Gastric dilatation volvulus (GDV) is a relatively common emergency presentation in small animal veterinary practice. Typically, deep-chested large and giant breed dogs are affected. The exact cause of the condition is unknown (Monnet, 2003), however many risk factors have been identified. Risk factors include (Glickman et al, 1994; Glickman et al, 2000; Beck et al, 2006; Pipan, 2012):
- Having a first-line relative that has had GDV
- Being underweight relative to the breed standard weight
- Increasing age
- Splenomegaly or having had previous splenic torsion
- Use of a raised feeding bowl
- Being an anxious dog
- Feeding food particles <30 mm
- Exercising shortly after feeding
- Feeding frequency of once a day.
The reported mortality rate for dogs with GDV ranges from 3.2–33.3% (Glickman et al, 1998; Belch et al, 2017), with more recent reports demonstrating improved survival rates.
Dogs presenting with GDV will typically exhibit signs of restlessness, retching and unproductive vomiting, distension of the cranial abdomen, ptyalism and respiratory compromise. Some dogs present collapsed. Clinical examination findings are consistent with hypovolaemic shock, including pale mucous membranes with prolonged capillary refill time, tachycardia and weak peripheral pulses (Williams, 2015). Cranial abdominal distention with or without tympany may not always be easily detected, particularly in very deep chested dogs where the stomach may be within the costal arch. Prompt recognition, stabilisation and surgical correction of the GDV are required for successful treatment. This article discusses the approach to investigation, stabilisation, surgical correction and postoperative management of GDV.
Preoperative management
Before anaesthetising the patient for corrective surgery, preoperative stabilisation is essential. The following factors should be considered, although there is no prescriptive order in which they should be performed. Multiple aspects can be addressed simultaneously, so that the patient is stabilised as quickly as possible and gastric decompression is not delayed longer than necessary. The longer it takes to decompress the stomach, the higher the risk for developing irreversible gastric necrosis.
Bloodwork
A minimum database blood panel should be performed and includes: packed cell volume, total plasma solids, urea, creatinine, glucose and electrolytes. Alternatively, a full biochemistry and electrolyte panel can be performed, in addition to haematology, depending on the availability of these tests.
Hypokalaemia is a common finding and will worsen following high-volume intravenous fluid resuscitation, so ongoing monitoring is required. Intravenous supplementation of potassium chloride may be provided, but high-volume bolusing or infusion rates are not recommended as they can have fatal consequences. The rate of intravenous administration of potassium chloride should not exceed 0.5 mmol/kg/hour (Plumb, 2018), and it is often preferable to provide intravenous potassium via a separate infusion if necessary.
Lactate has been investigated as a predictor of outcome (Zacher et al, 2010, Beer, 2013), but there is no reliable cut-off value that will determine survival following surgery. It may be beneficial to measure serial blood lactate levels to help determine whether a patient is responding to the current stabilising interventions, but more attention should be given to the clinical status of the patient in terms of normalising heart rate and blood pressure.
Fluid therapy
Wide bore (18G) intravenous cannulas should be placed in both cephalic veins. Veins within the hind limbs should not be used for initial fluid resuscitation in patients with GDV because of the impaired venous return to the heart via the caudal vena cava, which occurs as a result of mechanical compression by the gas-distended stomach.
An initial bolus of 10–20 ml/kg of isotonic crystalloid (Hartmann's solution) should be given over 15 minutes. The patient should then be re-evaluated and further intravenous fluid boluses provided until cardiovascular parameters have improved, with good quality peripheral pulses and systolic blood pressure maintained between 110 and 190 mmHg (Thomas and Boller, 2018).
If further fluid boluses are required, the aforementioned volume and rate of isotonic crystalloid can be repeated. Alternatively, a single 5 ml/kg bolus of hypertonic saline (7.5% sodium chloride) can be administered over 15 minutes (Williams, 2015), which is then followed with more isotonic crystalloid fluid at an appropriate rate (Schertel et al, 1997). Use of hypertonic saline results in fluid being drawn from the interstitial tissue and from the intracellular space, resulting in a rapid expansion of intravascular volume. The effects of hypertonic saline are not long lasting, but it may be useful for more rapid resuscitation of larger dogs, enabling them to reach a state of cardiovascular stability faster, allowing for anaesthetic induction.
Analgesia
Progressive gastric distension results in abdominal pain, tachycardia and poor cardiac output. Appropriate preoperative analgesia should be administered early as this will reduce the heart rate and extend diastole, which will potentially improve cardiac output and myocardial oxygenation.
Opioid analgesia, such as 0.2–0.3 mg/kg methadone, administered intravenously provides good analgesia with minimal deleterious cardiovascular effects. Fentanyl is also appropriate, but owing to its short half-life it should be administered as a continuous rate infusion.
Non-steroidal anti-inflammatory drugs should be avoided in patients with GDV because of the initial hypovolaemic shock and the risk of gastric wall necrosis. Their use can be considered once normal circulating fluid volume has been restored and the presence of gastric necrosis has been ruled out.
Continuous rate infusion of lidocaine provides adjunctive analgesia, while reducing the patient's requirements for induction and maintenance anaesthetics. A 1–2 mg/kg intravenous bolus is administered, followed by a continuous rate infusion of 25–50 µg/kg/minute (Plumb, 2018). At higher doses, lidocaine is a potential anti-arrhythmic medication for ventricular tachycardia - this property will be discussed later.
If required, additional adjunctive analgesia can be provided using injectable paracetamol (provided that the patient does not have liver dysfunction), or using a continuous rate infusion of ketamine.
Oxygenation
Owing to the physical effects of a markedly distended stomach, resulting in compression of the caudal aspect of the diaphragm, patients with GDV will often be dyspnoeic on presentation. If tolerated, oxygen supplementation can be provided via flow-by, face mask or nasal prongs.
Electrocardiography
Continuous monitoring with electrocardiography is important when managing GDV as abnormalities including ventricular premature complexes, accelerated idioventricular rhythm and ventricular tachycardia (where the heart rate exceeds 200 beats/minute) are reported in 10-20% of affected patients (Muir 1982; Muir and Bonagura, 1984; Miller, 2000). These abnormalities occur as a result of myocardial ischaemia following impaired coronary vessel blood flow, and in response to released myocardial depressant factor.
Ventricular tachycardia, ventricular premature complexes interfering with cardiac output (poor peripheral pulse quality), patients with multiform ventricular premature complexes, or patients where ‘R on T’ phenomenon is observed on electrocardiography should be treated with lidocaine (Monnet, 2003, Sharp and Rozanski, 2014; Williams, 2015). An initial 2–4 mg/kg bolus of lidocaine is administered over 1–2 minutes. If this results in the resolution of the electrocardiography abnormalities, an intravenous constant rate infusion of lidocaine at 25–100 µg/kg/minute is administered (Plumb, 2018). If the abnormalities persist, a further lidocaine bolus may be necessary.
Radiography
While fluid boluses are being administered, a right lateral abdominal radiograph can be taken to aid differentiation between gastric dilatation and GDV. The radiographic appearance of gastric dilatation is a single, large, gas-filled structure within the cranial to mid abdomen. In GDV, there are two gas-filled structures; the fundus, and the gas-filled pylorus and proximal duodenum located dorsally. A line of soft tissue extends between these two areas of gas (Figure 1).
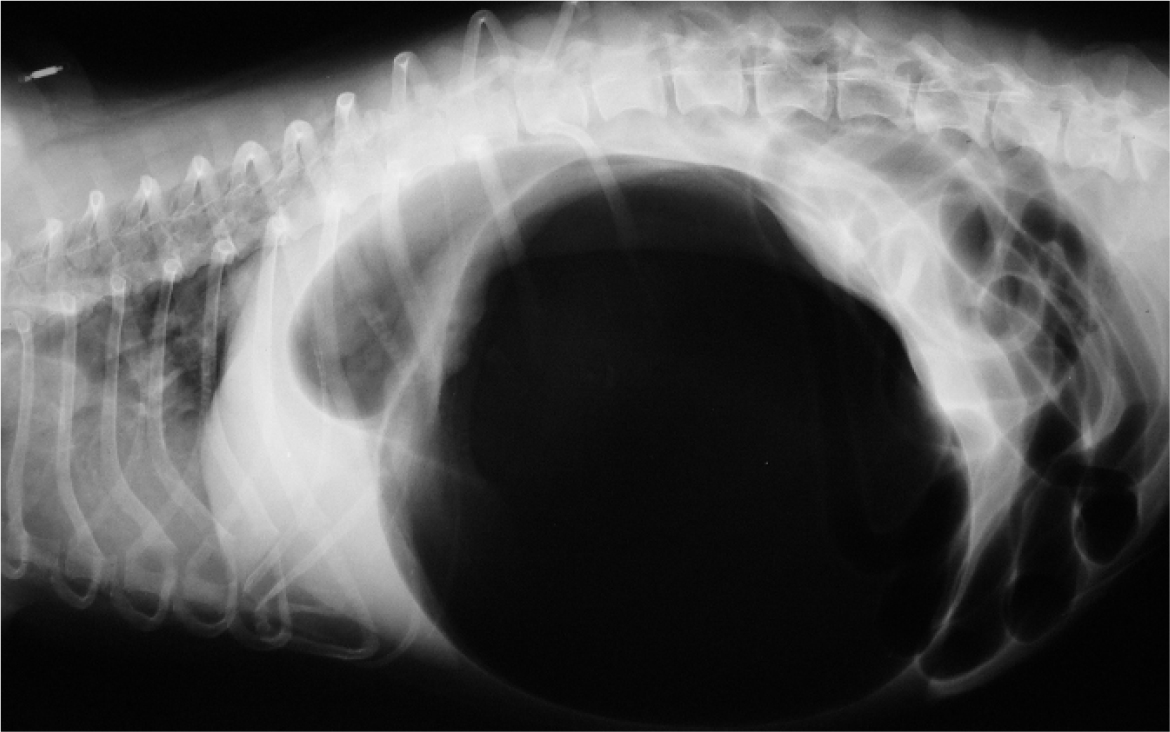
Patients with GDV typically present following a period of retching and non-productive vomiting, so are at risk of aspiration pneumonia. The prevalence of aspiration pneumonia on pre-operative radiographs reported in one study was 12% (Green et al, 2012). Screening thoracic radiographs may be useful to screen for aspiration pneumonia.
Temporary gastric decompression
Gastric decompression can be performed while the patient receives intravenous fluid therapy. It improves gastric perfusion, improves the patient's ability to ventilate and reduces the mechanical obstruction on the caudal vena cava. It can be achieved via placement of a suitably sized orogastric tube, or via percutaneous gastrocentesis (Williams, 2015).
For orogastric tube decompression, a roll of cohesive bandage with a central cylindrical tube can be used to hold the mouth open and prevent the patient from chewing the orogastric tube. The orogastric tube should be pre-measured from the tip of the nose to the level of the 11th rib, appropriately marked and well lubricated before attempting placement. The tube is slowly advanced down the oesophagous towards the gastro-oesophageal sphincter. As it reaches the sphincter, a twisting of the tube is performed to try to encourage it to go through the distal oesophagous and into the cardia. If resistance is felt, attempts to place the tube should be abandoned to prevent iatrogenic damage to the oesophagus or stomach. Orogastric tube placement should be performed with the patient either in sternal or right lateral recumbency. Some patients will tolerate this without sedation, others will not and may require induction of anaesthesia to allow this.
For percutaneous gastrocentesis, the abdomen immediately caudal to the last rib should be percussed on the right side to determine an area of tympany which will be suitable for placement of a wide bore (14–18G) intravenous cannula. The proposed site should be clipped and aseptically prepared before placing the cannula and removing the stylet. Gastric decompression can be aided by manual application of pressure to the abdomen. Ultrasound examination of the proposed gastrocentesis site can be performed prior to cannula placement to ensure the spleen is not between the abdominal wall and the gastric wall.
If gastrocentesis is successful, placement of an orogastric tube can be attempted following the reduction in gastric pressure or, if necessary, a repeat gastrocentesis can be performed before surgery.
Once fluid resuscitation has been completed and gastric decompression achieved, the minimum database and electrolytes should be rechecked. Any abnormalities should be corrected.
Gastric decompression and gastropexy
General anaesthesia considerations
If oxygen has not already been supplemented, pre-oxygenation should be provided to patients for 5 minutes before the induction of anaesthesia to increase their oxygen reserve. Attempts should be abandoned if the patient does not tolerate oxygen supplementation.
Patients with GDV are at an increased risk of developing aspiration pneumonia (Green et al, 2012), so rapid induction and intubation with prompt cuff inflation is recommended. Mask inductions should be avoided. Where possible, electrocardiography, blood pressure, capnography and pulse oximetry should be used to monitor the patient throughout surgery. Nitrous oxide should be avoided in patients with GDV as it will expand gas-filled spaces and exacerbate gastric distension.
Surgery
Perioperative antibiosis should be administered immediately following induction of general anaesthesia and repeated every 90–120 minutes intraoperatively. The authors use cefuroxime (Zinacef®, GlaxoSmithKline, UK) at 20 mg/kg. Assuming there has been no spillage of gastro-intestinal contents and the surgery remains a ‘clean’ procedure, antibiosis is discontinued at the end of the surgical procedure, or continued postoperatively every 8 hours for a maximum of 24 hours.
A ventral midline celiotomy is performed – a large incision is essential and should extend from the xyphoid cartilage to the pubis. The stomach, oesophagus and pylorus are palpated to identify the direction of rotation.
Clockwise rotation (180–270°) is most common and often results in greater omentum covering the gastric surface. In a clockwise torsion, the pylorus will be located on the left side of the abdomen (Figure 2). The spleen often displaces to a position in the right ventral abdomen. The pylorus should be gently lifted ventrally (upwards) and towards the right side of the abdominal cavity, while the fundus is simultaneously pushed dorsally (downwards) and to the left. The opposite should be performed for patients with anti-clockwise rotation.
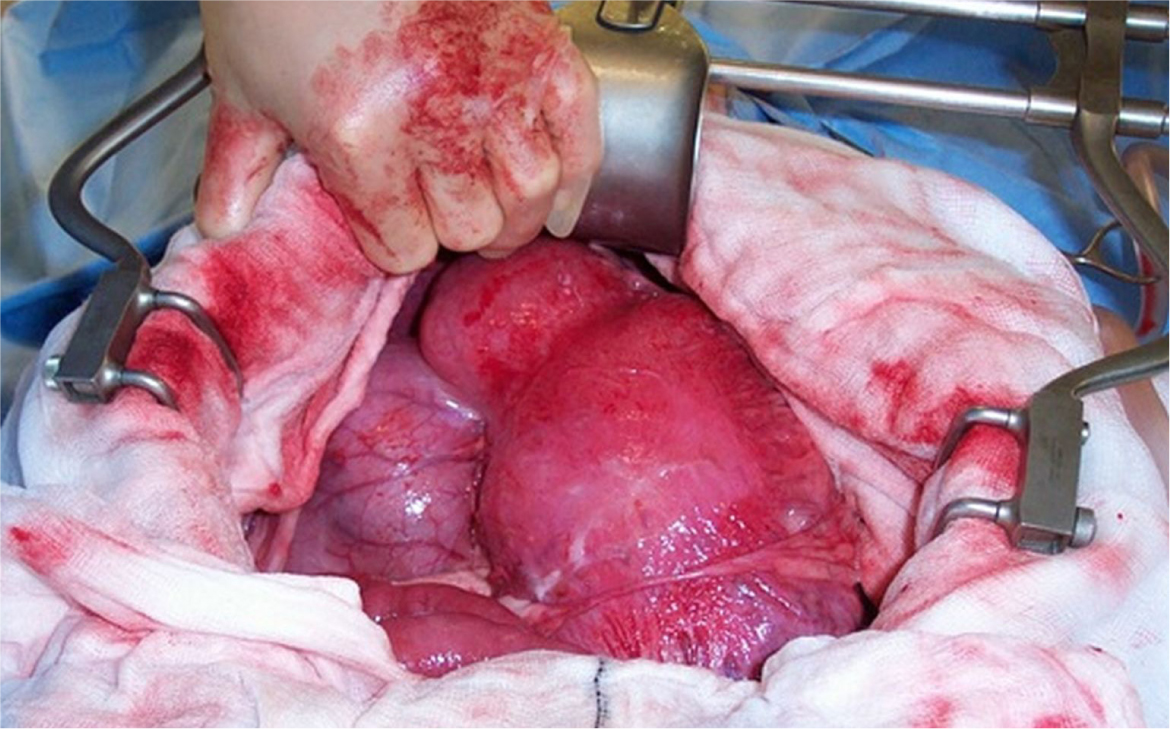
An orogastric tube should be placed at this time to allow gastric decompression. Should food material be present within the stomach, gastric lavage with warm water can be performed via the orogastric tube. Should the stomach be severely gas distended and de-rotation not possible, a wide bore intravenous cannula (14–18G) can be placed through a healthy area of the stomach wall to facilitate gastric decompression before re-attempting de-rotation.
Following gastric repositioning, the remainder of the abdominal cavity should be explored to rule out concurrent abnormalities. This allows time for the spleen and stomach to recover following repositioning, before their viability is evaluated. Splenectomy is rarely required, but should be performed where significant necrosis or splenic infarction is present, or if splenic blood vessels have avulsed, or if the spleen remains persistently congested despite having been returned to its normal anatomical position. Non-viable gastric wall will appear thin with a grey, green or black appearance. Where gastric vessels have avulsed or become thrombosed and the stomach appears haemorrhagic and red, gastric viability is questionable. In such circumstances, assessment can be aided by making a small partial thickness incision in the area(s) of concern using a scalpel blade – viable tissue will actively bleed (Figure 3). Healthy gastric wall will have palpable pulses with regular peristaltic waves. Where the viability of stomach wall is questionable, the abdominal cavity should be filled with warm saline for 5–10 minutes before re-evaluation. Assessing gastric wall colour changes is often beneficial in determining whether resection is required.
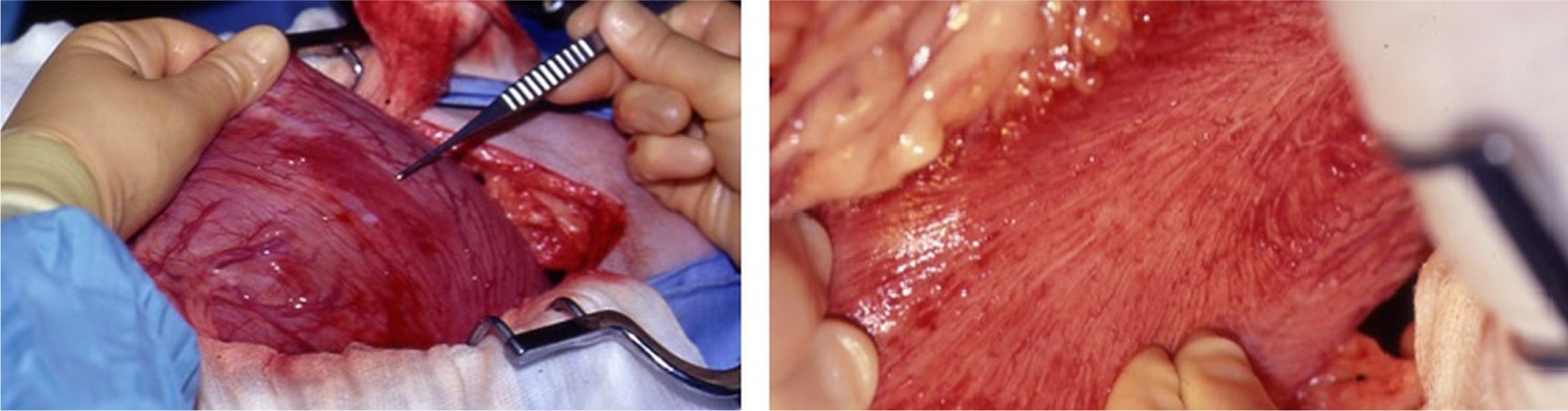
Necrosis of the gastric wall can occur, although this is a relatively uncommon finding, with a reported prevalence of 10.5% in one study (Matthiesen, 1985). Typically, the fundus and greater curvature are most affected. However, any area of the stomach is susceptible. Should the viability of an area of the gastric wall be questionable, gastric resection or invagination should be performed.
Partial gastrectomy is preferred to invagination because the compromised area of gastric wall is removed. Stay sutures are placed in the grossly normal gastric wall, adjacent to the necrotic area, and the site is packed off with moistened laparotomy pads. The area of necrotic gastric wall is grasped with forceps and excised until arterial bleeding is observed from the cut edges of the gastric wall. The gastric wall should be closed in two layers with absorbable monofilament suture material (such as poliglecaprone-25 or polyglyconate). First, the gastric mucosa is closed with a continuous appositional pattern, then the seromuscular layer is closed with an appositional or inverting suture pattern.
Invagination of the gastric wall is a useful method for sole surgeons to manage gastric necrosis. This is achieved by placing a continuous suture pattern in the gastric wall on either side of the necrotic area, causing the necrotic portion of the stomach to become inverted into the gastric lumen (Figure 4). The necrotic tissue will slough eventually, so a degree of melaena is expected. Some patients may have large areas of gastric necrosis or necrosis in areas which are non-resectable (such as the lesser curvature, cardia and pylorus). In these cases, intra-operative euthanasia may be the only option.
The abdominal cavity should be thoroughly and systematically explored. Correct positioning of the stomach should be confirmed before performing a gastropexy. Placement of an orogastric tube enables this.
Gastropexy should be performed in all patients following gastric decompression and de-rotation, as it reduces the risk of recurrence from 80% to less than 5% (Glickman et al, 1998; Williams, 2015). Several methods of gastropexy exist, including incisional, tube, belt-loop, circumcostal, gastrocolopexy and incorporating (Allen and Paul, 2014). Iizuka et al (2013) recommend performing a gastropexy that is quick and straight forward in these patients. Incisional gastropexy will therefore be described in more detail:
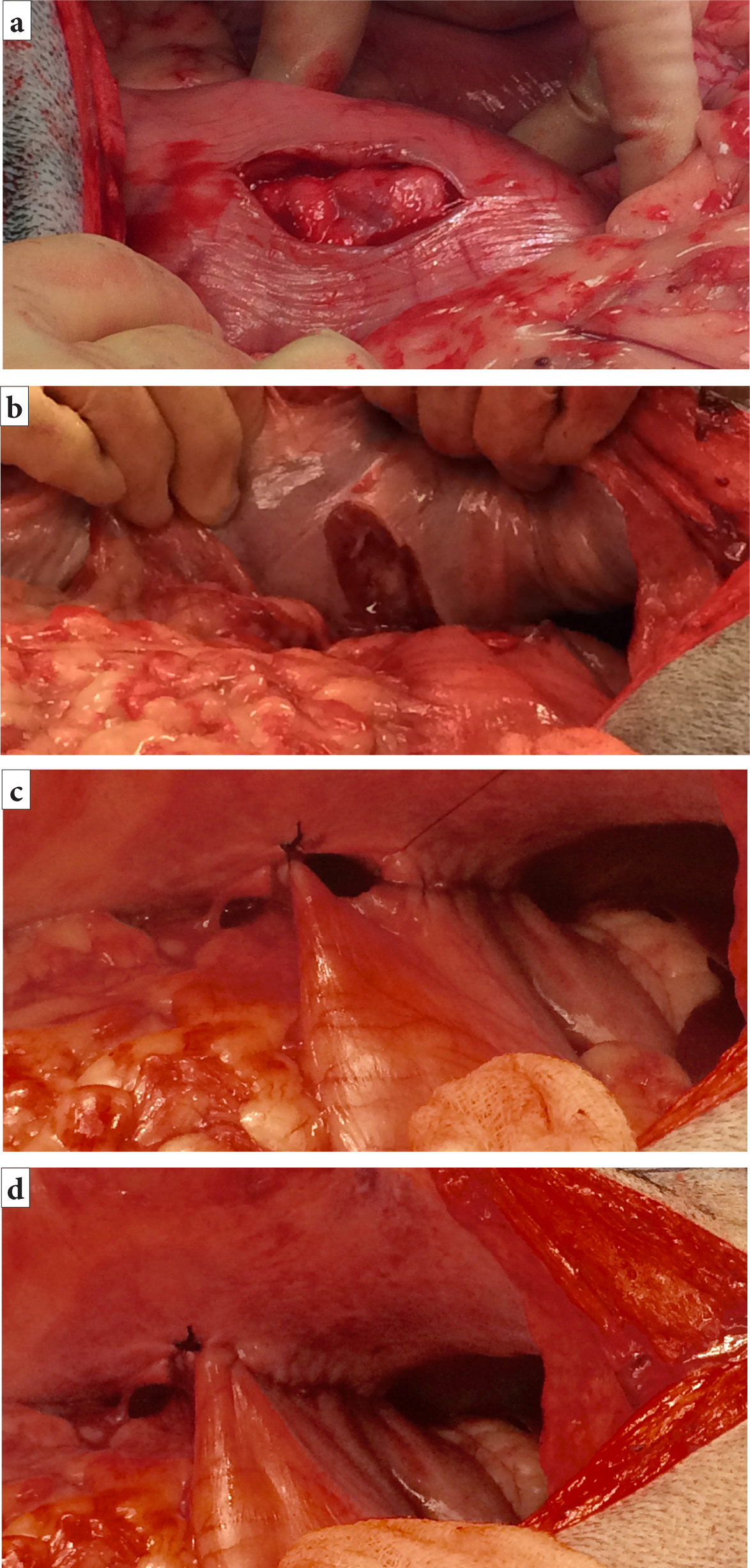
- The stomach is grasped and held in apposition to the right abdominal wall, just caudal to the 13th rib, to ascertain the position of the gastropexy incisions. The stomach should not be over-stretched to reach the incision site
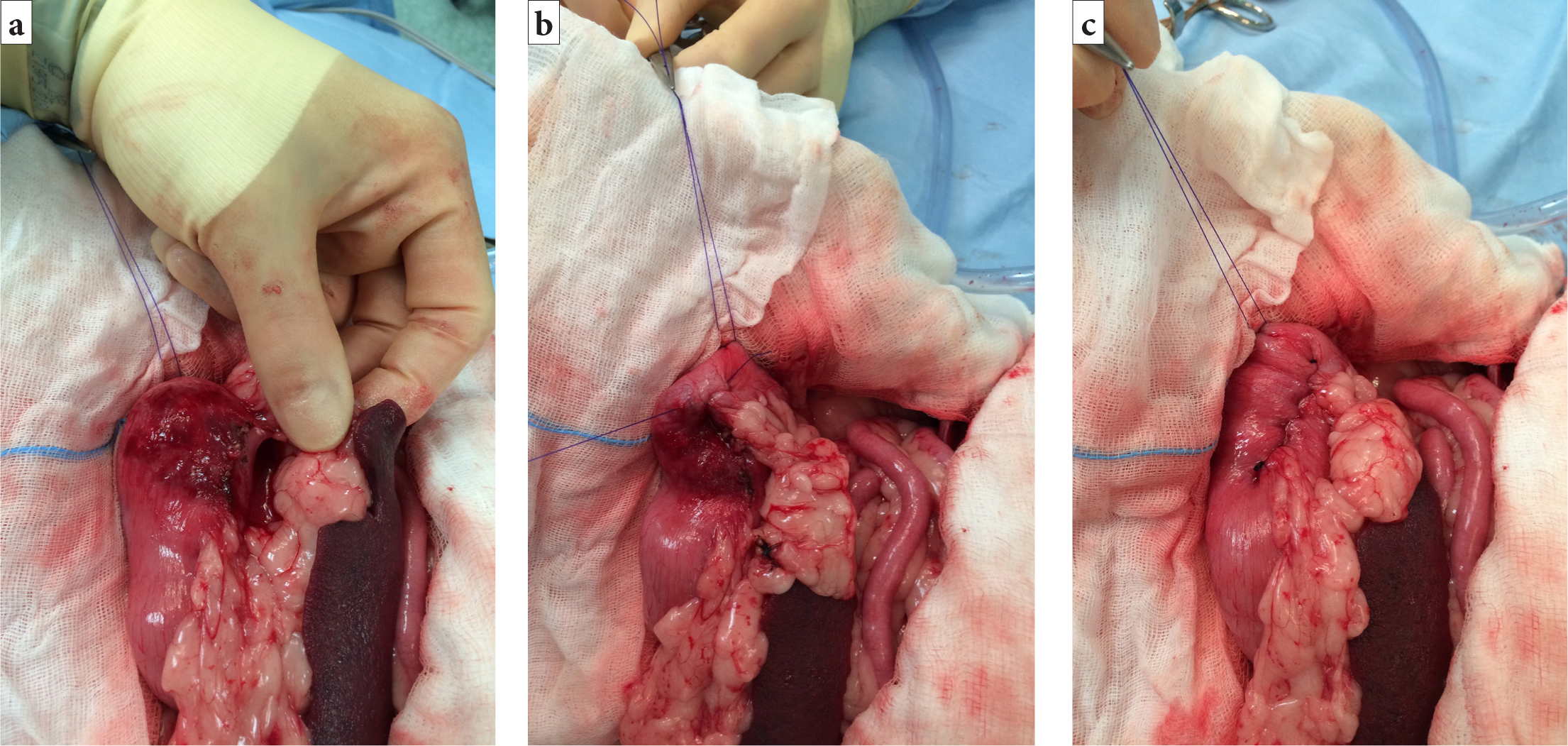
- A vertical incision (4–7 cm in length) (Allen and Paul, 2014) is made in the seromuscular layer of the gastric wall within the pyloric antrum (Figure 5a). Care should be taken to avoid penetrating the gastric lumen. This incision is made by sharply incising the seromuscular layer with a scalpel and extending the incision with either the scalpel blade or Metzenbaum scissors
- A corresponding vertical incision is made in the abdominal wall to include the peritoneum and first layer of abdominal musculature (Figure 5b)
- The edges of the seromuscular layer incision and the abdominal wall incision are apposed in a simple continuous pattern. The cranial edges should be sutured first, starting at the most dorsal (deepest) aspect of the incision (Figure 5cand5d)
- An absorbable or non-absorbable monofilament suture material (such as polydioxanone or polypropylene) is used for the suture
- The caudal part of the gastropexy is performed in the same manner. It is important that the gastric lumen is not penetrated by the suture material
- Before abdominal closure, the abdomen is lavaged with warm, sterile saline
- The omentum is placed around the completed gastropexy
- Swabs are counted and the abdomen is routinely closed.
Postoperative management
Postoperatively, the minimum database bloodwork should be repeated, and any significant abnormalities addressed. Food should be offered to patients following recovery from general anaesthetic. Use of the oesophageal feeding tube can help in providing early enteral nutrition. Intravenous fluids should be continued until the patient is normovolaemic and is eating consistently well. Opioid analgesia should be provided during the initial postoperative period, with adjunctive analgesia administered as necessary. Non-steroidal anti-inflammatory drugs can be considered in patients without gastric necrosis, but they should be used with caution.
Electrocardiography abnormalities are a common finding following GDV, particularly in patients with gastric necrosis, so continuous or intermittent electrocardiography monitoring is recommended (Matthiesen, 1985). Treatment indications for these electrocardiography abnormalities are the same as those discussed previously.
Complications
Septic peritonitis following GDV surgery occurs as a result of leakage of gastrointestinal contents into the peritoneal cavity. This can be because of gastric necrosis, but could occur secondary to inadvertent full thickness incision of the gastric wall during gastropexy. Septic peritonitis has a high mortality rate, but specific statistics are not comparable between retrospective studies owing to significant variations in their design and inclusion criteria. Patients with septic peritonitis are often dull with pyrexia, tachycardia and hypotension, and may collapse or become hypothermic and bradycardic. Vomiting is often observed.
Septic peritonitis following abdominal surgery cannot be definitively diagnosed, but the following investigations can be supportive of a clinical suspicion. Abdominal ultrasound will reveal free peritoneal fluid. Although, as this is a common finding following any abdominal surgery, abdominocentesis is recommended. A portion of the sample should be submitted for bacterial culture and sensitivity, and cytology should be performed on the remainder. High numbers of degenerate neutrophils with, or without, intracellular bacteria are highly suggestive of septic peritonitis. Blood and peritoneal fluid concentrations of glucose and lactate can be used to support the diagnosis, but no definitive diagnostic ratios have been determined. Where septic peritonitis is suspected, the patient should be stabilised with intravenous fluid boluses, appropriate analgesia and intravenous antibiotics. The patient should then be anaesthetised for further abdominal exploration to identify the source of the leak. Resection of gastric wall necrosis or repair of iatrogenic gastric wall perforation should be performed and the abdominal cavity lavaged with high volumes of warm saline (until the suctioned fluid becomes clear). If gastric wall necrosis is extensive or non-resectable, intra-operative euthanasia should be performed.
Broad spectrum antibiotics should be continued with subsequent modification according to the bacterial culture and sensitivity results. Recommended antibiotic combinations include (British Small Animal Veterinary Association, 2018):
- Metronidazole and marbofloxacin
- Amoxicillin clavulanate and marbofloxacin
- Ampicillin, amikacin and metronidazole.
Postoperative ileus can occur and is characterised by inappetence, vomiting and regurgitation. It is clinically suspected by a lack of borborygmi on abdominal auscultation and confirmed on ultrasound by the absence of intestinal peristalsis. It should be identified early and actively managed with prokinetic therapy (for example, metoclopramide 1–2mg/kg/day constant rate infusion) (Woosley, 2004); avoidance or antagonism of opioid analgesia, where possible, with provision of alternative analgesics, anti-emetics and correction of acid-base disturbances and fluid imbalances. Early ambulation and enteral nutrition should be encouraged within 48 hours of surgery (Whitehead et al, 2016).
Vomiting should be treated symptomatically with anti-emetics (such as maropitant) and gastroprotectants (such as omeprazole or ranitidine), provided that underlying causes (septic peritonitis and ileus) have been ruled out.
Conclusions
GDV is a relatively common emergency presentation in small animal practice. Prompt diagnosis and initiation of effective treatment are essential to managing these patients successfully.
KEY POINTS
- Initial stabilisation of patients with gastric dilatation volvulus includes treatment of hypovolaemic shock, correcting metabolic derangements and performing gastric decompression.
- Cardiac abnormalities are relatively common in patients with gastric dilatation volvulus. Electrocardiography monitoring should be used, with treatment being initiated where clinically indicated.
- Surgical exploration to correctly reposition the stomach and perform a gastropexy is always required in patients with gastric dilatation volvulus. A quick and simple gastropexy should be performed as soon as possible to avoid complications.


