the tick Hyalomma lusitanicum imported in the UK on a dog from Portugal and several cases of Rhipicephalus sanguineus from Mediterranean European countries (Buczek and Buczek, 2020). When the circumstances are favourable (climate change contributes to optimising conditions) and particularly when engorged females are transported, it is easy for new tick species to establish in new areas. For example, R. sanguineus – the most common tick species in Mediterranean areas and the vector of important pathogens (eg Ehrlichia canis, Anaplasma spp., Rickettsia spp., Babesia spp., Hepatozoon spp.) – can complete its life cycle indoors. Accordingly, heavy household infestation by R. sanguineus, after the importation of an infected dog from Spain to the UK, where approximately 100 ticks were found crawling on the walls and the furniture of the house, has been documented (Hansford et al, 2018). This means that this tick species can be easily established in new areas when imported with a pet animal (Szymański, 1979). Furthermore, there are reports of ticks that, although considered endemic, were transferred with dogs that acquired the infestation abroad. For example, the species Ixodes ricinus, Ixodes hexagonus, Ixodes canisuga and Dermacentor reticulatus were recorded in the UK in dogs returning from other European countries (Hansford et al, 2018; Buczek and Buczek, 2020).
In an effort to monitor the tick species in the UK, a Tick Surveillance Scheme was established in 2005. The Tick Surveillance Scheme has recorded an increase of ticks isolated from animals that have recently travelled abroad. Although the overall percentage of such specimens is low, the scheme is still of great importance as it demonstrates the flow of ticks and tick-borne pathogens imports in the country (Hansford et al, 2018).
Prevention of tick infestation for travelling pets is relatively straightforward as there is a great variety of effective veterinary products, both repellents and acaricides, that can protect the animals from such infestations and tick-borne pathogens. Owners should be advised to apply such products regularly and before travelling to areas where infestation risk is high.
Other parasites on the rise
Strongyloides stercoralis is a nematode parasite of the intestine that mainly affects dogs, humans and other primates. Infection happens when infective third stage larvae (L3) found in the soil penetrate the host's skin. Only female worms develop as parasites, and a free-living cycle, involving both male and female worms, also exists. The female parasites reproduce parthenogenetically in the intestine and larvae can migrate in many different organs, rendering this parasitism particularly pathogenic in immunocompromised hosts, that can experience severe, often fatal disease. Strongyloidosis in dogs mainly causes gastrointestinal (diarrhoea, vomiting, anorexia, dehydration, weight loss) and respiratory (cough, tachypnoea and sneezing) disorders (Schnyder et al, 2022).
In Switzerland, there have been several reports of cases diagnosed in the last decade, with the majority imported from Mediterranean countries, Eastern Europe and Germany (Basso et al, 2019; Schnyder et al, 2022). Similarly, three cases of canine strongyloidosis have been diagnosed in the Laboratory of Parasitology and Parasitic Diseases of the School of Veterinary Medicine, Aristotle University, in Greece. All the cases were puppies (3–8 months) imported from breeders located in Balkan countries (unpublished data). It could be that commercial dog breeding facilities, characterised by poor hygiene and overall conditions (known as ‘puppy mills’), are a hub of various dog pathogens and a source of S. stercoralis infection.
In the last decade, other parasites have been increasingly reported in southern European countries. Noteworthy examples are the cat lungworms, ie Aelurostrongylus abstrusus and especially Troglostrongylus brevior. The latter parasite is relatively ‘new’ in the sense that its prevalence and impact in cats have only recently been described, and its current distribution in cats includes southern and eastern European countries (Morelli et al, 2021c; Traversa et al, 2021). Furthermore, Angiostrongylus chabaudi, a nematode that primarily inhabits the pulmonary arteries and right chambers of the heart in European wildcats, holds limited significance for domestic cats. Although no confirmed cases of patent infection have been recorded thus far, it is essential not to disregard this parasite when compatible clinical signs are observed and for precise identification of larvae in cats' faeces (Diakou et al, 2016b; Traversa et al, 2021; García-Livia et al, 2023).
All three feline cardio-pulmonary nematodes have an indirect life cycle with terrestrial gastropods and small vertebrates acting as intermediate and paratenic hosts respectively (Dimzas et al, 2020), meaning free roaming cats in enzootic areas are in higher infection risk. These are also the kind of animals that are being adopted and rehomed in a different country. Therefore, it is important for cats imported from enzootic areas to be tested for cardio-pulmonary nematodes.
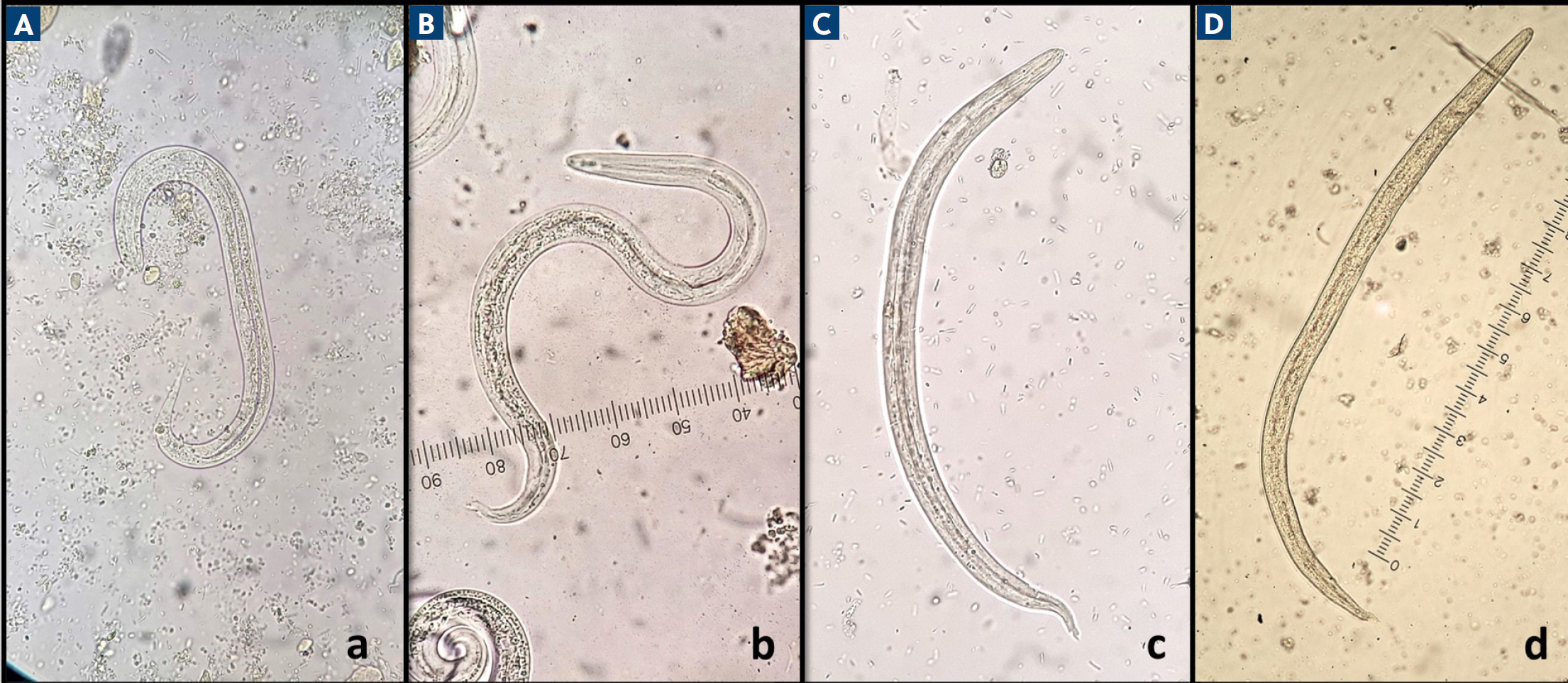
The diagnostic stage for S. stercoralis, A. abstrusus, T. brevior and is the first stage larvae (L1) in the faeces. The specific diagnostic test for the detection of these larvae is the Baermann method (Morelli et al, 2021c). Morphological identification of the larvae is feasible, as each one of these species displays different morphological characteristics (Figure 6). However, molecular confirmation through polymerase chain reaction may be required in some cases (Diakou et al, 2016b; Morelli et al, 2021c).
Conclusions
A number of parasites have expanded to new locations in Europe, primarily from southern and eastern areas, to central and northern countries. This expansion is facilitated by both climate change that favours intermediate hosts, and the long-distance travels and importation of pet animals. In some cases, local conditions favour the establishment of recently imported parasites. In this context, it is crucial to protect travelling animals with adequate preventive measures and to perform suitable preventive examinations in rehomed animals. Furthermore, veterinarians should be vigilant and aware of clinical presentations of common imported parasitic diseases so that they can suspect and set them in their differential diagnosis. Prevention of pet animals' infection in enzootic areas and timely diagnosis and treatment in imported or returning animals is the key for successful monitoring and control of parasites' expansion in Europe.
KEY POINTS
- Expansion of parasites in new areas is facilitated by climate change and travels or importation of pet animals.
- Local conditions may favour the establishment of recently imported parasites.
- Veterinarians should be vigilant for clinical signs of common imported parasitic diseases so that they can set them in their differential diagnoses.
- Prevention of infection when pet animals visit enzootic areas and timely diagnosis and treatment in imported or returning animals is the key for successful control of parasites' expansion in Europe.


