An absence or reduction of circulating myeloid cells is known as pancytopenia; this most commonly manifests as anaemia, thrombocytopenia and neutropenia. A combination of two of the aforementioned cytopenias is termed bicytopenia (Nelson and Couto, 2014).
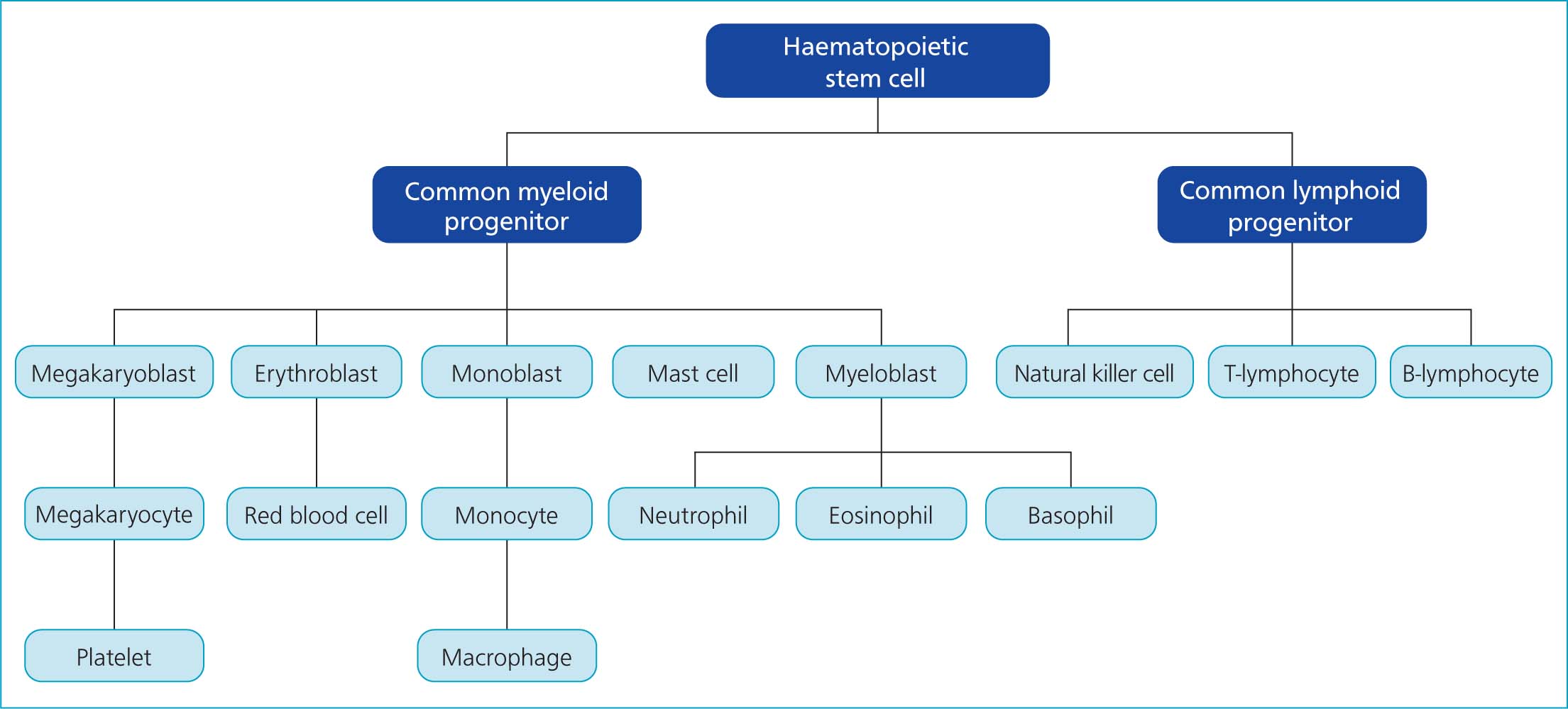
A review of haematopoiesis can aid with understanding these conditions. A simplified flow chart of this process is presented in Figure 1. This article summarises aetiologies, case management and prognoses for feline pancytopenia, using reports within the literature.
Clinical presentation and diagnosis
Cases of feline pancytopenia reported in the literature typically presented with vague clinical signs, including reduced appetite and lethargy (Stanley and Eatroff, 2017; Lyraki and Wilson, 2020), which may also depend on the underlying cause of the pancytopenia. Reported physical examination findings include pale mucous membranes, weight loss and peripheral lymphadenopathy (Shimoda et al, 2000; Takeuchi et al, 2010). The presence of pyrexia was variable across the published reports (Richter et al, 2014; Lyraki and Wilson, 2020), depending on the underlying disease or if secondary infections were present. Heart murmurs have been reported in 30% of anaemic cats (Korman et al, 2013), and while no specific studies into prevalence of heart murmurs in cases feline pancytopenia were identified in the literature, this may be found on physical examination (Stanley and Eatroff, 2017). Signs attributable to thrombocytopenia may include epistaxis, petechiation and haematuria (Shimoda et al, 2000; Bianco et al, 2008).
Pancytopenia will be identified on haematological evaluation. Exact reference ranges may vary depending on the haematology analyser used, and results should be interpreted according to the references for each laboratory. However, previous authors have used the following values as a definition of pancytopenia in cats (Weiss and Evanson, 2000):
- Packed cell volume <26%
- Neutrophil count <3x109/litre (3000/µl)
- Platelet count <200x109/litre (200 000/µl)
Care in interpreting results is advised. For example, errors in haematology machine platelet counts have been reported; with as few as 3.1% of automated thrombocytopenias being corroborated by manual platelet counts (Norman et al, 2001). Blood smear evaluation to assess cell structure and confirm cell populations/counts is strongly advised, and is frequently included in the diagnostic processes described in the available literature (Shimoda et al, 2000; Fujino et al, 2009).
Differential diagnoses and investigations
Lack of production within the bone marrow is the main broad category of pancytopenia. Destruction of circulating cells can also be considered. Table 1 provides a comprehensive list of reported causes of feline pancytopenia. The authors' suggestions for appropriate diagnostic steps are presented in Box 1.
Table 1. Differential diagnoses for feline pancytopenia
| Reduced production: | Bone marrow disorders (primary or secondary) | Myelofibrosis |
| Myelodysplasia | ||
| Aplasia | ||
| Myelonecrosis | ||
| Neoplasia (such as multiple myeloma or leukaemia) | ||
| Infection | Feline leukaemia virus | |
| Feline immunodeficiency virus | ||
| Vector borne disease: | Leishmaniasis | |
| Histoplasmosis | ||
| Cytauxzoonosis | ||
| Drug- or toxin-induced | Phenobarbital | |
| Griseofulvin | ||
| Azathioprine | ||
| Methimazole | ||
| Mycotoxins (T-2 toxin) | ||
| Propylthiouracil | ||
| Chemotherapeutic agents | ||
| Other | Hypocobalaminaemia | |
| Idiopathic | ||
| Increased destruction/utilisation: | Haemophagocytic syndrome | |
| Immune-mediated disease – peripheral and bone marrow | ||
| Sepsis or endotoxaemia | ||
Box 1.Recommended diagnostic approach to confirm pancytopenia in a cat
| Review history | |
| Physical examination | |
| Routine complete blood count + blood smear + biochemistry | |
| Infectious disease screens | Feline leukaemia virus/feline immunodeficiency virus point-of-care |
| Vector borne disease if appropriate | |
| Thoracic and abdominal imaging | |
| Urinalysis | |
| Bone marrow biopsy if no other cause found | Cytology |
| Histopathology | |
| Polymerase chain reaction test for feline leukaemia virus if serum tests are negative | |
Infection
Feline immunodeficiency virus pancytopenia has been noted in cases of infection with the lentivirus feline immunodeficiency virus (FIV), with a prevalence of 8% in a population of 50 FIV-positive cats (Fujino et al, 2009). It has also been reported in cats with myeloid dysplasia in bone marrow samples (Fujino et al, 2009). In-clinic combination tests for FIV antibodies and feline leukaemia virus antigens can be used as an initial screening for these viruses. For FIV, sensitivity and specificity reportedly range from 91.5–97.9% and 99–100% respectively, with no significant difference in accuracy of FIV detection across four point-of-care tests on the market (Levy et al, 2017). Guidelines published in 2020 advise that positive results for feline retroviruses by these tests should be corroborated via a second serological evaluation, or other diagnostic method (Little et al, 2020). The need for corroboration is supported by the findings of a study by Levy et al (2017) that reported poorer positive predictive values in ‘hypothetical populations of cats’ with lower seroprevalence. While virus culture is used as the standard test to confirm diagnosis in studies (Crawford et al, 2005), polymerase chain reaction (PCR) tests on bone marrow (Fujino et al, 2009) or blood (Crawford et al, 2005) are more accessible. Sensitivity and specificity of blood PCR has been reported to range from 41–93% and 81–100% respectively (Crawford et al, 2005).
Feline leukaemia virus
Pancytopenia has been reported in association with progressive feline leukaemia virus infection (Weiss and Evanson, 2000; Stavroulaki et al, 2020). In a case report of a cat with feline leukaemia virus, marked neutropenia was reported. In addition, worsening thrombocytopenia and anaemia, which was non-regenerative and identified by lack of reticulocytes on blood smear, were reported with disease progression (Stavroulaki et al, 2020). Bone marrow cytology findings from cats with feline leukaemia virus can be variable, with reports of hypercellularity and signs of immune-mediated destruction being identified as phagocytosis of mature neutrophils and red blood cells (Stavroulaki et al, 2020), alongside myelophthisis and myelofibrosis (Weiss and Evanson, 2000). To the authors' knowledge, no cases of pancytopenia have been reported in the published literature with latent feline leukaemia virus infection. However, non-regenerative anaemia was identified in two cases of latent feline leukaemia virus infection (Stützer et al, 2010).
Sensitivity and specificity for the feline leukaemia virus p27 antigen ranged from 85.6–100% and 85.7–100% respectively in a study comparing four point-of-care serological tests (Levy et al, 2017). False negative tests can occur in some feline leukaemia virus-infected cats, and in the presence of a cytopenia without identified cause, further testing such as PCR or immunofluorescence assay on bone marrow cells is required to definitively rule out feline leukaemia virus infection (Stützer et al, 2010). As with FIV, confirmation of diagnosis is advised before making definitive decisions on management of a case of suspected feline leukaemia virus (Little et al, 2020).
Feline parvovirus (panleukopenia) has a reported association with pancytopenia, and has been identified in one cat during a retrospective study of 36 cats with pancytopenia (Weiss and Evanson, 2000).
Vector-borne disease
In cats with a history of travel outside the UK, or for clinicians practicing in endemic areas, vector-borne diseases can be considered. Pancytopenia associated with both ocular (Richter et al, 2014) and visceral (Marcos et al, 2009) manifestations of leishmaniasis, and Cytauxzoon felis infection (Birkenheuer et al, 2006) have been reported. Fungal infections, such as histoplasmosis, could be considered in the USA (Schaefer et al, 2019).
Bone marrow disorders
Bone marrow evaluation, with collection of aspirates and core biopsy, is indicated in cases of persistent peripheral cytopenias and can assist with understanding the mechanisms of disease (Byers, 2017; Turinelli and Gavazza, 2018).
Bone marrow samples (aspirates and core biopsies) are commonly collected from the proximal humerus or the trochanteric fossa. The techniques for collection and sample handling have been previously described (Byers, 2017). Other authors have previously reviewed how bone marrow samples are evaluated. However, in summary samples can be evaluated for cellular populations and morphology, the ratio of erythroid to myeloid cells, and infiltration with other cell types (Stacy and Harvey, 2017). Core biopsies also allow the assessment of stromal changes within the bone, such as fibrosis or necrosis (Stacy and Harvey, 2017). Bone marrow aspirates can also be used for infectious disease screening such as PCR for feline leukaemia virus (Stützer et al, 2010).
Bone marrow dysfunction can occur via a number of mechanisms. For example, bone marrow precursors may be replaced (myelophthisis) by neoplastic or inflammatory cells or fibrosis tissues, damaged by immune-mediated mechanisms, hypoxia or direct toxic effects by xenobiotics including pharmaceuticals and natural toxins. Disorders of bone marrow, which have been identified in pancytopenic cats, are summarised below.
Myelodysplastic syndrome refers to disease caused by dysplastic changes to bone marrow precursor cells resulting in ineffective haematopoiesis and peripheral cytopenias. The bone marrow is normocellular or hypercellular (Weiss, 2003). Myelodysplastic syndromes can be primary, congenital, or secondary to other pathologies (Weiss, 2003; Weeden et al, 2016). Feline leukaemia virus infection is frequently reported in cats with myelodysplastic syndrome, at rates of 93.75% (Hisasue et al, 2001), 36% (Weiss, 2006b) and 22.2% (Turinelli and Gavazza, 2018), although in the last study feline leukaemia virus status was not known in all cases, highlighting the importance of testing for feline leukaemia virus in these cases. Dysplastic changes to the bone marrow have also been observed in cats with FIV (Shimoda et al, 2000; Fujino et al, 2009).
Sub-classification of myelodysplastic syndrome can be determined based on specific changes in bone marrow cell populations. Guidelines from the French-American-British Cooperative (Bennett et al, 1982) describe myelodysplastic syndrome in humans as one of the following; refractory anaemia, refractory anaemia with ringed sideroblasts, refractory anaemia with excess myeloblasts, refractory anaemia with excess myeloblasts in transition, and chronic myeloid leukaemia. This method has been adapted for use in a feline study to myelodysplastic syndrome with refractory cytopenia, myelodysplastic syndrome with sideroblastic differentiation, and myelodysplastic syndrome with excessive myeloblasts (Weiss, 2006b). Classification of myelodysplastic syndrome is advised because it can affect prognosis (Weiss, 2006b).
Myelofibrosis is a form of myeloproliferative disease with reduced myelopoiesis which occurs as a result of replacement of bone marrow cells with reticulin (Blue, 1988). Few case reports of feline myelofibrosis exist within the literature. However, myelofibrosis has been associated with bicytopenia in a feline leukemia virus-negative cat (Iwanaga et al, 2012), and in another cat which was feline leukaemia virus-positive, with anaemia and leuko- and thrombocytosis (Breuer et al, 1999). Myelofibrosis has also been observed in cats with myelodysplastic syndrome and acute myeloid leukaemia, with greater than 75% of these cats reportedly feline leukaemia virus-positive (Blue, 1988).
Aplastic anaemia, also known as aplastic pancytopenia, is a failure of haematopoiesis, with hypo-to acellular bone marrow and adipose tissue deposition (Weiss, 2003). Of 16 cats included in one study, 13 were pan- or bicytopenic on presentation and all but one of those initially presenting with bicytopenia progressed to become pancytopenic (Weiss, 2006a). Associated conditions included chronic kidney disease, feline leukaemia virus infection and methimazole use. In 5 out of 13 cats in the aforementioned study, an underlying cause was not identified.
Myelonecrosis occurs as result of ischaemia or damage to precursor cells (Stacy and Harvey, 2017). It is identified on cytology as a cell population lacking nuclear definition and has been associated with feline leukaemia virus infection (Shimoda et al, 2000).
Multiple myeloma is a neoplasm of the bone marrow, rarely reported in cats, and pancytopenia is a frequently identified abnormality associated with this condition (Patel et al, 2005; Takeuchi et al, 2010). The diagnosis of multiple myeloma is supported by the presence of two or more of the following; monoclonal gammopathy, light-chain proteinuria, osteolytic lesions and bone marrow plasmacytosis (MacEwen and Hurvitz, 1977). Patel et al (2005) diagnosed multiple myeloma in cats wherein plasma cells make up >20% of a bone marrow population or where plasma cells made up 10–20% of the population, if these plasma cells were of atypical morphology. Reported irregularities of plasma cells in cats with multiple myeloma include anisocytosis and anisokaryosis (Patel et al, 2005; Takeuchi et al, 2010). Table 2 summarises the frequency of identified criteria in the diagnosis of multiple myeloma.
Table 2. Prevalence of previously reported diagnostic criteria in feline multiple myeloma in three case series
| Article reference | Study size | Prevalence of cases with abnormality (%) | |||
|---|---|---|---|---|---|
| Bone marrow plasmacytosis* | Monoclonal gammopathy | Light-chain proteinuria | Osteolysis | ||
| Patel et al, 2005 | 16 | 93.3 | 78.5 × | 44.4∂ | 50 |
| Takeuchi et al, 2010 | 1 | 100 | 100 | 100 | 0 |
| Hanna, 2005 | 9 | 100 | 77 | 55 | 66 |
Adapted from MacEwen and Hurvitz (1977).
*Bone marrow plasmacytosis identified on ante- or post-mortem assessment.
14/15 individuals tested,
×11/14 individuals tested,
∂4/9 individuals tested, 6/12 individuals tested.
Drug- or toxin-induced
Pancytopenia is associated with different toxicities in cats, including in commonly prescribed medications. Selected examples are outlined below. Pancytopenia in association with phenobarbital use, where serum drug concentrations were within the recommended therapeutic reference range, has been documented in one cat (Lyraki and Wilson, 2020). Improvement in pancytopenia occurred following discontinuation of phenobarbital in this case and no bone marrow samples were collected. However, literature in canines sets a precedent to categorise phenobarbital toxicity as a cause of defective haematopoiesis; dogs treated with phenobarbital that had pancytopenia had left shift in one or more of the erythrocyte, megakaryocyte and neutrophil lines within the bone marrow, demonstrating poor haematopoiesis in spite of attempts at regeneration, alongside phagocytosis of neutrophils and erythrocyte precursors (Scott et al, 2021).
Cytopenias have been reported in hyperthyroid cats treated with methimazole. In a study of 262 cats treated with methimazole at doses of 10–15 mg per day, 3.8% had concurrent thrombocytopenia and leukopoenia, although no cats were reportedly anaemic. These haematological abnormalities were identified during the first 90 days of methimazole treatment (Peterson et al, 1988). However, pancytopenia has been reported in three cats receiving methimazole at doses described as being within therapeutic reference ranges, according to the ranges reported by Petersen et al (1988) (Weiss and Evanson, 2000, Weiss, 2006a). One of these cases is described as receiving a dose as high as 15 mg/kg, split into three doses per day (Weiss and Evanson, 2000). In the cat described in a study by Weiss (2006a), the dose of methimazole had been doubled to 2.5mg twice daily one week before identification of pancytopenia. The datasheets for licensed methimazole preparations and the pro-drug of methimazole and carbimazole in the UK recommend monitoring of haematology, with particular care recommended in cats receiving methimazole doses greater than 10 mg daily. The manufacturers do not advise use of the medication in animals with thrombocytopenia and neutropenia, and recognise a link between the drug and immune-mediated haemolytic anaemia (Dechra Veterinary Products, 2018; MSD Animal Health, 2020; Norbrook Laboratories Ltd, 2021). Rare associations (incidence of 0.01%) between methimazole and pancytopenia are also documented in humans, where 80% of these patients had reduction in cells of the myeloblast lineage before identification of pancytopenia (Watanabe et al, 2012). No such finding has been documented in the feline literature to the authors' knowledge. Propylthiouracil, another anti-thyroid medication, has been associated with severe thrombocytopenia and anaemia and is therefore not a widely advocated treatment for hyperthyroidism in cats, with one study reporting occurrence in 9/105 (8.5%) cats treated with this drug (Peterson et al, 1984). An immune-mediated cause was suspected in this study, as 7/9 bicytopenic cats tested were Coomb's positive and 5/8 tested positive for anti-nuclear antibodies; as haematological values normalised with the cessation of propylthiouracil therapy, Coomb's and anti-nuclear antibody tests also became negative (Peterson et al, 1984).
At licenced doses, albendazole and griseofulvin have been linked to pancytopenia in a retrospective study by Weiss and Evanson (2000). While myelosuppression is thought to be the pathological process in both of these toxicities, in the aforementioned study regenerative changes in one cat receiving each of the drugs indicated the potential for destruction of mature erythrocytes, although concurrent immune-mediated disease was not ruled out in this case. A further case of albendazole-associated cytotoxicity, when administered at recommended doses, was reported; in this case, bone marrow cytology was consistent with acute toxic injury (Stokol et al, 1997). Reports of griseofulvin use leading to pancytopenia are variable; in one case report, a cat receiving 40 mg/kg every 12 hours became profoundly pancytopenic, with hypoplastic bone marrow (Rottman et al, 1991), whereas in an experimental study of eight cats receiving griseofulvin at doses of 110–145 mg/kg daily for eight weeks, none developed pancytopenia (Kunkle and Meyer, 1987). Griseofulvin toxicity in cats appears to be inconsistent, but should still be considered as a differential diagnosis for pancytopenia.
Azathioprine has been documented as a cause of neutropenia in cats, with hypocellularity of the bone marrow. In the same study, azathioprine was reported to cause thrombocytopenia and in one case, pancytopenia (Beale et al, 1992). In two kittens that received azathioprine in error, at doses of 12–12.5 mg/kg for more than 2 weeks, pancytopenia was documented (Paul et al, 2008). It is for this reason that azathioprine is contraindicated in cats.
Many chemotherapeutic agents can lead to myelosuppression, and regular haematological assessment is advised when administering a course of chemotherapy. A few examples of myelotoxic chemotherapy agents are detailed here. Doxorubicin can cause cytopenia and one case report documented a cat that received a 2x overdose of doxorubicin on one occasion (received 2 mg/kg instead of the 1 mg/kg typically used) during L_CHOP protocol for the treatment of lymphoma, and subsequently developed pancytopenia. The cat survived this overdose and did not require hospitalisation (Musser et al, 2021). In one case series, six healthy cats were given doxorubicin as single chemotherapeutic agent at a dose of 30mg/m2 every 21 days, to a cumulative dose of 300 mg/m2 (O'Keefe and Schaeffer, 1992). In this case study, neutropenia occurred following 30% of administered doses and a mild thrombocytopenia was reported after 11.3% of doses. These values normalised within 14 days of administration in all but two cases, which required deferring one treatment cycle, followed by a dose reduction of the doxorubicin to 25 mg/m2. One cat in this study died of septic peritonitis after receiving an accumulative dose of 225 mg/m2. The exact cause was not determined, but the authors of the study deemed it unlikely to be related to any myelosuppressive effects of the doxorubicin, as this cat had not been neutropenic during the previous two doxorubicin cycles (although a neutrophil count was not performed in the days leading to death following the most recent doxorubicin administration) (O'Keefe and Schaeffer, 1992). The dosage of doxorubicin in the aforementioned study exceeds the other reported doses of 25 mg/m2 or 1 mg/kg used in cats (Reiman et al, 2008; Oberthaler et al, 2009). Vincristine administered at an accidental 10x overdose for a cat treated for gastric lymphoma using a cyclophosphamide-vincristine-prednisolone protocol led to death as a result of pulmonary oedema and acute kidney failure within 72 hours of overdose.
While haematology was not performed to confirm pancytopenia following this overdose, post-mortem bone marrow histopathology was consistent with myelonecrosis despite haematology values before the administration of vincristine being reported as un-concerning (Hughes et al, 2009). Cyclophosphamide has also been documented to produce a reversible pancytopenia in three cats receiving therapeutic doses of the drug at 50 mg/m2 orally once a day, 4 days a week, for treatment of lymphoma (Weiss and Evanson, 2000). These examples of drugs that can cause iatrogenic pancytopenia highlight the importance of obtaining a thorough clinical history; if the patient presenting with bi- or pancytopenia has recently received any chemotherapeutic or immunosuppressive medication, including those not listed above, consideration should be given to this drug being the underlying cause, and specific guidelines for the use of individual drugs should be consulted.
Cats have been reported to be extremely sensitive to the mycotoxin T-2, which is produced by Fusarium species of fungi that invades crops and may therefore be ingested in food. Toxicity is associated with many effects in the body, including apoptosis of proliferating cells in the bone marrow (European Food Safety Authority, 2011). The sensitivity of cats to this toxin is likely to be as a result of the inability of cats to perform the glucuronide conjugation needed to excrete the toxin (European Food Safety Authority, 2011). The European Food Safety Authority state ‘health risk from the exposure to T-2 and HT-2 toxins cannot be assessed’. In two prospective studies, T-2 toxin has been documented to cause pancytopenia in previously healthy cats receiving doses in the range of 0.06–0.1 mg/kg every 48 hours for a maximum of 40 days, or until death, as detailed in Table 3. Post-mortem bone marrow evaluation performed in these cats identified profound hypocellularity on histopathology (Lutsky et al, 1978; Lutsky and Mor, 1981).
Table 3. Survival data for feline pancytopenia based on an underlying or associated cause, where available in the literature
| Condition | Reported prognostic data | Comments | Reference | Study type and sample size |
|---|---|---|---|---|
| Aplastic anaemia | Death or euthanasia in 10/13 cats within 2 weeks of diagnosis. Survival times for the other three cats ranged from 5 months to 6 years | In the 10 cats with poorer survival times, one cat was euthanised as a result of splenic sarcoma, and one because of a mast cell tumour. The longer surviving cats all had pancytopenia associated with chronic kidney disease. | Weiss, 2006a | Retrospective studyn=16 in total, n=13 were pan- or bicytopenic |
| Albendazole | A cat was discharged after 11days of hospitalisation. Haemogram normalised 1 month post-discharge | Dose received before identification of pancytopenia was 25mg/kg twice daily, for a total of 8 doses | Stokol et al, 1997 | Case reportn=1 |
| Azathioprine-associated | Improvement in bone marrow suppression noted at 19 days post-azathioprine cessation in one 7-month-old kitten. Discharged home at day 38.l | Dose received was 12mg/kg once dailyDose received was 12.5mg/kg once daily | Paul et al, 2008 | Case seriesn=2 |
| No improvement in pancytopenia after 39 days post-cessation of azathioprine in a 6-month-old kitten. Patient went into cardiac arrest at day 40, and attempts at CPR were unsuccessful | Dose received was 12.5mg/kg once daily | |||
| Cytauzoonosis | 32/34 cats died or were euthanised | Time to worsening of clinical signs and euthanasia was not reported | Birkenheuer et al, 2006 | Case seriesn=34 (n=8 were pancytopenic) |
| Hypocobalaminaemia-associated | Haematological values normalised within 2 months of initiation of cobalamin supplementation | No long-term follow-up data available | Stanley and Eatroff, 2017 | Case reportn=1 |
| Immune mediated neutropenia | Asymptomatic more than 2 years after cessation of prednisolone therapy | Associated with feline leukaemia virus | Stavroulaki et al, 2020 | Case reportn=1 |
| Immune mediated neutropenia + immune mediated thrombocytopenia | Worsening of haematology parameters below prednisolone 1.6mg/kg per os every 12 hours, so the cat was kept on this dose longer-term | No follow up haematology reportedNo long-term follow-up data | Best and Fry, 2014 | Case reportn=1 |
| Leishmaniasis: Ocular | Normal values on haematology within 2 months of initiating treatment for leishmaniasis | Richter et al, 2014 | Case reportn=1 | |
| Leishmaniasis: Ocular Visceral | Euthanasia within 20 days | Marcos et al, 2009 | Case reportn=1 | |
| Multiple myeloma | Death or euthanasia ranging from 5–387 days post-diagnosisDeath or euthanasia ranging from 0–6 months post-diagnosis | Not all cats in these studies were pancytopenic. No specific data on whether presence of pancytopenia affects prognosis | Hanna, 2005 Patel et al, 2005 | Retrospective study n=9Retrospective study n=16 |
| Mycotoxicosis (T-2 toxin) | Average survival time 3 weeks.Survival time varied from 6–40 days in 19/20 cats receiving a form of T-2 toxin. n=1 cat survived (receiving 0.076mg/kg every 48 hours) | Cats receiving a dose of 0.08mg/kg per os every 48 hours until death Inverse correlation between dose of T-2 toxin received and survival time.Surviving cat had received 15 doses of the toxin. Within 2 weeks of cessation of toxin, was clinically normal, with return to normal haematology parameters by 2 months. Was clinically well 1 year later | Lutsky and Mor, 1981 Lutsky et al, 1978 | Animal model of human disease prospective study. Number included in study not listed. Prospective study n=24 (n=20 receiving toxin) |
| Myelodysplastic syndrome | Specific survival data not available | Prognosis dependent on classification of myelodysplastic syndrome, with those with excess blast cells on bone marrow cytology associated with death or euthanasia sooner post-diagnosis than other classifications | Weiss, 2006b | Retrospective studyn=34Retrospective studyn=16 |
| Survival times ranging from 0-74 months post-diagnosis | Although no statistically significant difference in survival times between groups with high vs low blasts when categorising myelodysplastic syndrome in this study, those with high blasts had a shorter range of survival times (1 week to 6 months), compared to the low blast group (10 days to 74 months) | Hisasue et al, 2001 | Retrospective studyn=16 | |
| Phenobarbital-associated | Normalisation of red blood cell and neutrophil counts within 10 weeks post-cessation of phenobarbital. Thrombocytopenia improved but wasstill present. | No long-term follow-up data available | Lyraki and Wilson, 2020 | Case reportn=1 |
Other
Pancytopenia, with hypoplastic bone marrow identified on histopathology, has been reported in a cat with a history of chronic diarrhoea (Stanley and Eatroff, 2017). No cause of the pancytopenia was identified, but the cat had hypocobalaminaemia. Improvement in pancytopenia was documented following several weeks of cobalamin supplementation in this case, although true causation cannot be confirmed on the basis of this finding.
An outbreak in feline pancytopenia was reported in the UK in 2021. Anecdotally, at their own institution and from discission with colleagues at other centres, the authors have found that in multi-cat households, more than one cat was often affected. Reviewing the history and survey results from owners and vets treating these cats, the Royal Veterinary College (RVC, 2021) identified that these cats had been fed a limited number of commercial diets. As a result of this association, a recall of these foods was undertaken. The Food Standards Agency has analysed these diets and at the time of writing this article, the most recent update on these investigations is that the Food Standards Agency (2021) have not demonstrated a causative link between these diets and cases of pancytopenia, but have not ruled out a link either. The RVC have so far reported 565 known cases based on information entered into their survey of this condition, but commented that this is most likely an underrepresentation of the true extent of the outbreak nationally. Reported mortality rates as of September 2021 were 63%. Investigations remain ongoing (RVC, 2021).
Destruction of circulating cells or precursors
Haemophagocytic syndrome is a disease of inappropriate phagocytosis of red blood cells. In two reported cases, haemophagocytic syndrome was associated with a diagnosis of multiple myeloma (Webb et al, 2008; Dunbar and Lyles, 2013), whereas in a third case, there was no evidence of neoplasia or other identifiable cause of the haemophagocytic syndrome (Wilkinson et al, 2018). All three of these case reports documented non-regenerative anaemia and leukopenia, but thrombocytopenia was only reported in one case (Wilkinson et al, 2018), wherein phagocytosis of megakaryocytes within the bone marrow was observed. Thrombocytophagia was also reported in the spleen, liver and bone marrow of one cat with multiple myeloma, despite the absence of thrombocytopenia on complete blood count (Dunbar and Lyles, 2013). In each case, bone marrow biopsies and fine needle aspirates were taken from the liver and spleen, and phagocytosis of mature cells, or myeloid precursor cells, was evident cytologically. Furthermore, a case series reported that haemophagocytic syndrome can occur in cats infected with a histoplasma-like fungus in the USA (Schaefer et al, 2019). In this series, 2/32 cats had pancytopenia, while 20/32 had a deficiency in at least one cell line.
Immune-mediated pancytopenia is poorly described in cats, although a retrospective study documented pancytopenia associated with immune-mediated haemolytic anaemia in three cats (Weiss and Evanson, 2000). Concurrent immune-mediated thrombocytopenia and immune-mediated neutropenia was reported in a 21-week-old Maine Coon cat (Best and Fry, 2014) and investigation, including endemic infectious disease screens and abdominal and thoracic imaging, revealed no identifiable cause. Bone marrow aspiration yielded a normocellular population, with neutrophil left shift, suggesting peripheral loss of neutrophils. In cases where immune-mediated disease is suspected, assessment of autoagglutination (Coomb's or in-saline) and antinuclear antibodies should be performed (Weiss and Evanson, 2000).
Sepsis has been associated with development of pancytopenia (Weiss and Evanson, 2000). Sepsis may result in pancytopenia as a result of the effects of inflammatory mediators on haematopoiesis, bone marrow necrosis and the effects of endotoxins on bone marrow precursors (Kearns and Ewing, 2006). In cats presenting with pancytopenia and sepsis, it is also important to consider that sepsis could be a consequence of the neutropenia associated with another cause of pancytopenia, rather than the primary cause of the pancytopenia itself.
Treatment
In the presence of a specific underlying cause for pancytopenia (infectious disease, toxin exposure), targeted therapy or removal of toxins forms the mainstay of treatment (Weiss and Evanson, 2000; Richter et al, 2014; Lyraki and Wilson, 2020).
Therapeutic strategies, including doses, for specific neoplastic conditions or bone marrow disorders are not discussed in this article, but treatments given within the case reports and series are noted. Immunosuppressives are reportedly used in cases where there is evidence of immune-mediated disease, either peripherally (Best and Fry, 2014) or within the bone marrow (Stavroulaki et al, 2020), and in primary bone marrow disorders (Weiss, 2003). Corticosteroids, such as prednisolone, at immunosuppressive doses, are often used; with doses typically being tapered every 3–4 weeks, ideally following haematology review (Best and Fry, 2014; Stavroulaki et al, 2020). Ciclosporin has been prescribed in cases of myelodysplastic syndrome (Hisasue et al, 2001).
Chemotherapeutic agents can be used in certain scenarios, although careful monitoring is advised, given that pancytopenia has been associated with their use (Weiss and Evanson, 2000). Cases of multiple myeloma have reportedly been prescribed melphalan (Hanna, 2005; Takeuchi et al, 2010), doses used varied, but the medication was consistently discontinued if a worsening of neutropenia was noted. Additional agents reported to be used in multiple myeloma are chlorambucil (Takeuchi et al, 2010), and prednisolone, either as an adjunct or sole agent (Hanna, 2005; Takeuchi et al, 2010). Cytarabine can be considered in myeloproliferative neoplasia, and its use has been reported in feline myelodysplastic syndrome (Hisasue et al, 2001).
Managing complications of pancytopenia
Supportive treatment may be indicated, while awaiting final diagnosis or for specific therapeutic agents to take effect. Patients with profound anaemia resulting in clinical signs such as weakness, tachycardia and tachypnoea will require blood transfusion to provide cardiovascular stability. Specific cut-off values indicating the need for transfusion are lacking within the feline literature, although it appears that the decision on when to perform a transfusion will vary depending on duration of anaemia and how well the patient is adapting to the anaemia; with the authors of one review paper transfusing at a packed cell volume of <10% in cases of prolonged non-regenerative anaemia, compared to 15–20% in cases with acute or ongoing blood loss, and at a range of 10–20% in euvolaemic patients with signs of decompensation, such as tachycardia (Barfield and Adamantos, 2011). Discussion of blood product selection and administration falls outside the scope of this article.
Platelet counts below 30x109/l are associated with an increased risk of spontaneous haemorrhage (Ettinger et al, 2017). Handling of cats with severe thrombocytopenia must be gentle and minimised as much as possible. Blood samples should be collected from peripheral veins, and pressure bandages applied. Care should be taken when considering whether subcutaneous and intramuscular administration of drugs is required, with it being preferable to administer medications via an intravenous catheter or orally if cats will accept oral medication in food or without struggling. As discussed above, clinical signs of tissue hypoxia in the presence of anaemia, or as a consequence of haemorrhage caused by thrombocytopenia, may warrant blood transfusion.
The risk of secondary infection, potentially progressing to sepsis, must be considered in neutropenic patients. Guidelines by the Veterinary Cooperative Oncology Group (2016) categorise a neutrophil count of <1 x109 cells/litre as ‘severe or medically significant’. A neutropenia of this severity, with or without concurrent pyrexia, justifies broad spectrum antibiotic prophylaxis in patients receiving chemotherapy (MacDonald, 2009). The authors suggest that these guidelines be applied to cats with other causes of neutropenia. Excellent hygiene and infection control, with reverse barrier nursing, should also be implemented.
Other authors have suggested the use of exogenous granulocyte colony stimulating factor (G-CSF) in cases of neutropenia. The rationale for its use is that this cytokine increases the rate of neutrophil maturation and release from the bone marrow. Recombinant human G-CSF was reportedly administered to seven cats with feline panleukopenia virus, six of which were neutropenic on presentation. Each cat was treated with 5µg/kg for 5 days, administered subcutaneously. Repeated haematology profiles were performed 24 hours after the first injection, 24 hours after completing treatment, and again 5 days after the drug course was complete. Of the six cats surviving to the end of the treatment course, neutrophil counts increased from pre-treatment values in all cats following one dose of recombinant human G-CSF. Neutrophil levels remained within or above reference range in all cats at all time points within this study, with the exception of one cat who had an increase in neutrophils following the first dose of treatment, but was still below reference limits (Felix et al, 2005). A separate case report has associated the use of recombinant human G-CSF with the development of thrombocytopenia in a cat treated at 5µg/kg once daily for 3 days (Kim et al, 2021). This cat had received recombinant human G-CSF for treatment of asymptomatic neutropenia several months after receiving phenobarbital, zonisamide and levetiracetam for management of idiopathic epilepsy. The thrombocytopenia was transient, asymptomatic, and no therapy was required. Similar cases of thrombocytopenia related to G-CSF treatment have been described in people, with recovery from this being documented following cessation of G-CSF therapy (Kovacic et al, 2007; Minelli et al, 2009). Long-term use of recombinant human G-CSF (5µg/kg daily for 3 weeks) has been associated with antibody production against the protein in cats with FIV (Phillips et al, 2005). Experimental use of feline G-CSF and its pegylated form has been reported in FIV-infected cats to overcome the development of anti-G-CSF antibodies (Coleman et al, 2014). Further investigation into the efficacy of this treatment modality in cats is needed.
Prognosis
Specific prognostic data for feline pancytopenia is lacking within the literature. Reported outcomes appear to be dependent on underlying cause and requirements for intensive treatment, with toxicity and immune-mediated diseases generally carrying more favourable prognosis (Weiss and Evanson, 2000). At the time of writing, the RVC had reported a mortality rate of 63% with the recent UK outbreak of feline pancytopenia (RVC, 2021), though specific data relating to survival times was not available at the time of writing. Table 3 provides a compilation of survival times reported in the papers referenced in this review, where available.
Conclusions
Pancytopenia is infrequently reported within the feline veterinary literature. Many of the articles referenced in this review paper report bicytopenia, with later progression to pancytopenia occasionally observed. For the purposes of this article these reports were included, as a bicytopenia still involves more than one cell line, so the diagnostic and therapeutic approach appears to be similar. Awareness of the differential diagnoses will aid the clinician in investigative steps; the discussion presented in this article highlights the need for a step-wise approach to these cases. Further studies are indicated to improve understanding pancytopenia in cats, as published data is limited in some areas.
KEY POINTS
- Pancytopenia is a clinical syndrome in which there is a reduction in circulating cells from erythroid and myeloid lines.
- Causes of reduced cell production include primary bone marrow disorders, bone marrow derangements secondary to neoplasia, infection and toxins, and peripheral destruction of cells.
- Treatment of pancytopenia aims to manage the underlying aetiology, and provide supportive or symptomatic care.
- Prognosis appears to be dependent on the underlying cause.


