Leishmania spp. are intracellular protozoan parasites in the family Kinetoplastidae and they cause of a range of clinical disease known as leishmaniosis. Different Leishmania spp. are endemic in parts of Europe, South America, Africa and Asia. Canine leishmaniosis is predominantly caused by Leishmania infantum in Europe, which can also infect humans and cats. Transmission occurs primarily through the bites of infected phlebotomine sandflies. Other less frequent forms of transmission include blood transfusion, congenital and venereal routes, and possibly dog bites (Tabar et al, 2008; Naucke and Lorentz, 2012; Naucke et al, 2016).
In the mammalian host, Leishmania occurs as amastigotes (Figure 1) preferentially within phagocytic cells in the skin, bone marrow and visceral organs. In the gut of sandflies, Leishmania occurs as flagellated promastigotes (Figure 2). L. infantum infection occurs when a feeding sandfly deposits promastigotes into the dermis of the mammalian host. Following a local multiplication in dendritic cells and macrophages in the skin, the parasites disseminate to various organs via the lymphatic system and blood.
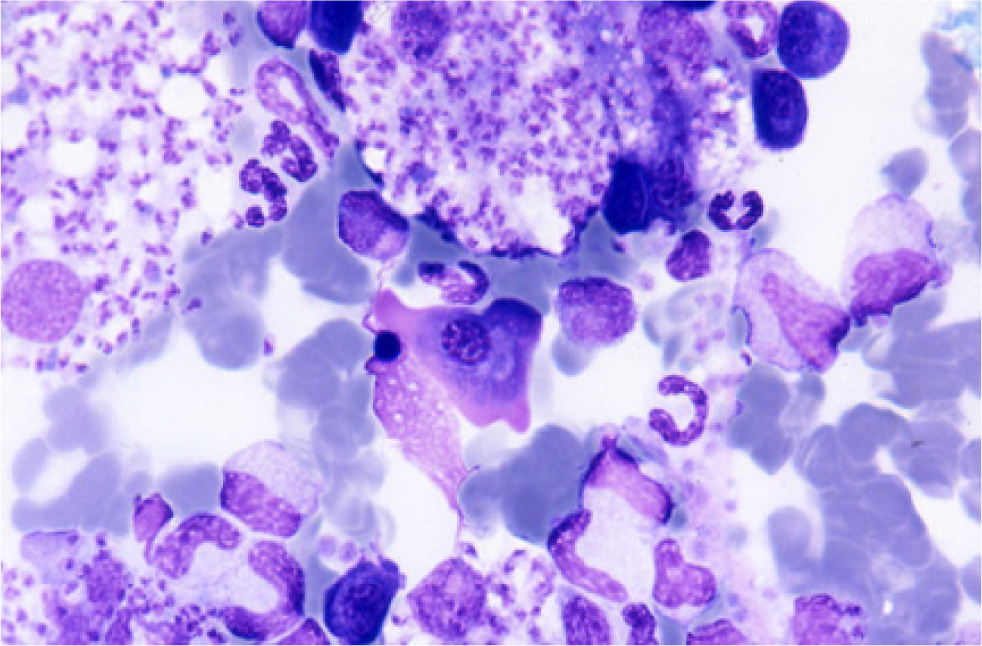
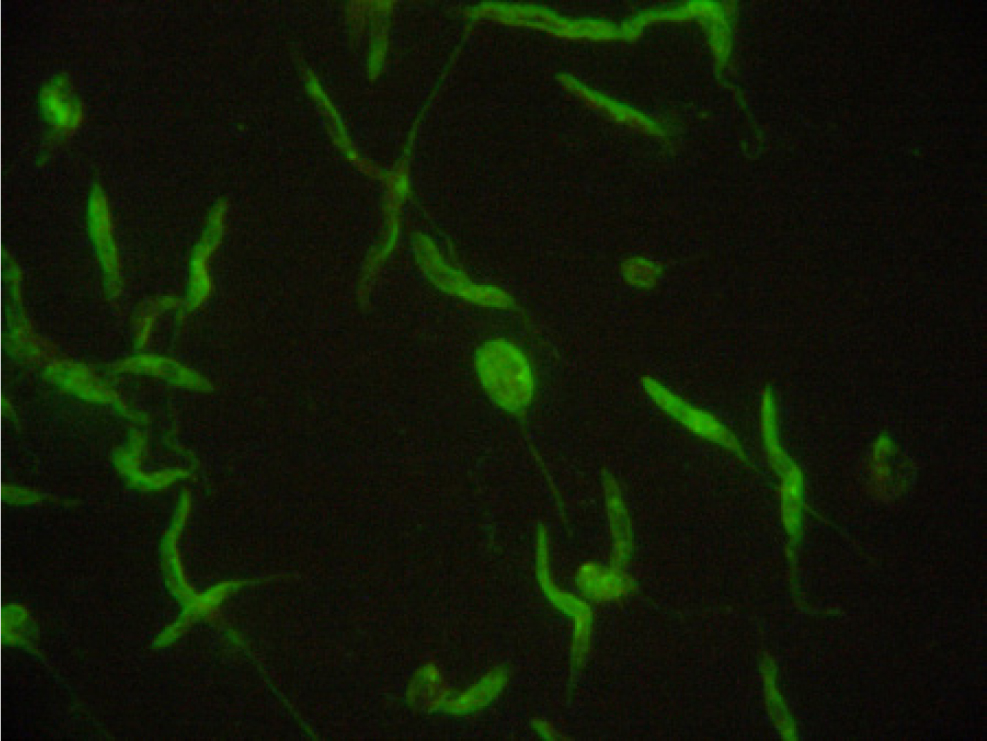
A wide spectrum of clinical disease presentations can result from infection, ranging from mild to severe, and can have potentially fatal outcomes from glomerulonepthritis, thromboembolism or thrombocytopenia. Leishmaniosis is classically chronic in nature and signs can progress from mild to severe over long periods of time.
Preventing animals' exposure to infection is desirable to reduce the risk of chronic and potentially fatal canine and feline disease.
Preventing the parasites from establishing in the UK will minimise infection and zoonotic risk. Increasingly, infections are being diagnosed in dogs rescued from Europe and imported into the UK. Rapid diagnosis of infected dogs is important for long-term management and to limit the risk of transmission.
Clinical presentation
The signs associated with Leishmania infection are immune mediated and in dogs commonly include lymphadenopathy, alopecia, dermatitis, hyperkeratosis, dermal ulcers, anorexia, weight loss, conjunctivitis, uveitis, epistaxis and anaemia. Atypical manifestations include mucosal, osteolytic and osteoproliferative lesions, splenomegaly and hepatomegaly. Many Leishmania infections are subclinical with highly variable periods occurring from initial infection to development of clinical leishmaniosis.
Clinical signs can develop months or years after initial exposure. Some breeds of dogs such as the Ibizan hound rarely develop the clinical illness, while other dog breeds are more susceptible to the disease. These include Boxers, Cocker Spaniels, German Shepherds and Rottweilers. In cats, the most frequent clinical manifestations reported are skin nodules, dermal ulcers, exfoliative dermatitis, mucocutaneous lesions, chronic gingivostomatitis and enlarged lymph nodes. Uveitis and other ocular lesions have also been reported. Other non-specific signs include weight loss, dehydration, lethargy, pale mucous membranes, fever, vomiting and diarrhoea.
Diagnosis
Clinical signs are only observed in a proportion of infected animals and, where clinical leishmaniosis is suspected, diagnostic tests should be interpreted in light of clinical signs and travel history. The travel might not have been recent and is easily lost if dogs have been rehomed or moved from one veterinary practice to another. The potential for vertical transmission also means that the travel history of parent animals should also be considered. A number of different diagnostic tests are available.
Direct diagnosis
The amastigote stage of Leishmania can be identified in Giemsa or DiffQuick stained smears obtained from superficial lymph nodes or bone marrow aspirates of clinically affected animals. The presence of amastigotes confirms infection but sensitivity is variable depending on the extent of parasitaemia and the number and quality of aspirates.
Histopathology.
Biopsies taken from clinically affected skin and lymph nodes or from bone marrow and spleen show signs of lymphoplasmacytic inflammatory reaction associated with macrophages infected with Leishmania amastigotes. Samples taken from multiple sites carry high sensitivity and specificity, but the absence of amastigotes does not rule out the possibility of infection.
Serological detection
Serological detection of specific anti-Leishmania antibodies is routinely used for diagnosis of leishmaniosis. Antibodies are detectable in dogs 6–8 weeks after infection. Indirect immunofluorescence assays and enzyme-linked immunosorbent assay tests are both commercially available. The presence or absence of antibodies does not confirm or rule out active infection but quantitative serology allows the size of the response to be measured. If this climbs over time in non-endemic countries, then it strongly suggests active infection and clinical leishmaniosis being present or likely to develop. These assays may give false-positive reactions with sera of dogs imported from endemic areas that have been vaccinated against Leishmania and in dogs infected by Trypanosoma cruzi, another protozoan that infects dogs in the Americas.
Polymerase chain reaction
A wide range of samples can be used for Leishmania infection by PCR. These include samples of lymph node, bone marrow, spleen, skin and conjunctival swabs. These all carry higher sensitivity than samples of blood, buffy coat or urine (Maia et al, 2009; Solano-Gallego et al, 2011). Many pets that have spent prolonged periods of time in endemic areas will be positive by PCR as sub-clinical carriers; a positive result in these pets does not mean that Leishmania is necessarily the cause of any presenting clinical signs. Negative PCR does not rule out infection. However, conjunctival swab PCR is non-invasive and useful in combination with serology when screening imported pets or attempting to confirm infection in dogs with low but positive antibody titres.
Treatment and long-term case management
Before starting treatment, kidney function should be assessed, especially proteinuria. Cases with high proteinuria and/or poor renal function carry a poor prognosis. Zoonotic risk should also be discussed but this is very low in the immune-competent owner and a human case of leishmaniosis contracted directly from an infected dog has never been confirmed. Owners undertaking treatment for their pets should be aware that a lifetime of treatment and monitoring is likely to be required at considerable cost. Treatment consists of allopurinol at 10mg/kg bid orally in combination with meglumine antimonite (100mg/kg intravenous or subcutaneously) every 24 hours for 3–6 weeks or miltefosine orally (2mg/kg every 24 hours for 4 weeks). The latter has the advantage in renal compromised patients of being metabolised solely by the liver. Gastrointestinal side effects however, from its use are common. Treatment with allopurinol alone may be required for up to 6 months after resolution of clinical signs to prevent relapse and some patients will need to remain on the drug indefinitely. Supportive treatment for hepatic and renal function may also be required. In cats, allopurinol is used alone as other drugs used for treatment in dogs are not well tolerated. The immune-regulating drug domperidone is licensed for treatment of leishmaniosis in continental Europe and dogs imported already on treatment may be being managed with a regimen including this drug. Evidence on the efficacy of domperidone is mixed but is an alternative to conventional treatment where none of the drugs required are tolerated.
Once treatment is initiated, routine monitoring is essential for long-term management. A full health check should be performed alongside comprehensive blood biochemistry and haematology to monitor liver and renal parameters, electrolytes, and blood proteins. Haematology is also important, particularly given the potential for Leishmania-positive dogs to become anaemic and thrombocytopenic over time. Analysis of urine sediment, specific gravity and protein/creatinine ratio is important to check renal function and also the potential development of xanthine crystals in animals on allopurinol treatment for the long term. This should then be repeated at least every 3 months, extending to every 6 months if the patient is responding well and xanthine crystalluria has not developed.
Quantitative serology should be repeated every 6 months to monitor the course of infection or when clinical relapse is suspected. Given the ongoing risk of xanthine crystalluria, stopping allopurinol treatment should be considered if clinical signs have resolved and antibody titres remain low. Stopping treatment increases the risk of clinical leishmaniosis flaring up, and a risk/benefit analysis should be made with the owner before deciding to stop treatment. If treatment is stopped, routine quantitative serology and urinalysis should be continued to give early warning of any deterioration in the animal.
Prevention in travelling pets
Disease prevention is essential for dogs travelling to endemic countries. The use of insecticides with ‘knock down’ capabilities to prevent flies from biting are required. Pyrethroids are the drug class of choice for fly ‘knock down’ and permethrin spot-on licensed preparations are available for dogs in the UK (Advantix [Elanco], Vectra 3D [Ceva], Frontline tri-act [Boehringer Ingelheim]. Scalibor (MSD) collar is long acting (up to 5 months) but efficacy will be reduced by frequent swimming and/or shampooing of the pet. Although it is not fully licensed for repelling sandflies, Seresto (Bayer) collar provides useful fly repellent efficacy in cats, when products containing other pyrethroids are not an option. The collar also has data on its licence now supporting prevention of Leishmania transmission in dogs. Treatment should be applied 1 week before travel as pyrethroids take time to reach full distribution and activity. No fly repellent is 100% effective and so other preventative measures should also be taken for dogs and cats travelling frequently or for long periods to endemic countries. CaniLeish (Virbac) and LetiFend (MSD), are commercial vaccines aimed at disease prevention and can be used in addition, but not as a substitute, to a fly repellent. Avoiding activity outside at dawn and dusk, sleeping upstairs and camping in breezy locations will also reduce sandfly bites because they are poor fliers.
Risks and prevention:
Despite the absence of the sandfly vector in the UK, the risk of Leishmania introduction and transmission are increasing because of a number of factors, including:
- Increasing distribution in Europe: the prevalence of L. infantum in the South of Europe, its seasonal expansion into central France and possibly Eastern Europe, and the arrival of clinical cases in travelled pets in non-endemic countries have all increased in recent years (Maia and Cardoso, 2015). This is the result of a combination of factors including increased movement of infected animals and climate change supporting spread of the sandfly vector. Likewise, the past few years has witnessed the emergence of an increasing number of diagnosed Leishmania infections in cats in endemic countries. The prevalence of leishmaniosis is predicted to continue to grow, creating more opportunities for the establishment of new endemic Leishmania foci in central and northern Europe (Pennisi, 2015).
- Potential establishment of the sand fly vector: while the sand fly vector is not currently thought to be endemic in the UK, several routes of introduction of the parasite into the UK are possible including vehicles, clothing and luggage. This may have occurred in one UK case where the infected animal had not been abroad, but the owners had (Wright & Baker, 2019). Climate change is also making conditions for the sand fly and leishmania establishing in the UK more favourable (Koch et al, 2017).
- Increased numbers of travelling dogs: the numbers of dogs increasing on the pet travel scheme is increasing year on year (Table 1) and L. infantum endemic countries such as Spain, Greece, Italy and Southern France remain popular tourist destinations for people taking their pets abroad. Therefore, there is an increased risk of pets from the UK being exposed to L. infantum.
- Increased importation of Leishmania-positive dogs: in the UK, increasing numbers of L. infantum infections in travelled and imported dogs have been documented, creating a potentially significant reservoir of infected animals. Reviews of veterinary records have indicated a prevalence of between 0.007 and 0.04% of dogs confirmed with clinical leishmaniosis and, given the difficulties in diagnosis and sub-clinical carriers, then this is likely to be a significant underestimate (Shaw et al, 2009; Silvestrini et al, 2016). If undiagnosed, positive dogs may unknowingly be used for breeding or as blood donors.
- Unexplained horizontal transmission: infected dogs in the UK also pose an unquantified transmission risk. Horizontal transmission has been recorded in the UK in the absence of the sand fly vector (McKenna et al, 2019; Wright and Baker, 2019) and without the dogs in question having been used for breeding or as blood donors. It is currently unknown how these infections occur but it has been speculated that transmission may occur through dog bites (Karkamo et al, 2014; Daval et al, 2016) or through mechanical fly transmission, as has occurred with Trypanosoma vivax in South America (Dávila and Silva, 2000). If large numbers of dogs are kept together in the UK or are kept in areas densely populated with canines, then the odds of transmission by these routes will increase. This is a concern, because many of the Leishmania infected dogs in the UK are in urban areas of the south of England, where large numbers of positive dogs socialise together (Shaw et al, 2009; Silvestrini et al, 2016).
Table 1. Number of dogs imported to the UK annually on the pet travel scheme and Balai directive
| Year | Number of dogs imported via the Pet Travel Scheme | Number of dogs imported via Balai | Mixed dog/cat |
|---|---|---|---|
| 2011 | 85 299 | 4625 | |
| 2012 | 139 643 | 5555 | |
| 2013 | 152 075 | 3596 | |
| 2014 | 155 444 | 26 399 | |
| 2015 | 164 836 | 28 344 | |
| 2016 | 275 876 | 34 012 | |
| 2017 | 287 016 | 39 998 | 336 |
| 2018 | 307 357 | 37 144 |
This combination of factors makes preventative measures essential to minimise the risk of Leishmania establishing itself in the UK.
Health checks and screening in imported dogs
Imported dogs with a history of travel presenting with relevant clinical should be tested for evidence of L. infantum infection. Screening all imported dogs from endemic countries by quantitative serology and/or PCR is also useful, both for individual case management and to ensure positive dogs are not used for blood transfusion or breeding. Because Leishmania infections are known to have long incubation periods and no test is 100% sensitive, owners should be advised to re-test their imported pets for infection 6 months after importation and, ideally, for at least 2 years after importation (Paltrinieri et al, 2010).
Neutering positive dogs can prevent congenital or transplacental transmission. as well as screening for blood donors. Serology would be a relatively sensitive and specific test to look for possible exposure to infection before dogs are used as blood donors. Although a lack of clinical signs and travel history will reduce the risk of transmission, the long incubation periods before disease manifests means that clinical signs may not be present in infected dogs and travel history can be potentially lost.
Conclusions
Despite the availability of treatment and preventive measures, clinical infections with Leishmania will likely remain common in dogs, cats and humans. UK cases are also likely to increase as the trend for importing rescue dogs continues to grow. Therefore, veterinary professionals need to be vigilant in these dogs but also consider the possibility of Leishmania infection in cats, as well as in dogs that have been in contact with Leishmania-positive animals and animals bred from parents with a history of travel. More information on leishmaniosis is available at http://www.leishvet.info and http://www.esccap.org.
KEY POINTS
- Leishmaniosis is a serious vector-borne disease, which is expanding its distribution across Europe.
- Increasing numbers of rescue dogs are being imported into the UK from endemic countries, with increasing instances of Leishmania infantum entering the country as a result.
- Treatment of clinically infected dogs should only be undertaken after prognostic assessment for the patient and when the owner understands the potential lifetime costs and commitment involved.
- The zoonotic risk to individuals in the absence of the sand fly vector is low.
- Although the sand fly vector is not currently present in the UK, other means of transmission can occur including venereal, blood transfusion, transplacental and dog bite routes.
- Screening of imported dogs and vigilance for relevant clinical signs is essential for case management and to minimise the risk of transmission.


