Part 1 of this 2-part review article discussed the aetiopathogenesis and diagnosing of gallbladder mucoceles (Figure 1). This part will look at treatment options and prognosis.
Stabilisation
Although many gallbladder mucoceles are discovered during abdominal ultrasonography while screening for other diseases, patients with mature gallbladder mucoceles or gallbladder rupture can present as an emergency. When a patient, especially one whose breed is genetically predisposed to gallbladder mucoceles, presents with signs of acute abdominal pain, increased body temperature and sometimes icterus, gallbladder mucocele with potential rupture of gallbladder wall should be suspected (Jaffey et al, 2019). The incidence of gallbladder rupture in dogs with gallbladder mucoceles ranges from 20–60% (Pike et al, 2004; Worley et al, 2004).
Bile peritonitis
Peritonitis is an inflammation of the peritoneal cavity and its classification depends on the underlying cause, extent and presence of infectious agents. To help with the diagnosis and classification of peritonitis, cytological, biochemical and microbiological tests should be performed with collected peritoneal effusion sample (Volk, 2015).
Bile peritonitis develops when the gallbladder contents come into contact with the peritoneum. If gallbladder rupture and subsequent bile peritonitis is suspected, bilirubin concentration should be measured in paired abdominal free fluid and in the blood. If the latter is 2.5–10 times lower, then this indicates presence of bile in the peritoneal cavity (Ludwig et al, 1997).
A glucose concentration difference of more than 1.1 mmol/litre between paired blood and peritoneal fluid glucose (lower in the latter) indicates the presence of bacteria in the peritoneal fluid (Volk, 2015). It is important to remember that if dextrose has been administered to the patient before sample collection, these results might not be reliable. More than 2.0 mmol/litre difference in lactate concentration in blood and peritoneal effusion (higher in the latter) can also be a useful tool for diagnosing septic peritonitis in dogs, but not in cats (Levin et al, 2004). Postoperatively, these parameters have shown to be unreliable (Szabo et al, 2011). If samples for microbiological testing are collected before initiating therapy, further sampling should be unnecessary.
One of the most reliable ways to diagnose septic peritonitis is by visualising intracellular bacteria on cytology. If the patient has already been on antibiotic therapy, then the absence of bacteria on cytological sample does not exclude septic peritonitis (Volk, 2015). Culture and sensitivity test should always be performed from the effusion. Postoperatively, increasing neutrophil counts, or increasing morphological features of toxicity in neutrophils, is an indication for exploratory celiotomy (Volk, 2015).
Medical management
Although the first-line treatment for gallbladder mucoceles is cholecystectomy (especially in patients with complicated forms of the disease), medical management has shown to be effective in certain cases (Mayhew and Weisse, 2018). Medical management can be considered in patients without clinicopathological abnormalities, but bacterial culture should still be acquired via percutaneous ultrasound-guided fine needle aspiration (Besso et al, 2000).
There are reports of successful medical management in dogs with concurrent hypothyroidism (Walter et al, 2008). Specific treatment involves ursodiol, S-adenosyl-methionine (SAMe) and antibiotics; omega fatty acids and famotidine have also been used as adjunctives (Walter et al, 2008). If a predisposing disease (such as an endocrinopathy) has been diagnosed, this should be treated accordingly.
Ursodiol is mainly used in small animals as a choleretic medication, to reduce cholesterol synthesis and secretions from the liver and decrease cholesterol's absorption in the small intestine (Plumb, 2019). It has also been shown to reduce inflammation and fibrotic changes in the liver cells (Plumb, 2019). Care should be taken in patients who have complete biliary obstruction because this can result in biliary tract rupture, thus ursodeoxycholic acid should only be used in patients with mild cases of gallbladder mucocele (Aguirre, 2017).
Although no studies have shown efficacy of SAMe in the treatment of gallbladder mucocele, it is used as an adjunctive treatment for liver disease in small animals. It is an important molecule for the synthesis and reduction of glutathione, which protects hepatocytes against oxidant stress (Center et al, 2005).
The most commonly isolated species are Escherichia coli, Streptococcus spp., Enterococcus spp., Pseudomonas aeruginosa, Bacillus spp., Gram-negative bacilli and Gram-positive cocci (Crews et al, 2009). Although the need for and selection of antibiotics should be based on culture and sensitivity, empirical use can be considered if there is a high suspicion of suppurative disease (Aguirre, 2017). When empirical therapy is considered, then appropriate antibiotic choices are amoxicillin-clavulanate and fluoroquinolones with metronidazole (Maddison et al, 2008).
There is no specific diet indicated for medical management of gallbladder mucoceles, but based on pathophysiology of gallbladder mucoceles in dogs, a low fat (<10% crude fat), high protein (>40% crude protein) diet can be recommended. The diet should inhibit mucin production and increase gallbladder motility and emptying (Beynen, 2019).
Surgical management
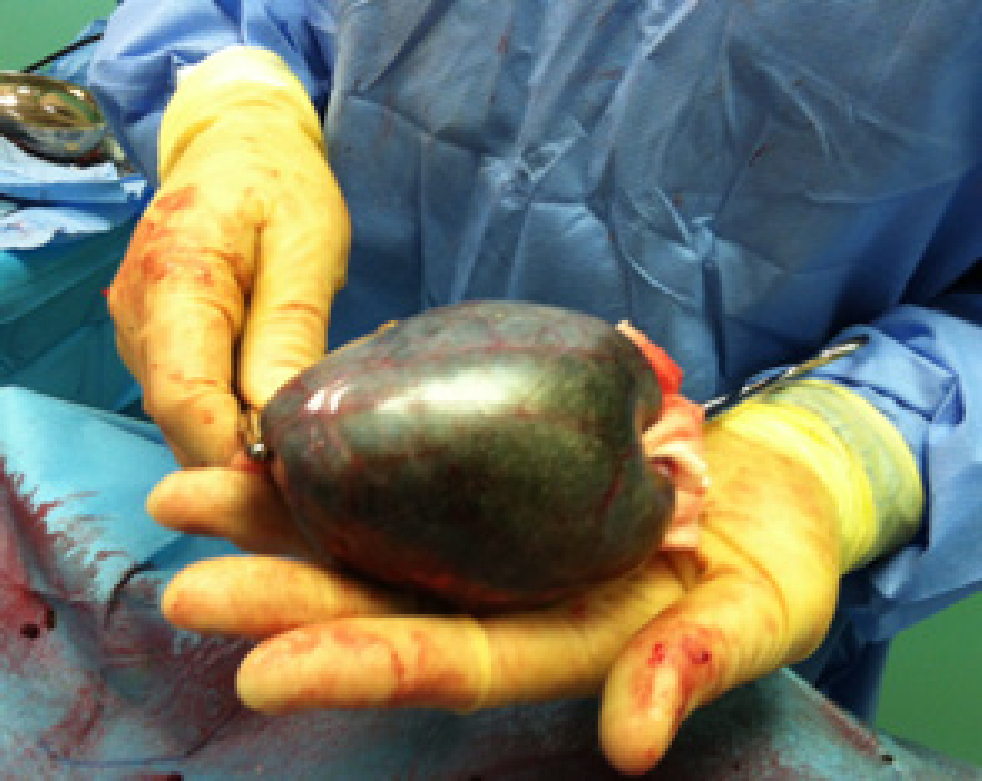
Cholecystectomy is preferred over cholecystotomy. The latter should not be performed in patients with gallbladder mucocele because of the high incidence of wall necrosis. During the procedure, the gallbladder is first bluntly dissected out of the hepatic fossa (Figure 2); large vessels or small bile ducts can be ligated or cauterised. If the gallbladder is extremely inflamed, blood vessel-rich adhesions to the liver or omentum may occur. After dissection, the cystic duct and artery are double ligated, a clamp is placed on them, and a cut is made between the ligatures and the clamp. The gallbladder is then removed (Figure 3) (Mayhew and Weisse, 2018).
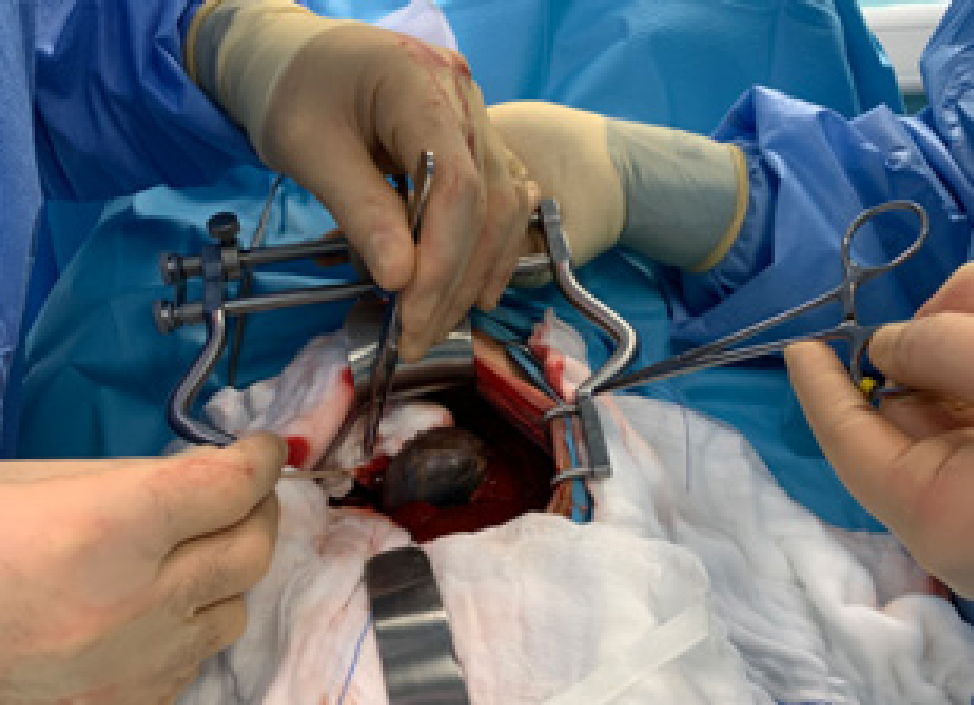
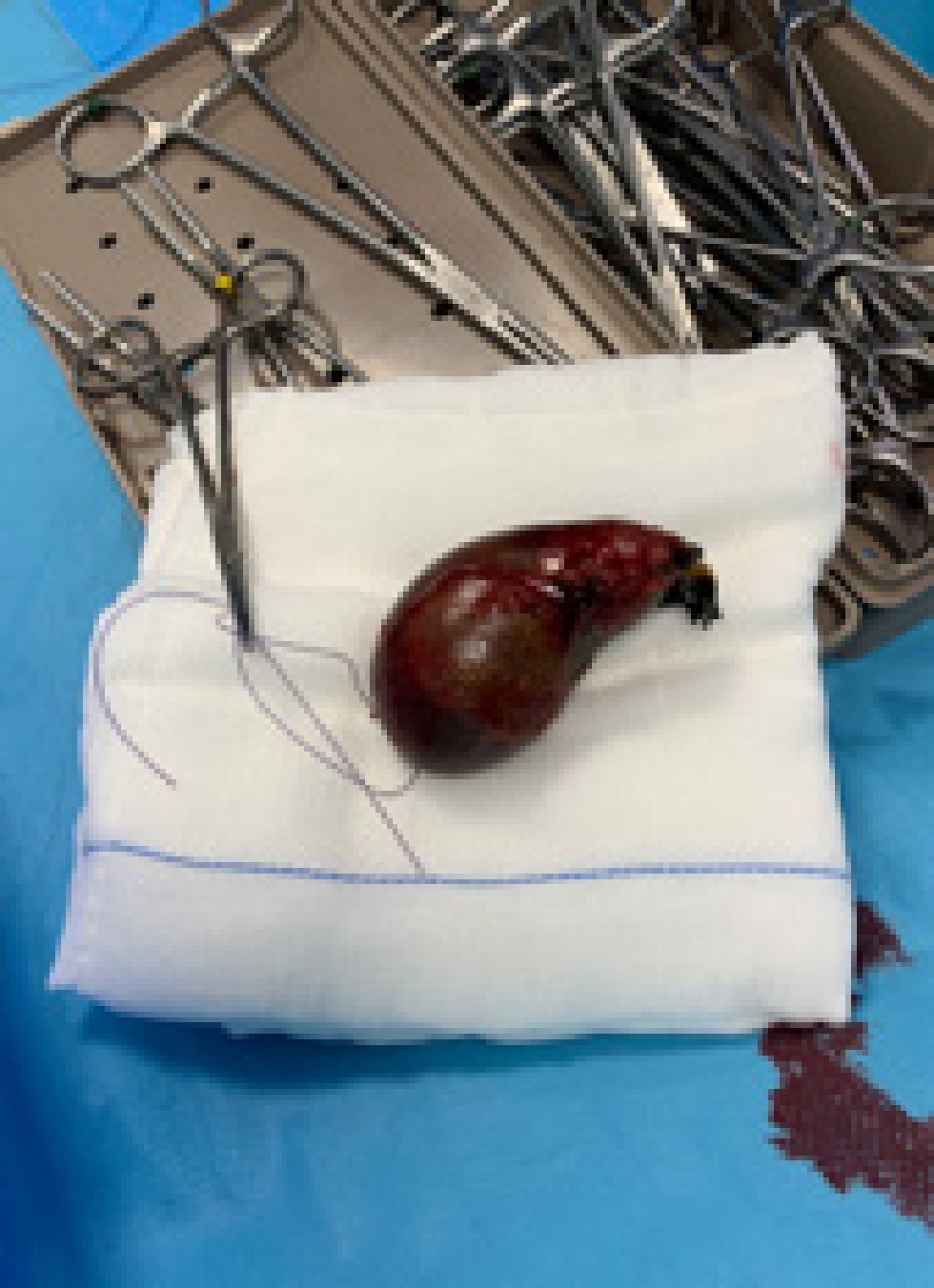
Common bile duct catheterisation and flushing (Figure 4) with sterile saline has been described (Mayhew and Weisse, 2018), but remains controversial. A study by Piegols et al (2020) showed that overall incidence of postoperative complications between catheterised and non-catheterised groups were similar, but the incidence of postoperative pancreatitis was significantly higher in dogs where common bile duct catheterisation was performed intraoperatively. The authors of this study proposed three mechanisms for the development of acute pancreatitis:
- Reflux of biliary secretions into the pancreatic duct as a result of high pressures during flushing
- Direct injury to duodenal papilla with the catheter, causing inflammation and obstruction of the duct
- Direct trauma to the pancreas caused by repeated handling of the area during flushing.
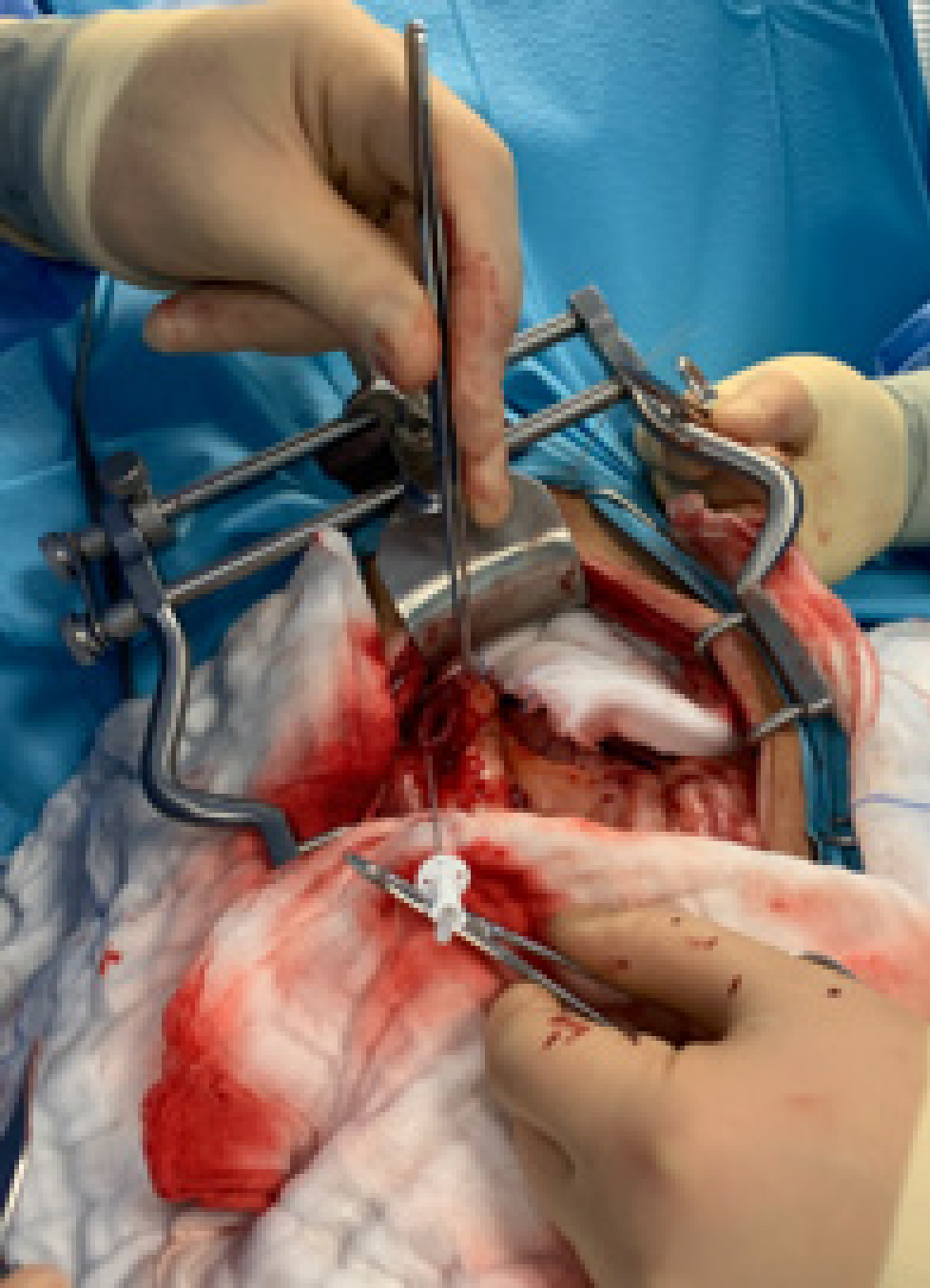
Flushing of the common bile duct was therefore only recommended where there was a direct indication to do so; in cases of concurrent cholelithiasis, bile duct obstruction or common bile duct rupture. The area should be carefully inspected for bile leakage or haemorrhage and rigorously lavaged before routine closure.
Laparoscopic gallbladder removal has been described and has shown very good results, even in small dogs (>4kg) (Mayhew et al, 2008). Mayhew et al (2008) reported that after 2 months, liver enzyme activity improved in 75% of patients. The researchers concluded that if the liver enzyme activity is still high 2 months after surgery, then additional liver pathology is present. As a result, the authors recommended always taking a liver biopsy, either laparoscopically or during open cholecystectomy.
Performing cholecystectomy in humans in the first 72 hours after symptoms of acute cholecystitis arise has shown to improve outcomes and reduce the risk of complications (Cao et al, 2016). In dogs, the same has not yet been proven to be true, but researchers admit that more detailed research is needed to completely rule out the association (Rogers et al, 2020). Owing to the high risk of complications, urgent cholecystectomy should still be recommended to owners (Jaffey et al, 2019).
Prognosis
The mortality rates after gallbladder removal vary from 16–40% (Besso et al, 2000; Burns et al, 2014). Patients with septic and bile peritonitis have the highest mortality rate, although long-term prognosis is variable (Aguirre, 2017). With early diagnosis, the best long-term prognosis is associated with treatment by cholecystectomy (Parakanzky et al, 2019. The same study also showed that medical management can be an option in patients with lower alkaline phosphatase activity and gallbladder mucocele scores, with a mean survival time of 1340 days.
Jaffey et al (2019) found that mortality was 2.3 higher in Pomeranians than in other breeds. The authors hypothesised that the main reason for this might have been increased incidence of tracheomalacia in this breed, because the most common cause of cardiac arrest was hypoxaemia and refractory hypotension in the postoperative period. In the same study, another factor that was connected to mortality was increased age, although researchers admitted this might have been a result of owners deciding to euthanise an older animal because of more frequent comorbidities.
Concurrent pancreatitis is connected to a worse prognosis (Amsellem et al, 2006). This is most likely because of the effect of inflammatory mediators and not secondary to extrahepatic biliary obstruction (Mehler et al, 2004). Postoperative dyspnoea and hypotension have also been reported to be associated with poorer prognosis (Amsellem et al, 2006). Causes of dyspnoea after biliary tract surgery include pulmonary thromboembolism, aspiration pneumonia, overhydration and acute respiratory distress syndrome (Amsellem et al, 2006), most likely caused by the presence of inflammatory mediators and endotoxins (Carpenter et al, 2001).
Septic bile peritonitis has been shown to be associated with a poorer prognosis and higher mortality rate (Mehler et al, 2004). Sterile bile peritonitis has not been named as a risk factor for death (Amsellem et al, 2006). This also confirms the importance of obtaining a peritoneal fluid sample for bacterial culture and sensitivity testing to be able to estimate a prognosis and inform better clinical decision making.
Concurrent hyperadrenocorticism has been connected to higher mortality in dogs with gallbladder mucocele, but it has not been statistically proven because of low numbers of patients (Jaffey et al, 2019). Further studies are needed to confirm this connection.
KEY POINTS
- Gallbladder mucoceles can be diagnosed incidentally while screening for other diseases but patients with mature gallbladder mucoceles or gallbladder rupture can present as an emergency.
- Although medical management can be attempted in patients without clinicopathological abnormalities, gold standard treatment is cholecystectomy.
- Mortality rates after cholecystectomy vary from 16–40%, higher mortality rates have been associated with septic bile peritonitis, hyperadrenocorticism and pancreatitis as a comorbidity.


