Trematodes (phylum: Platyhelminths), with their colonies, comprise a division of labour among soldiers and pools of reproductive individuals, defined as the specialisation of individuals to perform some tasks because they are unable to perform others (Duarte et al, 2011). When we think about specialised behaviour, polymorphism and caste formation, we immediately think of social insects (Michener, 1969). One of the most extreme cases of specialisation being the formation of a reproductive caste and a non-reproductive caste, which are diametrically opposite not just in behaviour but also morphologically (Oster and Wilson, 1978). In groups of individuals that are separate or mobile, this had only been described in insects (Korb and Heinze, 2008; Queller and Strassmann, 1998), snapping shrimp (Duffy, 1996), sea anemones (Ayre and Grosberg, 1996; Francis, 1976), and mole rats (Jarvis, 1981; O'Riain et al, 2000). However, it has also been described experimentally in the first intermediate host of digenean trematodes (Hechinger et al, 2010; Leung and Poulin, 2011; Miura, 2012).
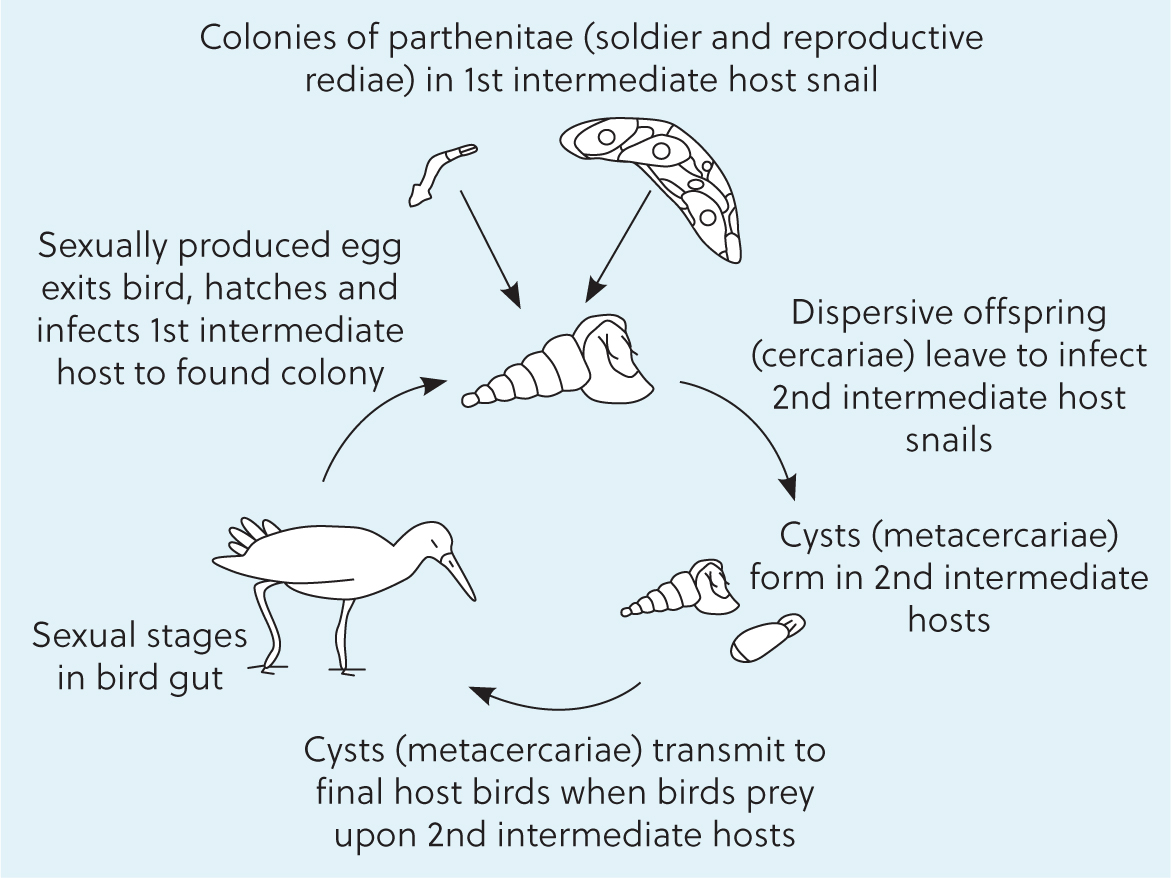
Parthenitae is the medical term used to describe the different larval stages present in the gastropod during the parasite's life cycle, which are, miracidium, mother sporocysts, and rediae (Dickerman, 1945). These display repeated clonal reproduction in gastropod hosts, following infection by a single, diploid larva that was sexually produced by a hermaphroditic parent. Parthenitae produce more and disperse offspring (cercariae), each emerging from a single unfertilised cell (Galaktionov and Dobrovolskij, 2003). These trematode larval stages are able to block host reproduction and can comprise 16–41% of the gastropod's soft tissue mass (Hechinger et al, 2009). These can also form a colony, by cohabitating, replicating, and operating the stolen host body (Kuris and Lafferty, 2005). Antagonism between heterospecific trematode species that contemporaneously infect the same gastropod was described many years ago (Lim and Heyneman, 1972; Lie, 1973). However, this was only recognised as a dominance hierarchy. With species of rediae that have mouth parts dominating and actively preying upon parthenitae of subordinate species, which often have smaller mouths or lack mouths altogether, no actual division of labour was recognised.
While studying the ecology of marine snail habitats, Hechinger and his colleagues at the University of California found two very distinct rediae within colonies of different trematode species in diverse locations across the globe (Hechinger and Lafferty, 2005; Hechinger et al, 2007). Figure 1 illustrates this observation. There were two type of morphologies: a primary one, which had the features of replicating rediae and secondary ones, with characteristics of immature reproductive rediae, with these traits having been already described in the literature (Galaktionov and Dobrovolskij, 2003). However, there were some major differences in the secondary rediae, which led to thoughts that these could be more than just immature reproductive rediae. These did not actively replicate, they were small and thin, had massive muscular pharynxes and guts, were very active, and were found to be very common in regions of the gastropod where new trematode invasions could occur (Figure 2). Interestingly, the trait that was very particular about these secondary rediae, was that, on several occasions, they were found aggregating and attacking invading heterospecific trematode parthenitae (Hechinger and Lafferty, 2005; Hechinger et al., 2007). This led to several studies that reported colonies of trematode species with an actual division of labour: a large reproducing caste specialised in reproduction, producing offspring (cercariae) in a clonal form and, in addition, a small non-reproducing caste that had morphological and behavioural differences from the former, as individuals of this caste appeared to be specialised for defence against hetero or conspecific colonies trying to establish within the same gastropod. This trait was found to be similar to the caste organisation of many social insect colonies (Hechinger et al, 2010; Leung and Poulin, 2011; Miura, 2012).
In parallel with colony organisation of social insects, demographic traits such as the ratio of the small, non-replicating individuals compared to the large, replicating individuals, are expected to vary and adjust to local conditions. The optimal caste ratio theory (Oster and Wilson, 1978), which predicts that with highly specialised castes, variation in ratios will be associated with the overall success of the colony. Consequently, caste ratios should be adaptive and optimised according to various conditions.
A study carried out using a trematode, reported to use a division of labour, such as Philophthalmus spp. (Leung and Poulin, 2011), and using the common New Zealand mudsnail, Zeacumantus subcarinatus, provided evidence that the optimal caste ratio theory used in social insects, can also explain caste structure of trematode colonies with this division of labour. The small to large caste ratio in colonies of this trematode species was significantly different among geographically distinct colonies in a way that exactly matches the prediction concerning the selective pressures from the local risk of competition (Lloyd and Poulin, 2014a). This shift in caste ratio in favour of a higher number of soldiers was also described in cases of both perceived threat of competition and actual competition.
Establishment of the competitor may not be prevented, because the host is immunologically compromised, but it would prime the colony for defense against an imminent threat. Moreover, in the face of actual competition, Philophthalmus colonies were shown to invest in more soldiers in order to accelerate the reduction in colony size of a competitor. Furthermore, investments in defense, did not translate in a fitness cost, as the trade-off between numbers of soldiers and dispersing offspring (cercariae) was only noticeable when secondary rediae became too numerous (Lagrue et al, 2018). This lends further support with regards to parallel adaptations of colonial organisms against competitive threats in phylogenetically disparate organisms. In situations where there are mixed genotype infections, as it has been identified in the case of Philophthalmus spp. in their first intermediate host Zeacumantus subcarinatus, intraspecific competition does take place. In 5.12% of the infected snails, a mixed genotype infection was found, but the total number of rediae was not significantly different between single or mixed infections. Thus, if no intraspecific competition took place, each individual colony in mixed genotype infections would reach approximately the same number of individuals, irrespective of the presence of a conspecific colony, and the total number of rediae would then be at least twice in snails with multiple infections. However, the authors reported that the colonies in the mixed infections had less rediae per colony in comparison to single geno-type infections (Lloyd and Poulin, 2014b).
There is a hypothesis that intraspecific competition can actually be stronger than interspecific competition. Studies looking into interspecific competition between Maritrema novaezealandensis and Philophthalmus spp. found that the number of rediae was not significantly different between single and mixed species infections. However, the infection of M. novaezealandensis did have a negative impact on dispersive offspring output (cercariae) (Keeney et al, 2008). Nevertheless, intraspecific completion did not influence small-to-large caste ratio in Philophthalmus spp. colonies, suggesting that division of labour in this species does not play an important role in competition against conspecific co-infections. This is not entirely unexpected, as secondary rediae (soldiers) in two other philophthalmid species do not engage in attacks against conspecific rediae, but do attack heterospecific rediae when reared in an in vitro culture (Miura, 2012). Nevertheless, rediae soldiers have been reported to identify and attack conspecific individuals in another trematode species from a different family (Hechinger et al, 2010).
Another aspect that has also been studied is the impact of ocean acidification on not just caste ratio, but on offspring output in parasitic trematodes in their first intermediate host. In this era of global warming and environmental consciousness, it is predicted that low pH in the ocean will affect the structure and function of all marine ecosystems, including marine parasites. The first study using a realistic ocean acidification simulation system to determine the effect on parasites reported that there was a significant decrease in the longevity of cercariae for different trematode species under acidic conditions, as well as a reduction in metacercarial survival (MacLeod and Poulin, 2015). Transmission success was also measured under ocean acidification conditions (Harland et al, 2015).
With the same simulation system, the effect of long-term exposure to acidic conditions on caste ratio was explored in gastropod hosts for two philophthalmid species (Parorchis spp. and Philophthalmus spp.) known to have a division of labour. The results were found to be species-specific and not always unimodal. For Parochis spp. reduced pH affected the colony composition by decreasing the number of secondary (soldiers) rediae. Therefore, the stress caused by low pH conditions may alter investments towards reproduction. In the case of Philophthalmus spp. the caste ratio was higher at pH 7.8 than at higher or lower pH, suggesting a shift in the direction of investing in colony defense under mild external stress, but towards reproduction under more extreme stress. This shift to reproduction also translates into an increase in cercarial output, in long-term exposure to low pH conditions. Another trait that was reported was the decrease in metacercarial size after 60 days of exposure to the most acidic condition, suggesting a trade-off between the quantity and the quality of offspring produced. This could have been the result of a decrease in the availability of energy for the parasite colony, as the host needs more to maintain physiological processes in this adverse condition. This reduction was not seen in Parochis spp., suggesting species-specific responses to ocean acidification (Guilloteau et al, 2016).
The caste ratio effect on the reproductive output was also studied. It was found that there was a clear fitness benefit of maintaining the secondary rediae in the presence of a competitor. Looking at a much longer time scale, the higher reproductive output of colonies with a high proportion of soldier rediae in competitive situations means that adjusting caste ratios according to competitive pressure over an evolutionary timeframe should be possible, given that this selective pressure is constant, and populations are isolated enough. If the variation within a population should exceed the temporal fluctuations of competitive pressure, selection should favour adaptive shifts of caste ratios on an evolutionary time scale (Kamiya and Poulin, 2013).
Environmental factors, such as shell damage in gastropod hosts can influence the caste ratio in trematode species as well, as well as the host–parasite interactions that could derive from there on. A study using the Philophthalmus spp. model reported that shell damage, trematode infections and the combinations of both, affected life-history traits of the common New Zealand mud snail Zeacumantus subcarinatus. Snail survival was significantly affected by shell damage and trematode infections. However, it was not the actual damage to the shell that increased this mortality, but the damage that caused by being exposed, probably owing to secondary bacterial pathogens. Interestingly, there were higher mortality rates of uninfected snails (both undrilled and patched) in comparison with trematode-infected snails (MacLeod et al, 2018). This could have been a result of fitness costs between survival and reproduction in uninfected snails, something that parasite-castrated snails do not have to worry about. Also, parasites in general may tamper with the amount of resources taken from its host in response to the stress and health levels to avoid over-exploitation and potentially killing its host (Lloyd and Poulin, 2013). Furthermore, no significant differences were found when comparing the degree and haste of shell repair among snails from all infection statuses. Uninfected snails also showed significantly lower shell growth than trematode-infected snails, regardless of whether the shell damage was patched right away or if it was left exposed (MacLeod et al, 2018). This has been reported before as the energy made available by parasitic infection used for growth (Minchella, 1985). The proportion of soldiers was found to be much higher in snails with concurrent infections. In addition, because the cost of shell damage with possible secondary infections is so high for the gastropod, and consequently their parasites, parasites with these primary and secondary castes then need to invest heavily in producing soldiers to defend the colony, as well as their host, from bacterial and fungal pathogens.
To date, the only species in which there is evidence describing the existence of a reproductive division of labour involving a soldier caste among the parthenitae of trematode parasites, belong to two closely related families, the Echinostomatidae and the Philophthalmidae (superfamily: Echinostomatoidea) (Hechinger et al, 2010; Leung and Poulin, 2011; Miura, 2012; Nielsen et al, 2014; Mouritsen and Halvorsen, 2015). However, all these species occupy high positions in trematode interspecific dominance hierarchies. Moreover, the two species recognised as not having a soldier caste belong to the Echinostomatidae as well (Garcia-Vedrenne et al, 2016). The non-reproductive caste of soldier rediae has also been described in four trematode species of intermediate dominance, all belonging to the family Heterophyidae (superfamily: Opisthorchioidea): Euhaplorchis californiensis, Phocitremoide ovale, Pygidiopsoides spindalis, and Stictodora hancocki (Garcia-Vedrenne et al, 2017). Throughout all species, the primary (reproductive) rediae were 18–32x larger than secondary (soldier) rediae. Something to note was that the heterophyid soldiers examined here lacked the collars and locomotory extension that have been relatively prominent in soldier rediae in the Echinostomatidae and Philophthalmidae (Figure 2), suggesting that these features are not a requirement to be able to function as a soldier. This provides evidence that a soldier caste is more widely spread throughout the trematode phylogenetic tree and among species in dominant hierarchies than was previously known. Furthermore, this suggests that there was an independent evolution of soldiers, or conversely its loss, multiple times within the class Trematoda. This also indicates that selection for a soldier caste may be much more common amid digenean trematodes (Garcia-Vedrenne et al, 2017).
This newly described aspect of the biology of trematodes could have a very important implication for possible alternatives of biological control in species infecting companion animals, such as Nanophyetus salmincola, vector of Neorickettsia helminthoeca, the cause of salmon poisoning in dogs, Paragonimus kellicoti, Cryptocotyle lingua, Alaria canis, Platynosomum illicens, Metorchis spp. and Eurytrema procyonis. This could also translate into a public health matter, as blood flukes (Schistosoma haematobium, S. mansoni, and S. japonicum), lung fluke (Paragonimus westermani), and liver fluke (Clonorchis sinensis), among others, infect humans. If it is discovered that these organisms also have castes within the snail host, then a controlled release of a species whose soldier caste could outcompete them or are higher in the dominance hierarchy, could translate into effective biological control. However, the author understands that releasing parasites into bodies of water, even though they are virtually harmless to pets or humans, could pose a possible setback on this approach, as it could have some negative political and even environmental connotations. Having said that, further research is warranted in order to study its potential applications.
KEY POINTS
- There is an active division of labour in trematode colonies.
- Specialisation into reproducing and non-reproducing castes has been described experimentally in the first intermediate host of digenean trematodes.
- Non-reproducing individuals appear to be specialised for defence against hetero or conspecific colonies trying to establish within the same gastropod.
- The longevity of trematode offspring (cercariae) can be affected by factors such as pH.


