An open fracture is defined as any fracture accompanied by a break in the skin that communicates with the fracture or its associated haematoma. They are characterised by contamination of the fracture site with microorganisms, and sometimes the introduction of foreign bodies into the wound (Zalavras, 2003). Moreover, depending on the severity of injury, there may be extensive damage to the soft tissue envelope and bone vascularity, which compromises healing potential. As a result, open fractures are associated with an increased risk of complications such as infection and nonunion and present a therapeutic challenge. The incidence of such complications is directly influenced by the emergency treatment approach and as such, the focus of this article is to help practitioners optimise their emergency management of open fractures and minimise complication rates.
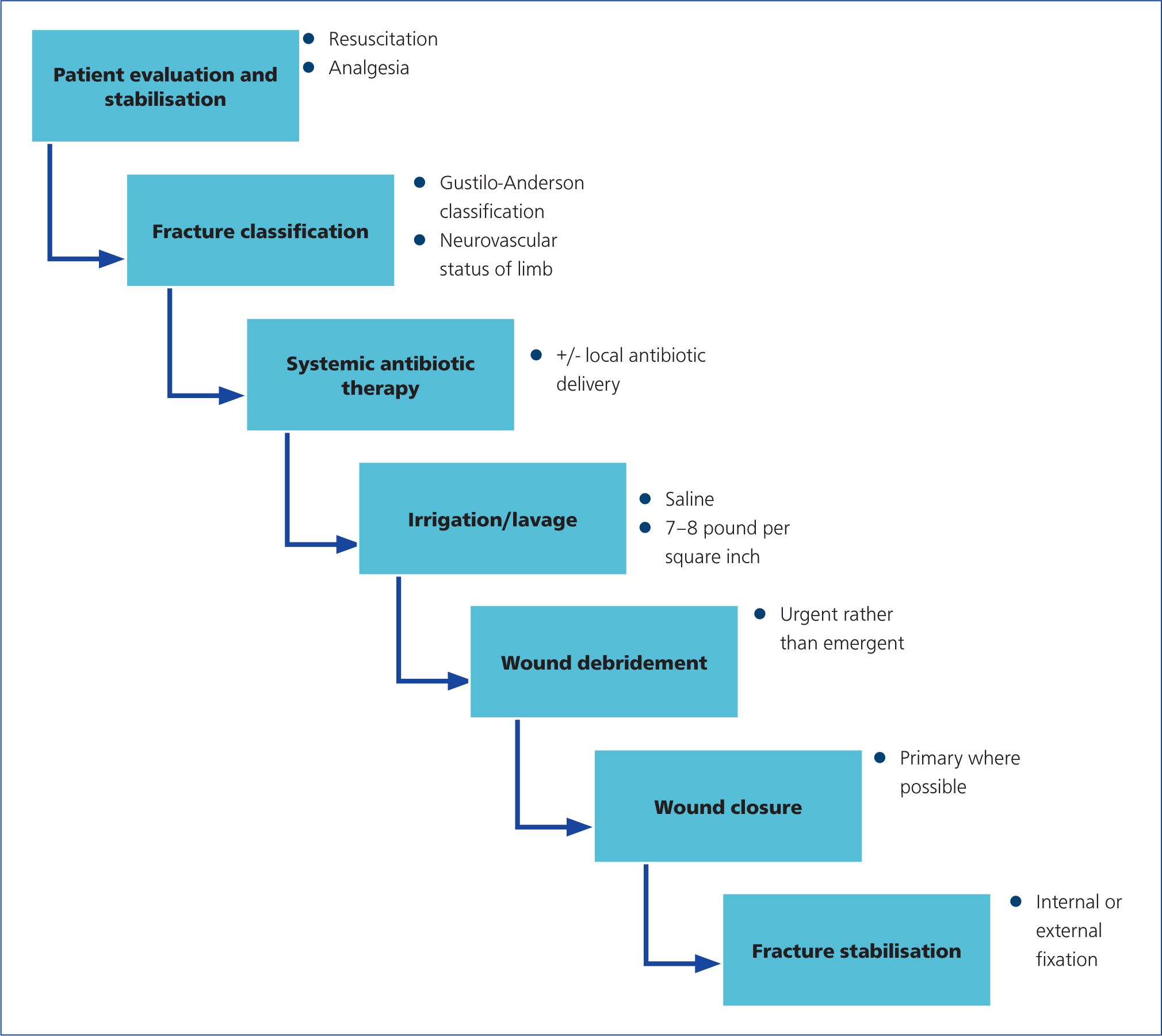
The goals of open fracture management are prevention of infection, fracture union and restoration of function. These goals are best achieved by careful patient and injury assessment, including:
- Fracture classification
- Early administration of systemic antibiotics
- Supplementation with local delivery of antibiotics in severe cases
- Thorough irrigation
- Surgical debridement
- Wound management, with soft tissue coverage if needed
- Stable fracture fixation (Zalavras, 2017) (Figure 1).
Pet owners are oft en anxious about a number of issues such as total length of treatment, the number of anaesthetics required, the quantity and frequency of re-checks, length of hospital stay, the likelihood of returning to an acceptable level of function and the risk of associated complications. The ability of veterinarians to provide guidance on these important issues is dependent upon experience and evidence. Unfortunately, evidence in the small animal literature regarding open fracture management is relatively lacking, prompting extrapolation from human medicine where appropriate. More evidence, specific to small animals, is required, but this review article uses the most recent information available, in conjunction with the most up-to-date data from human medical literature and the personal experience of the authors, to provide a comprehensive, evidence-based guide to emergency management of open fractures.
Patient evaluation and stabilisation
Patients presenting with an open fracture must be carefully assessed. Open fractures are typically the result of high-energy trauma (Anderson and Gustilo, 1980), such as automobile accidents, so any potentially life-threatening injuries should be investigated before addressing injuries to the limbs. A detailed and systematic evaluation of all organ systems should be performed, and resuscitation measures initiated where appropriate. Analgesia must also be attended to, although if possible, it is beneficial to withhold opioid medications until after the neurological status of the injured extremity has been assessed. While elaboration on this aspect of care is beyond the scope of this article, it represents a critical component of care which cannot be overlooked. While the assessment and resuscitation is ongoing, and until irrigation, debridement and wound care can be performed, a temporary sterile dressing should be applied to cover the open fracture wound and limit the risk of nosocomial infection.
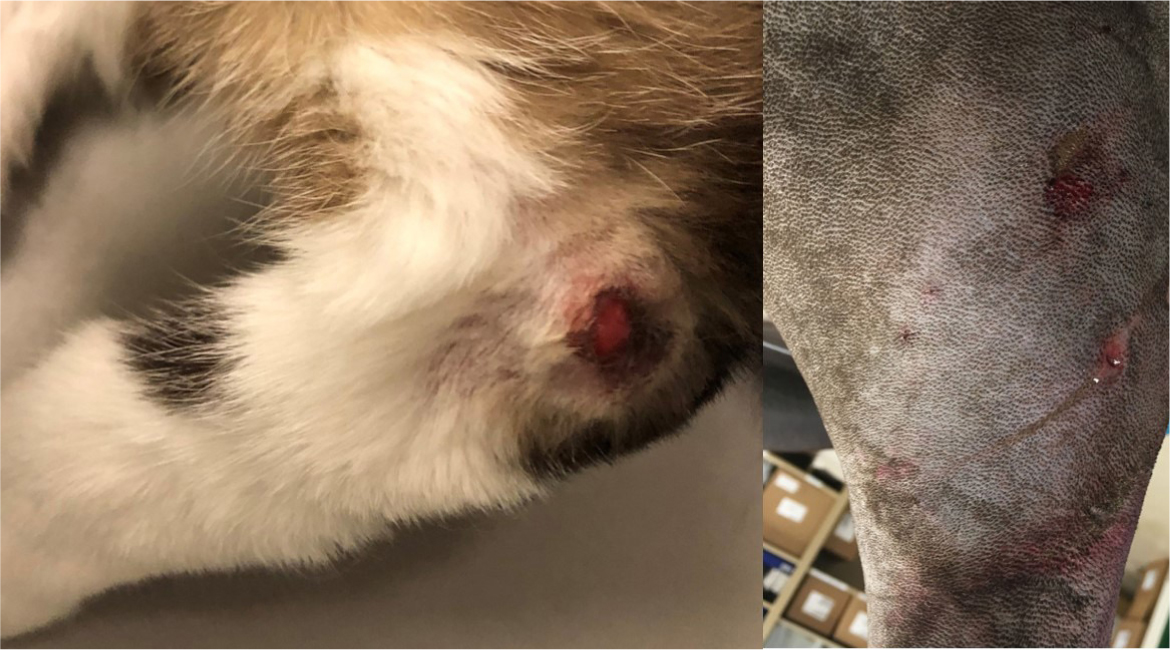
While high-energy trauma is the archetypal history for open fractures, it remains possible for less severe open fractures to occur with low-energy trauma. As such, all fracture patients should be assessed carefully for any evidence of an open wound that may communicate with the fracture site. Any soft tissue wound in proximity to a fracture should be treated as an open fracture until proven otherwise. The wounds associated with open fractures may be very small, so the limb may need to be clipped to identify them (Figure 2). Timely detection of such wounds, and the instigation of appropriate emergency management, can have a significant impact on the prognosis following such injuries.
Once stabilised, a full examination of the affected limb is performed. As a minimum, emergency management of the open fracture should include:
- Assessment and documentation of the neurovascular status of the injured limb
- Evaluation of the size, location and degree of gross contamination of the open fracture wound, leading to an initial classification of the fracture
- Placement of sterile, water-soluble lubricant within the wound, clipping of the hair widely and cleansing the peri-wound area with a 4% chlorhexidine solution
- Wound irrigation, removal of gross contamination and application of a sterile dressing. A full description of all the potential dressing types available is beyond the scope of this article but depending on wound characteristics, low adherent dressings, semipermeable films, hydrocolloids, alginates (with a secondary dressing), foam dressings or antimicrobial dressings may be appropriate
- Gross realignment of the fractured limb and, for fractures distal to the elbow or stifle, immobilisation with external coaptation. In the majority of cases, a traditional Robert Jones bandage is sufficient to minimise oedema and provide stability, thereby assisting with analgesia. In select cases, this may be reinforced with a splint or bivalved cast for additional stability but this is generally unnecessary as a temporary first-aid measure
- Systemic antibiotic therapy
- Imaging to characterise the fracture so that fixation can be planned
Fracture classification
Open fractures vary in severity depending on the mechanism and energy of injury. Classification systems have been developed to describe the injury, guide treatment, determine prognosis and compare various treatment methods. The classification system of Gustilo and Anderson (1976), subsequently modified by Gustilo et al (1984), has been extensively used in both human and veterinary medicine.
The Gustilo-Anderson classification was the first of its kind; a system through which clinicians could classify open fractures with some degree of reliability. The original article defined type I fractures as those with skin wounds <1 cm in length, which were clean with no overt evidence of contamination or infection (Gustilo and Anderson, 1976). Examples of type I fractures can be seen in Figure 2. Type II fractures involved lacerations >1 cm but without extensive soft tissue damage, flaps or avulsions. Another commonly included criterion was that type I fractures were generally created from inside to out, while type II fractures more commonly were created from outside to in. Type III fractures were defined as either segmental, with extensive soft tissue damage, or traumatic amputations. Special consideration was given to those involving gunshot injuries, or those requiring vascular repair.
Although the original description has stood the test of time, studies have highlighted that the way in which the classification was created and validated was not scientifically sound (Trompeter et al, 2020). The pooling of retrospective and prospective data, the use of a single outcome measure (infection) and the highly subjective descriptors for each group highlight significant limitations of the original system (Trompeter et al, 2020). Another limitation was that too many types were grouped together. The original severe type III covered a huge spectrum of injuries and in 1984, Gustilo expanded it into three subgroups. Type IIIa fractures were defined as having severe crushing soft tissue injuries and high degrees of contamination, yet were still closeable. Type IIIb were defined as having a loss of soft tissue cover and typically required a soft tissue flap, and type IIIc fractures had an associated arterial injury requiring repair. However, the significant use of subjective language that persists within this classification scheme remains a limitation (Trompeter et al, 2020). Gustilo further evolved the descriptions from the original paper, adding more subjective detail (Gustilo et al, 1990). Descriptions such as ‘extensive stripping of periosteum’ and ‘massive’ contamination appear. However, it remains impossible to quantify these terms and again this classification system is let down by subjective interpretation. This explains the major limitation of this system, in that the inter-observer reliability has been shown to be poor with only moderate (kappa 0.59) (Horn and Rettig, 1993) and average agreement of 60% (Brumback and Jones, 1994).
Despite these limitations, the Gustilo–Anderson classification scheme remains the most commonly used in veterinary medicine today (Figure 3) and classifications have been shown to determine the risk of infection in humans, ranging from 0–2% for type I open fractures, 2–10% for type II and 10–50% for type III (Gustilo et al, 1984; Patzakis and Wilkins, 1989). Multiple newer classification schemes have been proposed more recently, which may be advantageous for patient management, particularly in communicating accurate expectations to pet owners. A comprehensive review of all classification systems would require a separate article, but two particularly promising schemes warrant brief discussion.

In 2010, the Orthopaedic Trauma Association (OTA) open fracture study group published their classification system for open fractures in humans (OTA, 2010). This system takes into account five characteristic aspects of the pathoanatomy of an open fracture, irrespective of the anatomical site. These five aspects refer to the condition of the skin, muscle, vasculature, bone and degree of contamination. Again, there is some subjectivity and there remains some margin for error or misinterpretation (Agel et al, 2013; Trompeter et al, 2020). Again, there is some subjectivity: ‘extensive degloving’, ‘muscle loss’ and ‘muscle function’ are seen as descriptors and there remains some margin for error or misinterpretation (Trompeter et al, 2020). Nonetheless, this system was shown to be superior to the Gustilo-Anderson system in relation to the detail of description of open fractures (Ghoshal et al, 2018) and its prognostic value (Hao et al, 2016) and it has been reported to have moderate to excellent interobserver reliability.
The Ganga Hospital Open Injury Severity Score (GHOISS) was proposed in 2006 (Rajasekaran et al, 2006). Many of the shortcomings of the Gustilo-Anderson classification are addressed in this system. With these improvements, the GHOISS can provide a clear prediction of outcome in human patients with the most severe trauma to the lower leg. However, the primary focus of this score has been that of a decision making tool in limb salvage versus amputation, more than a true classification system aiming to offer guidance for reconstruction (Rajasekaran and Sabapathy, 2007). Good specificity and sensitivity (Madhuchandra et al, 2015) and ease of use have been reported, showing it to be simple to use in a clinical context (Rajasekaran et al, 2009). A framework such as this, which draws direct parallels between the type of injury and surgical treatment is, in theory, ideal. While the clear advantage of the GHOISS lies in its help in decision-making, therein also lies its downfall. The system does not function as a generalised classification system for open fractures, nor was it designed to. It was designed to address the poor sensitivity and specificity to predict salvage or outcome in Gustilo-Anderson type IIIa and IIIb fractures in people (Rajasekaran et al 2006). It cannot provide guidelines for surgical management or rehabilitation for less severe injuries (Rajasekaran and Giannoudis, 2012).
A major problem with all the classification systems to date is that they define groups of patients based on complication rates rather than functional outcomes. At present, there are extremely limited data, beyond anecdotal and personal experience, on which to base long-term patient prognoses. A full return to function represents a far greater measure of success than the presence or absence of infection and this should be considered in the development of future classification schemes (Trompeter et al, 2020). Additionally, while initial classification in the emergency room remains one of the best available ways to guide prognosis, it is important to remember that the true extent and severity of the injury cannot be fully assessed in the emergency room. The degree of contamination and soft tissue crushing injury are important factors for classifying an open fracture, which may be mistakenly overlooked in a wound of small size. Therefore, classification of the open fracture, and the impact on prognosis, should be finalised in the operating room following wound exploration and debridement (Zalavras, 2017).
Systemic antibiotic therapy
Early antibiotic administration is a key principle of open fracture management, because most patients with open fractures have contaminated wounds (Patzakis et al, 1974; Gustilo and Anderson, 1976). Systemic antibiotics should be administered in all patients with open fractures and local antibiotics in select cases. The role of antibiotics in reducing the infection rate in human patients with open fractures was demonstrated by Patzakis et al (1974) in a prospective randomised study. The infection rate when cephalothin was administered before debridement was 2.3% compared with 13.9% when no antibiotics were used.
Early antibiotic administration is very important as delayed administration of the first dose of antibiotic prophylaxis increases the risk of infection markedly (Wilkins and Patzakis, 1991). Patzakis and Wilkins (1989) initially reported that a delay longer than 3 hours post-injury was associated with increased risk of infection and subsequent animal and clinical studies have corroborated these findings. Penn-Barwell et al (2012a) used a rat femur model with a defect contaminated with Staphylococcus aureus and found that delaying antibiotics to 6 or 24 hours had a detrimental effect on the infection rate regardless of the timing of surgery. Lack et al (2015), in a retrospective study of 137 type III open tibial fractures in people, demonstrated that administration of antibiotics beyond 66 minutes from injury was an independent risk factor for infection with an odds ratio of almost four.
The optimal length of antibiotic therapy remains controversial. Evidence in the human field suggests that antimicrobial cover should commence within 3 hours of injury and should continue at least until first debridement. Antibiotics should continue to be administered until primary closure of the wound, or for a total of 72 hours, whichever is sooner (Lenarz et al, 2010; Venkatadass et al, 2017). A recent meta-analysis of comparative studies on antibiotic protocols demonstrated insignificant differences in the incidence of infection-associated complications with prolonged therapy (>72 hours), or a short course (<72 hours), irrespective of fracture severity. Comparable results were achieved when comparing even shorter courses (24–48 hours) to prolonged therapy (Messner et al, 2017). The recommendation in people is that antibiotic cover should not be extended beyond 72 hours unless there is an indicated need, such as the development of sepsis. An additional 2-day administration of antibiotics is recommended for subsequent surgical procedures, such as repeat debridement and wound closure (Patzakis and Wilkins, 1989; Templeman et al, 1998).
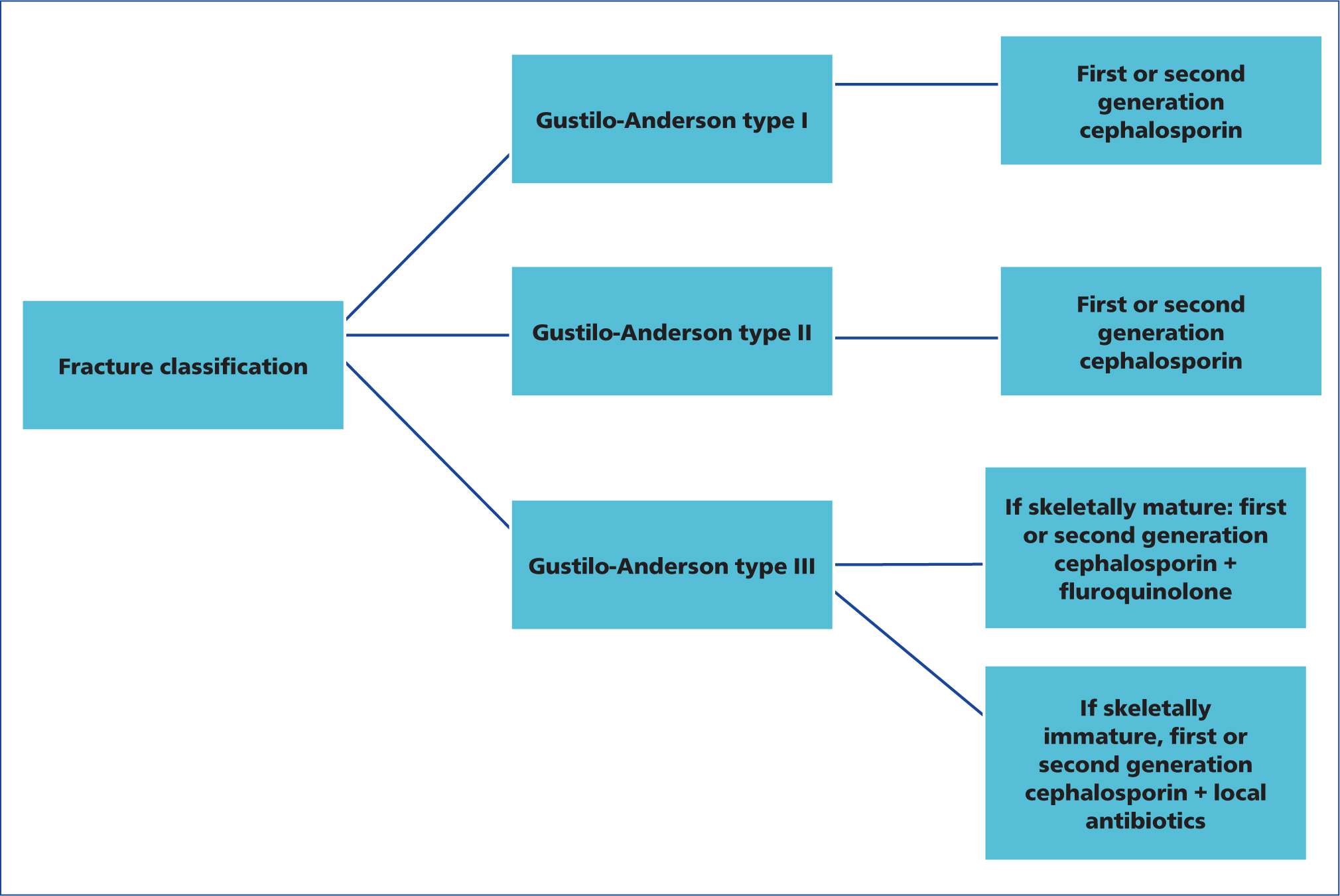
Although the necessity of antibiotics has been definitively established, there is no firm consensus on the optimal choice of agents and specifically, on the necessity of gram-negative coverage, especially for less severe injuries (Zalavras, 2017). In severe, type III fractures, there is wide agreement that gram-positive and gram-negative coverage is needed, and this is usually provided by a first-generation cephalosporin and historically, an aminoglycoside in people (Patzakis and Wilkins, 1989; Hoff et al, 2011; Luchette et al, 2000; Templeman et al, 1998; Zalavras et al, 2007). For less severe, type I and II open fractures, the necessity for gram-negative coverage remains controversial, with some authors recommending administration of a cephalosporin only (Hoff et al, 2011; Luchette et al, 2000; Templeman et al, 1998), and others maintaining that combination therapy is warranted (Patzakis and Wilkins, 1989; Zalavras et al, 2007). Some of the variation in these study findings may result from misclassification of the open fracture wound. As noted earlier, the severity of injury will be better appreciated in the operating room following wound exploration and debridement and when classified in the emergency room, it is possible that type IIIa fractures with a small open wound may be misclassified as a type I or II open fracture. As a result, a type IIIa fracture may be treated inappropriately with cephalosporin only, leading to a higher risk for infection.
Aminoglycosides for gram-negative coverage have fallen out of favour because of associated dose-dependent nephrotoxicity and irreversible ototoxicity. While these present less of a concern now that shorter courses of antibiotics are being recommended, alternatives have been considered and their use has become more widespread. Fluoroquinolones have been proposed as an alternative to aminoglycosides based on their broad-spectrum antimicrobial coverage, bactericidal properties, oral administration and good tolerance. A randomised prospective study in people showed that ciprofloxacin as a single agent in type I and II open fractures resulted in a similar infection rate (6%) compared with combination therapy with a cephalosporin and an aminoglycoside (Patzakis et al, 2000). However, in type III fractures, ciprofloxacin was associated with a higher infection rate of 31%, compared with 7.7% in the combination therapy group. While fluoroquinolones are commonly used as an alternative to aminoglycosides for open fracture management in companion animals, they do have a dose-dependent cytotoxic effect on bone healing in in vivo studies in rats (Huddleston et al, 2000), with histology indicating inhibition of endochondral ossification. Therefore, the use of fluoroquinolones may be contraindicated in juvenile open fracture patients (Garner et al, 2020).
Based on the aforementioned data or type I and II open fractures, either first- or second-generation cephalosporin is used by the authors. For type III open fractures in skeletally mature animals, a combination of cephalosporin and fluoroquinolone is used to provide both gram-positive and gram-negative coverage. In the face of a type III open fracture in a juvenile animal, where the use of fluoroquinolone prompts concern, a first- or second-generation cephalosporin in conjunction with local antibiotic administration is used to provide broad-spectrum coverage. This decision-making process is detailed in Figure 4.
Wound cultures obtained at patient presentation or intraoperatively do not help select the optimal antibiotic regimen, because they fail to identify the organism causing a subsequent infection in most cases (Fischer et al, 1991; Lee, 1997). Depending on the study, only 18–26.9% of infections that develop in people following open fractures are caused by an organism identified by perioperative cultures (Patzakis et al, 2000; Bosse et al, 2017). Studies have also investigated the effect of timing of culture and one such study reported that pre-debridement cultures identified the infecting organism only 22% of the time, with post-debridement cultures identifying the causative organism 42% of the time (Lee 1997). Based on this study by Lee et al (1997), post-debridement cultures would be preferable to pre-debridement cultures, but their accuracy remains suboptimal. In most cases, infections are not caused by the organisms initially present in the wound, but by nosocomial organisms that subsequently contaminate it (Zalavras, 2017). Indeed, one study reported that 92% of open fracture-associated infections were caused by bacteria acquired while the patient was hospitalised (Carsenti-Etesse et al, 1999). As such, routine cultures at the time of injury are not recommended (Garner et al, 2020). However, there is consistent agreement that cultures should be performed if active infection is noted during the healing process, in order to guide antibiotic use.
Local antibiotics
Local delivery of antibiotic therapy is commonly used as an adjunct to systemic antibiotics in people with open fractures and is becoming more common in veterinary patients also. A commonly used delivery vehicle is polymethylmethacrylate (PMMA) cement, which can be manually moulded by the surgeon into beads with diameters ranging from 5–10mm. Several antibiotics including aminoglycosides, vancomycin and cephalosporins can be successfully incorporated into PMMA cement for local delivery.
Morgenstern et al (2018) performed a systemic literature review with a pooled data analysis investigating local antibiotic prophylaxis in people. They compared standard systemic antibiotic treatment with standard antibiotic prophylaxis plus additional local antibiotic prophylaxis. In 2738 patients, an overall infection rate of 7.9% was determined. Patients treated with additional local antibiotics suffered infection in 4.6% of cases, while infection occurred in 16.5% of all patients with only systemic antibiotic prophylaxis. Owing to heterogeneity within the groups, a low level of evidence in the primary studies and a potential risk of bias, it was advised to interpret these results with caution (Morgenstern et al, 2018).
Since PMMA bead chains are not resorbable, removal of the beads requires a second surgery. Bioabsorbable delivery vehicles such as calcium sulphate, collagen fleeces and phospholipid gels appear to be promising alternatives (Sørensen et al, 1990; McKee et al, 2002; Penn-Barwell et al, 2014), although there is a limited evidence-base for their use in open fractures. Only two case series in humans have been published, using calcium sulphate pellets as antibiotic carriers in open fracture treatment (Cai et al, 2010; Helgeson et al, 2009), and to the authors' knowledge, there is only one small case series in dogs and cats that evaluates the use of antibiotic-impregnated calcium sulphate beads (Peterson et al, 2021). In this study 16 cases were included, 10 of which were treated therapeutically and six of which were treated prophylactically. Infections did not occur in any of the six cases treated prophylactically, while infection resolved in 6 out of the 10 cases treated therapeutically. Major complications associated with bead implantation were not encountered (Peterson et al, 2021). Further studies are certainly required before definitive recommendations can be made in humans or in companion animals, but implantation does appear to be well tolerated. The authors have used antibiotic-impregnated calcium sulphate beads during stabilisation of open fractures in a small number of cases (Figure 5), none of which have developed infection to date and as such, the preliminary findings appear to mirror those of the small case series mentioned above.

Modern bioabsorbable carriers are able to elute therapeutic concentrations of antibiotic for over seven days, based on pharmacokinetic studies (Stockley et al, 2008, Howlin et al, 2015; Stravinskas et al, 2016; Van Moojen et al, 2008). Hence, local antibiotic therapy may allow for shorter courses of systemic antibiotics to be used for orthopaedic infection (Buchholz et al, 1981; Calhoun et al, 1993; Stockley et al, 2008; Klemm, 2001; Taggart et al, 2002; Hoad-Reddick et al, 2004), limiting the frequency of side effects, cost and selection pressure for antimicrobial resistance. Shorter courses of systemic medications are also likely to improve client compliance, which in veterinary medicine is often suboptimal (Adams et al, 2005; Papich, 2013). A randomised, controlled open-label non-inferiority trial evaluating the duration of systemic antibiotics in adults with orthopaedic infection treated operatively and with local antibiotic therapy is ongoing in humans, comparing short or long systemic antibiotic regimes (Dudareva et al, 2019). If a strategy using local antibiotics in combination with a short systemic antibiotic regime is shown to be non-inferior, this will be advantageous for the patient, as well as contributing to antimicrobial stewardship.
Irrigation or lavage
Irrigation or lavage serves to decrease bacterial load and remove foreign material from the wound, another pivotal component of open fracture wound management. While most protocols recommend copious volumes of irrigation fluid, despite intensive preclinical and clinical research, no reliable data for the appropriate volume of lavage solution exists (Rupp et al, 2020).
Historically, there has been substantial debate regarding exactly which solution should be used for irrigation and at what pressure. The fluid lavage of open wounds trial (FLOW) was initiated to answer these questions, in a multicentre prospective randomised controlled trial, including 2447 patients at 41 sites (Bhandari et al, 2015). Three different wound irrigation pressures, as well as saline versus castile soap for irrigation solution, were compared. The primary endpoint was reoperation for the promotion of wound or bone healing and treatment of wound infection within a follow-up period of 12 months. Reoperation rates were not found to depend on irrigation pressures, but reoperation was more frequent after the use of a soap irrigant (14.8%) compared to saline (11.6%) (Bhandari et al, 2015). Patient-reported outcomes during 12-month follow-up did not depend on irrigation solutions or irrigation pressure (Sprague et al, 2018). Thus, the potential drawbacks of high pressure irrigation with bacterial seeding into the intramedullary canal (Bhandari et al, 1998) and myonecrosis of soft tissue (Chiaramonti et al, 2017) evidenced in animal experiments could not be proven to be clinically relevant and low pressure irrigation seems to be a viable and economical treatment option. This research indicates that the lavage pressures commonly adopted in veterinary medicine, of 7-8 pounds per square inch (psi), appear to be appropriate; this pressure can be reliably achieved using a 1-litre fluid bag with an external pressure sleeve at 300mmHg. While the use of large syringes and needles likely produces pressures substantially higher than 8psi, given the lack of significance of irrigation pressure in the FLOW trial mentioned above, this is not considered to be contraindicated.
Other authors have proposed the use of antibacterial or antiseptic solutions for wound irrigation, but this is no longer recommended as antiseptic solutions may be toxic to host cells and are better avoided (Bhandari et al, 2001). In a rat model of open fracture, when saline and the antiseptics iodophor and hydrogen peroxide were compared for irrigation, saline was found to be comparably effective and superior in minimising wound inflammation compared to both antiseptics (Cheng et al, 2015). Toxic effects against host tissue have been reported for chlorhexidine as well, in addition to a rebound effect of bacterial growth caused by chlorhexidine-induced tissue damage (Penn-Barwell et al, 2012b). When considering the use of antibiotics in irrigation solutions, clinical data comparing human patient outcomes such as fracture union, development of infection and healing of soft tissue wounds did not demonstrate an advantage of bacitracin solution. Indeed, significantly more wound healing disturbances were noted in the antibiotic additive group (Anglen et al, 2005).
Reasonable alternatives to saline appear to be distilled water, and even tap water. Comparing saline with distilled water in a clinical prospective trial (Olufemi et al, 2017) and with tap water in a preclinical porcine open fracture model (Penn-Barwell et al, 2012b), demonstrated comparable outcomes for both compared to saline solution. For open fracture cases with limited contamination, the authors use sterile isotonic saline in large volumes for wound irrigation. For severely contaminated cases, initial irrigation is carried out with tap water, in order to limit cost to the client, followed by large quantities of sterile saline for the final stages.
Debridement
Thorough surgical debridement plays a critical role in the management of open fractures (Swiontkowski, 1989; Tetsworth and Cierny, 1999). Devitalised tissue and foreign material promote the growth of microorganisms and constitute a barrier for the host's defense mechanisms and therefore should be removed. Debridement should be performed in the operating room. Surgical extension of the wound allows assessment of the degree of soft tissue damage and contamination. Skin and subcutaneous tissue are sharply debrided back to bleeding edges. Muscle is debrided until viable tissue is identified by its bleeding, colour and contractility. A repeat debridement can be performed after 24–48 hours based on the degree of contamination and soft tissue damage. The goal is a clean wound with viable tissues and no infection. In injuries requiring flap coverage, debridement should also be repeated at the time of the soft tissue procedure (Zalavras, 2017).
Traditionally, debridement has been recommended within 6 hours of presentation. The rationale behind this figure is believed to date back to early studies on microorganism load following contamination (Friedrich, 1898; Werner et al, 2008). In more recent literature, the 6-hour window does not show a clear evidence base. Many human studies do not show a significant difference in the incidence of infection, regardless of whether debridement is performed early or is delayed, as long as antibiosis is attended to appropriately (Halawi and Morwood, 2015; Ali et al, 2013; Werner et al, 2008; Schenker et al, 2012). Patzakis and Wilkins (1989) reported that the infection rate was similar in open fracture wounds debrided within (6.8%) and after (7.1%) 12 hours of injury and concluded that elapsed time from injury to debridement is not a critical factor for development of infection in patients receiving antibiotic therapy. Schenker et al (2012) performed a meta-analysis on the effect of timing on the overall risks of infection in open long bone fractures in people and again, found no difference in the rate of infection following delayed debridement irrespective of injury severity or anatomical location. Although bacterial populations in an untreated contaminated wound do increase over time, it appears early antibiotic administration and thorough surgical debridement can effectively reduce contamination. As a result, small delays in surgical management do not appear to translate to increased infection rates and may allow for stabilisation of the patient, as well as for treatment of the patient by experienced surgical teams with all the necessary equipment available (Zalavras, 2017).
Wound closure
The optimal timing of wound closure also remains controversial. Historically, concerns about deep infection by anaerobic organisms led to the practice of not closing wounds in open fractures (Trueta, 1976). Delayed wound closure prevents anaerobic conditions in the wound, permits drainage, allows for repeat debridement at 24–48 hours and gives time for tissues of questionable viability to declare themselves.
However, in recent years, primary wound closure has been proven to be beneficial in people, resulting in lower deep infection and nonunion rates. Scharfenberger et al (2017) demonstrated fewer deep infections (4% vs 9%) and nonunions (13% vs. 29%) in Gustilo-Anderson type I-IIIa fractures when comparing immediate wound closure to delayed soft tissue coverage. Jenkinson et al (2014) described similar results, with an infection rate of 4.1% in fractures treated with immediate closure, compared to 17.8% in cases treated with primary delayed closure. For fractures requiring soft tissue reconstruction by flap coverage, low infection rates of 1.5% were reported after flap reconstruction within 72 hours (Godina, 1986), compared to an infection rate of 60% in similar injuries treated via flap coverage after 7 days (Olesen et al, 2015).
Based on the literature, primary closure of open fracture wounds is recommended in select cases, provided there is no severe tissue damage or contamination, early administration of antibiotics has taken place, meticulous debridement has been executed and the wound edges can be approximated without tension (Zalavras, 2017). In such cases, where necessary, the use of muscle flaps appears to be superior to the use of fasciocutaneous flaps (Harry et al, 2008). Delayed closure remains recommended in cases with extensive soft tissue damage and gross contamination, in patients presenting with a considerable delay, in wounds with tissues of questionable viability and in wounds that cannot be approximated without tension (Zalavras, 2017). In cases where delayed wound closure is elected, the wound should not be left open to the outside environment, to prevent contamination with nosocomial pathogens. One option to seal the wound is a technique that involves the insertion of antibiotic-impregnated PMMA beads into the open fracture wound, which is then sealed by a semipermeable barrier; the so-called ‘bead-pouch technique’. Negative pressure wound therapy can also be used (Okike and Bhattacharyya, 2007).
Fracture stabilisation
As the focus of this article is on the emergency management of open fractures, significant discussion of stabilisation is beyond the scope, although stabilising an open fracture remains an important component of management. Stability at the fracture site prevents further injury to the soft tissues and enhances the host response to contaminating organisms (Worlock et al, 1994). Fracture stability facilitates wound and patient care and allows early motion and functional rehabilitation of the extremity. Fracture stabilisation can be accomplished with interlocking nails, plate and screw fixation or external fixation. Selection among these methods depends upon careful evaluation of the fracture, soft tissue and patient characteristics. More than one method may be applicable to a specific injury.
Future directions
While standards on antibiotic cover and management of soft-tissue injuries have helped to reduce the incidence of infection and consequent complications following open fracture, the risk remains higher than for closed injuries. Injuries complicated by infection are more likely to require further operative management (Zelle and Boni, 2015) and experience complications with bony union. As such, investigation of adjunctive therapies with the potential to further reduce complication rates and enhance functional outcomes are ongoing.
One promising area is in the use of recombinant human bone morphogenetic proteins (rhBMP) 2 and 7, which have shown promise in the treatment of open fractures in people. The use of rhBMP-2 enclosed in an absorbable collagen sponge has been shown to be safe and efficacious in the management of open fractures, with a lower need for secondary surgical intervention, faster fracture healing and fewer infections seen (Govender et al, 2002; Swiontkowski et al, 2006). This may hold promise in the future, pending further research for both people and companion animals.
Another area of ongoing research is that of antimicrobial functionalising of the implant surface, with a view to avoiding the formation of biofilm on metallic implants. Different antimicrobial coatings such as poly(d, l-lactide), silver and povidone-iodine have been tested in clinical studies (Schmidmaier et al, 2006, 2017; Hardes et al, 2010; Fuchs et al, 2011;; Tsuchiya et al, 2012; Shirai et al, 2014; Metsemakers et al, 2015). Except implants functionalised with gentamicin coatings, none of the clinically tested coatings have been used for open fractures. Two case series in people reported favourable outcomes in 8 and 19 patients treated for open tibial shaft fractures, respectively (Schmidmaier et al, 2006; Fuchs et al, 2011). Lower infection rates for silver coated megaprostheses in tumoral prosthesis surgery (Hardes et al, 2010) and salvage revision arthroplasty (Zajonz et al, 2017) suggest a clinical application of silver coated implants in fracture care. However, no clinical studies of silver coated implants in fracture care exist, yet. Additional research is required in this area but it may present a promising route to lowering complication rates for open fracture management.
Conclusions
Open fractures are associated with an increased risk of complications, such as infection and nonunion, and appropriate emergency management is critical to reducing these risks. The early instigation of broad-spectrum antibiosis is essential, as delays in administration directly correlate with increased infection rates. While debate persists regarding the optimal agent choice, for grade I and II fractures coverage with first- or second-generation cephalosporins appears to be appropriate, while fluoroquinolone should be added for type III fractures. Local antibiotics may play a valuable role in select cases based on the limited evidence presented and the ready availability of resorbable delivery vehicles renders their use inherently more attractive. Wound irrigation represents a pivotal component of emergency management and high-quality research indicates that sterile saline, at any pressure between 2 and 20 psi, leads to the lowest infection and reoperation rates, although distilled water or even tap water may represent reasonable alternatives. Provided appropriate systemic antibiosis has been administered, small delays in wound debridement do not translate to increased complication rates, so delaying this until an experienced surgical team is available is recommended. As the majority of open fracture infections appear to be caused by nosocomial bacteria that contaminate the wound, rather than bacteria present at the time of injury, wound cultures are generally not indicated to guide antibiotic choice and primary wound closure should be performed where appropriate. In cases with extensive soft tissue damage, gross contamination, tissues of questionable viability, or where wounds cannot be closed without tension, delayed closure remains the prudent approach, but every effort should be made to seal the wound from the external environment to limit contamination with nosocomial pathogens.
KEY POINTS:
- Open fractures are associated with an increased risk of complications, such as infection and non-union, and appropriate emergency management can significantly reduce these risks
- The most commonly used classification scheme for open fractures in veterinary medicine remains the Gustilo-Anderson classification. While limitations of this scheme are recognised, classification does determine the risk of infection and as such, can be used as a basis to discuss prognosis with owners
- Early instigation of broad-spectrum antibiosis is essential in animals with open fractures as delays in administration directly correlate with increased infection rates
- Neither pre- nor post-debridement wound cultures are recommended to guide antibiotic choice as the majority of open fracture infections appear to be caused by nosocomial bacteria that contaminate the wound, rather than bacteria present at the time of injury
- Wound irrigation is a critical component of emergency open fracture management with sterile saline representing the ideal irrigation agent at any pressure being 2–20 pounds per square inch.


