Chronic kidney disease (CKD) is a highly prevalent disease and common cause of morbidity and mortality in older cats. It is estimated to affect up to 30–40% of cats over the age of 10 years (Lulich, 1992) and is the cause of death of over 13% of cats (O’Neill et al, 2015). There are thought to be a number of potential underlying aetiologies contributing to disease, but the risk factors, other than age (Sparkes et al, 2016), and causes associated with the development of CKD are still not well elucidated and most cases are classified as idiopathic in origin. There is, however, an increasing awareness that early diagnosis and management of CKD can slow down the progression of disease and have a significant impact on longevity as well as quality of life. This has a number of challenges and controversies associated with it. Dietary management, ideally with a therapeutic renal diet, is arguably the most important aspect of treatment for cats diagnosed with CKD in International Renal Interest Society (IRIS) stage 2 onwards (Polzin and Churchill, 2016), and generally accepted as such by most veterinarians. However, given that until relatively recently veterinarians have not been able to identify early CKD (IRIS stage 1 disease), and that there are a limited number of studies looking at management of cats at this early stage in disease, the best diet for these cats is still unknown. This article explores the current understanding of therapeutic strategies and the impact this can have on progression of the disease, with a particular focus on dietary management, exploring potential dietary changes and at what point they should be made.
Nutritional management for International Renal Interest Society stage 1 feline patients
While dietary intervention is regarded as a cornerstone of management of CKD, the timing of renal diet introduction and the degree of protein restriction required is more controversial (Witzel, 2018). A number of different commercial renal diets is available in the UK (Figure 1), all with relatively similar nutrient adaptations to help slow progression of kidney disease, prolong survival time and maximise quality of life. These diets are generally recommended from IRIS stage 2 CKD onwards. The common characteristics of these diets are detailed in Table 1, with protein and phosphate restriction considered the most important feature of commercial renal diets, and responsible for conferring the major benefits seen (Sparkes et al, 2016). The evidence base for recommendation of most of these diets centres on starting in IRIS stage 2 of CKD: studies for clinical efficacy have historically focused on these patients since it is only recently that clinicians have started to identify cats with early, non-azotaemic CKD more often. Thus optimal dietary management in IRIS stage 1 is currently still unknown, and both identification of the best diet for a cat with IRIS stage 1 disease and timing for implementation can be a challenge for clinicians.

Table 1. The main characteristics of different commercially available diets for nutritional support of cats with chronic kidney disease and the rationale behind such modifications
| Nutrient modification | Rationale for modification and potential benefits for patient |
|---|---|
| Phosphorus restriction | Reduces plasma parathyroid hormone levels, slows the progression of chronic kidney disease, reduces morphological damage to the kidneys and minimises risk of secondary renal hyperparathyroidism. Unless treated, renal hyperparathyroidism progressively worsens and is associated with reduced survival time. High levels of parathyroid hormone may contribute to the uraemic state by acting as a uraemic toxin, and contribute to ongoing renal damage by increasing calcium influx into renal tubular cells and precipitation of calcium phosphate in the lumen of the tubules. Phosphate restriction is thought to be the most important influencer of survival, and is recommended by International Renal Interest Society (IRIS) in all cats from IRIS stage 2 onwards |
| Protein restriction | Restricted levels of high quality proteins minimise the levels of nitrogenous waste and formation of uraemic toxins which contribute to nausea and reduced quality of life. Reduced levels of protein can also reduce proteinuria, which is a key prognostic factor. Quality, as well as level, is important, and proteins fed should have a good amino acid profile and high bioavailability. Amount of protein restriction varies between different commercially available renal diets, and there is controversy about the degree of protein restriction required. Protein is not thought to be a key influencer of survival time per se, but has an important impact on quality of life |
| Increased potassium | Helps to compensate for potential excessive loss of urinary potassium in chronic kidney disease to help maintain normokalaemia and reduce — although not completely remove — the risk of hypokalaemia, which can contribute to on-going renal damage and to inappetance and lethargy |
| Non-acidifying | Acidosis is common in renal disease — diet may help to control this and cats fed a renal diet can show higher blood bicarbonate levels |
| Increased omega 3 fatty acids | Increased essential fatty acids may help reduce glomerular hypertension, and minimise glomerular inflammation. Renal diets with higher omega 3 fatty acid levels may be associated with a longer survival time, although a causal relationship has not been established |
| Increased B vitamins | Compensate for the loss of water-soluble B vitamins in diuresis which can otherwise contribute to deficiencies with resultant inappetance and other clinical signs |
| Antioxidants | Reduce oxidative damage to the kidneys, which is exacerbated in chronic kidney disease. Whether antioxidants within renal diets have a clear renoprotective effect in chronic kidney disease is still to be determined |
| Reduced sodium | May help control blood pressure in cats with concurrent hypertension, although evidence for a beneficial effect in cats with chronic kidney disease is still lacking |
Most commercially available renal diets are fairly similar, although there is some variability in exact nutrient levels, particularly protein. They are modified based on the impact of declining kidney function, and also supplemented with nutrients such as omega 3 polyunsaturated fatty acids and antioxidants thought to ameliorate complications of CKD. Most renal diets also tend to have an increased fat content to increase energy density and palatability, as appetite and maintenance of body condition score can be a challenge in cats with CKD. Ensuring adequate calorie intake in cats with CKD is critical: weight loss can be detected in cats before diagnosis of CKD, accelerates after diagnosis, and is associated with poorer prognosis (Freeman et al, 2016).
What evidence do we have to inform nutritional management?
From International Renal Interest Society stage 2 chronic kidney disease onwards
Several studies (retrospective and prospective) have looked at the effect of feeding cats with CKD on a renal diet and the potential benefits this has (Elliott et al, 2000; Plantinga et al, 2005; Ross et al, 2006), particularly with respect to minimising uraemic crises and extending survival. In three separate controlled studies, cats with IRIS stage 2 or later CKD fed on a therapeutic diet designed for renal disease (thus incorporating most or all of the characteristics in Table 1) lived for longer than cats on a control diet. Plantinga et al (2005) found an increased median survival time of 270 days for cats fed on the renal diet, while Elliott et al (2000) found an increased median survival time of 369 days. While all studies had limitations, the consistency of findings of an improved clinical outcome in cats with CKD when fed a renal diet across the studies have been useful to inform decision making on diet from the point at which cats with CKD develop azotaemia. Each study used slightly different diets and methodologies, and they were based in different countries using different populations of cats, but all found similar results — that feeding a renal diet appeared to offer a longer survival and reduce uraemic crises in cats compared with when fed a control diet.
Elliott et al (2000) conducted one of the first prospective studies done to examine the effect of feeding a veterinary renal diet (restricted in phosphorus and protein and differing in other nutrients, including increased levels of B vitamins and decreased levels of sodium) to a group of 50 cats with stable azotaemic CKD in IRIS stage 2–4. Twenty nine cats and their owners accepted the diet. Compliance was not achieved in 21 cats which were subsequently fed a diet considered an appropriate adult maintenance diet (control group). Cats were matched for age, bodyweight, and relevant blood and urine parameters, and every attempt was made to treat both groups of cats in the same way, regardless of the diet they were fed. If and when the veterinary diet alone became inadequate to control blood phosphate concentrations, cats in this group had an intestinal phosphate binder added to their food. No attempts were made to control blood phosphate levels in the control group. Cats fed the veterinary diet showed lower plasma phosphate and urea levels, indicating that the diet helped control hyperphosphataemia. They demonstrated a better ability to maintain plasma parathyroid hormone, whereas increasing levels were seen in cats in the control group, suggesting the diet helped control secondary renal hyperparathyroidism. They also had a markedly increased survival time (median survival times of 633 vs 264 days). While the authors acknowledged there were some limitations to the study, including the fact that it was not randomised or masked, and thus there could have been an element of bias introduced by the treating veterinarian, the effects of this were relatively minimal since the authors felt the effects of feeding the veterinary diet would have been evident from the biochemical results of the majority of cats involved, making the study challenging to conduct in a blinded manner. The authors concluded that their results suggested feeding of a veterinary diet specifically formulated for cats with CKD together with the use of phosphate binders if required significantly increased survival time from initial diagnosis (Elliot et al, 2000).
Ross et al (2006) found azotaemic IRIS stage 2 or 3 cats fed a renal diet had lower urea and phosphate levels, fewer uraemic crises and fewer renal-related deaths compared with cats fed an adult maintenance diet in a 2-year study. This was a randomised, masked clinical trial containing 45 cats (22 cats fed a manufactured renal diet and 23 cats fed a maintenance diet). The renal diet contained lower levels of protein, phosphorus and sodium and increased omega 3 fatty acid levels compared with the maintenance diet. There were no significant differences in bodyweight, haematocrit or urine protein:creatinine (UPC) ratio, or in serum creatinine, potassium, calcium, and parathyroid hormone concentrations between the two groups. During the study, none of the cats on the renal diet suffered a uraemic crisis, and there were no renal-related deaths; in comparison, five cats on the maintenance diet died of renal causes (Figure 2). The authors concluded that a renal diet was superior to an adult maintenance diet to minimise uraemic episodes and renal-related deaths.
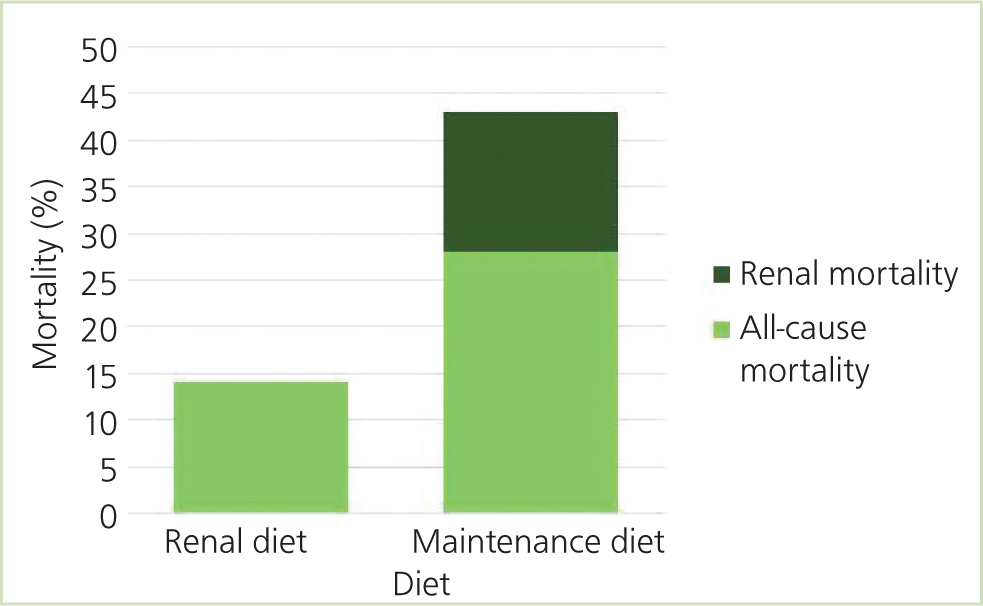
A retrospective study compared survival times for cats with IRIS stages 2, 3 and 4 fed on a commercial feline renal diet with cats not fed a renal diet (Plantinga et al, 2005). Median survival time for cats fed a renal diet was 480 days compared with 210 days on the maintenance diet (Plantinga et al, 2005).
There is now a relatively large evidence base to support the use of renal diets for cats with azotaemic CKD to help improve survival time, reduce the risk of uraemic crises, lower serum phosphate concentrations and prevent an increase in parathyroid hormone concentrations. Benefits are thought to be a result of the multiple nutrient adjustments, rather than a single dietary element, although protein and phosphate restriction together are likely to be most influential. Further studies are required to investigate the importance of different individual nutrient levels. Dietary protein levels are particularly debated, and the optimum level — if one exists — is still unknown (Boxes 1 and 2). Further studies are needed to look at the impact of different levels of dietary protein on bodyweight, body condition score and muscle mass.
Box 1.What evidence is there to suggest the optimal level of protein restriction in chronic kidney disease?
- Protein restriction offers the advantages of minimising uraemic toxin production and minimising protein filtered through the glomeruli which can exacerbate glomerular damage and proteinuria. However, cats are obligate carnivores and when fed inadequate dietary protein they catabolise their lean muscle to meet their metabolic needs. A balance needs to be struck between protecting the kidneys from further damage and maximising quality of life by minimising uraemic crises, while also trying to maintain diet palatability and body musculature
- Feline renal diets typically contain 60–70 g protein/1000 kcal
- The National Research Council (2006) minimum guideline protein level for cats is 50 g/1000 kcal. The European Pet Food Industry (FEDIAF, 2019) guidelines cite a minimum protein level of 62.5 g/1000 kcal
- Most feline maintenance diets contain 90–100 g protein/1000 kcal
- A study by Laflamme and Hannah (2013) demonstrated that cats consuming 95 g protein/1000 kcal maintained lean body mass but cats consuming 73 g protein/1000 kcal lost lean body mass (Box 2). However, it was unclear whether this suggests cats’ protein requirements may have previously been underestimated since cats in this group also consumed inadequate energy which may have contributed to muscle catabolism. The authors concluded that a typical healthy adult cat consuming 50–60 kcal/kg bodyweight per day, should have 30–40% of the dietary calories coming from protein (Laflamme and Hannah, 2013)
- One research abstract evaluated lean body mass in cats with early IRIS stage 2 chronic kidney disease (Yu et al 2011). After 30 weeks, serum albumin levels and lean body mass were maintained on a diet of 65.3 g protein/1000 kcal, suggesting this could be adequate at least in early stages of chronic kidney disease. However, this was a small study containing only seven cats
- A study of 10 cats with unstaged chronic kidney disease fed on diets containing 45 g, 57 g and 68 g protein/1000 kcal on rotation for 4-month intervals showed that when on the 45 g protein diet there was weight loss and reduced lean body mass despite higher energy intake compared with cats on the 68 g protein diet (Kirk and Hickman, 2000). Cats also showed lower albumin levels on the 45 g protein diet. This suggested levels closer to the higher protein content diet may be more beneficial for cats with chronic kidney disease. Again, however, this was a small scale study and chronic kidney disease was not staged
- At the time of writing, the optimal protein level in the early stages of chronic kidney disease and later in disease are both still unknown. Focus on protein restriction from the point of development of azotaemia can reduce uraemic crises, but caution may be needed not to implement protein restriction too early in disease and potentially cause or exacerbate loss of lean muscle mass or weight with a potentially negative effect on morbidity.
Box 2.Study set up looking at protein requirements of adult cats to maintain nitrogen balance and lean body mass (adapted from Laflamme and Hannah, 2013)20 healthy male neutered adult cats (aged 4–8 years) were fed a baseline (34% protein) diet for 1 month, and then one of three types of protein diet for 2 months. The veterinarians involved in the study were blinded to the specific dietary treatments. At the beginning and end of the study urine and faeces were collected over a 96-hour period for each cat to allow assessment of the nitrogen balance, and dual energy X-ray absorptiometry scans were performed on all cats to assess lean body mass. The results were:
| Diet | Results | Conclusions |
|---|---|---|
| 34% (95g/1000kcal ME) | Maintained nitrogen balanceMaintained lean body mass | Adequate protein and energy to maintain lean body mass |
| 26% (73g/1000kcal ME) | Maintained nitrogen balanceLost lean body mass | Lower energy intake vs 95g protein group — is loss of lean body mass a result of inadequate protein or insufficient energy? |
| 20% (57g/1000kcal ME) | Maintained nitrogen balanceLost lean body mass | Inadequate protein level to maintain lean body mass Nitrogen balance can be maintained but lean body mass lost, so nitrogen balance is a poor indicator of protein requirements in cats |
For ‘early’ International Renal Interest Society stage 1 chronic kidney disease?
Although compelling evidence exists for the benefits of renal diets in azotaemic cats, optimal dietary management for cats with early CKD and an absence of azotaemia is still highly debated. While it is known that early interventions in the disease can improve prognosis, the form that these interventions should take at stage 1 or early stage 2 with respect to dietary management is still unknown. It is becoming an increasingly important area of research as clinicians successfully diagnose early CKD more frequently.
One study investigated whether dietary interventions in non-azotaemic cats with increased serum symmetric dimethylarginine (SDMA) concentrations but normal creatinine levels (IRIS stage 1) could improve renal function based on a decrease in serum SDMA. A test diet designed to promote healthy ageing was fed, containing functional lipids (fish oil), antioxidants (vitamins C and E), L-carnitine, botanicals (as vegetables), high quality protein (wet meat chicken), and increased amino acids. Cats on the test food were more likely to have stable renal function (indicated by stable serum SDMA concentrations) than cats fed owner’s-choice food over a 6-month period. The test diet was not phosphorus restricted, and the study did not assess serum phosphorus levels (Hall et al, 2016).
A prospective study was conducted by Fritsch et al (2015) containing 81 pet cats primarily in IRIS stage 1–2 or classified as at risk of developing CKD. They were fed a therapeutic renal diet for 12 months and renal parameters measured. There were no major changes in renal parameters, plasma urea or creatinine concentrations, UPC or urine specific gravity (USG). In all groups, bodyweight remained stable and owner-assessed quality of life improved initially and then remained stable. Of note in this study 94% of cats were successfully transitioned onto the renal diet (compared with, for example, only 58% in the study conducted by Elliot et al (2000)) — hypothesised to be primarily because of the early stage of introduction, thus providing an argument for introducing a renal diet at an early stage of disease. However, while the authors concluded that the results of this study supported previous findings that renal food can help stabilise cats with CKD (Fritsch et al, 2015), there was no control group, so it is difficult to determine whether similar findings could have been seen on a control diet. Furthermore, muscle mass was not measured, so it was not possible to conclude whether protein restriction at an early stage of CKD resulted in any loss of lean body mass.
Geddes et al (2016) looked at the effect of a test diet with moderate dietary protein and phosphate restriction on calcium phosphate homeostasis in healthy older cats. They found that cats fed the test diet showed lower fractional excretion of phosphate and more stable plasma parathyroid hormone concentrations, but a slightly greater increase in ionised calcium compared with the control diet. There was no difference in the proportion of cats developing azotaemic CKD between groups, and plasma phosphate and fibroblast growth factor (FGF)-23 (a modulator of serum phosphate concentrations with phosphaturic effects) concentrations remained stable for all cats. It was not possible to determine whether feeding the test diet benefited renal function and the authors concluded that further investigations were needed (Geddes et al, 2016). Notably, in this study five of the 26 cats fed the test diet developed ionised hypercalcaemia (compared with only one in the control group). Concern has been expressed about starting phosphate restriction too early and increasing the possibility of ionised hypercalcaemia developing in the early stages of disease (Sparkes et al, 2016). Furthermore, protein restriction too early may also have adverse consequences. Protein restriction later in CKD can help to reduce nitrogenous waste (Polzin and Churchill, 2016; Table 1), and cats started on a protein-restricted renal diet from later in IRIS stage 2 onwards are able to maintain body condition score until at least midpoint in their survival after diagnosis. However, restriction too early may cause a loss of lean muscle mass and reduce diet palatability (Elliott et al, 2000; Ross et al, 2006). Loss of muscle condition and/or bodyweight could impact on long-term survival (Freeman et al, 2016). Nutritionally speaking, one of the primary goals for early management of cats with CKD is to minimise muscle mass loss and weight loss, and it is critical that cats consume sufficient amounts of protein to maintain lean muscle mass. Cats in IRIS stage 1 or early stage 2 are also unlikely to develop clinical or biochemical abnormalities and experience uraemia when fed diets unrestricted in protein — which is the main rationale for reducing protein later in disease.
Some components of a renal diet, such as increased omega 3 fatty acids, are likely to be of benefit in early CKD (Plantinga et al, 2005), but at this stage much is still unknown, and more evidence is needed to confirm whether a renal diet is of benefit to non-azotaemic cats with early CKD. However, some of the studies discussed have supported development of some commercially available early kidney disease diets and senior diets. Early kidney diets tend to incorporate many of the principles of renal diets, but with higher protein levels and less phosphorus restriction (Fritsch et al, 2015). These initial studies suggest potential benefits may justify use of such diets in some patients with early CKD, and they may also be helpful when, after diagnosis, clinicians or owners are keen to try to intervene and delay progression of disease, without transitioning to a more advanced renal diet (Sparkes et al, 2016). Increased research and studies are needed to determine clear benefits, however, and in every case patients should be evaluated individually before dietary advice is given. Given the relatively limited evidence base, at this stage the author would generally advise feeding an age-appropriate diet to cats with early CKD is still a very reasonable approach and may frequently be preferred. If already on one of these (usually a senior diet), further diet change to an early renal diet is unlikely to always be warranted at an early stage, particularly if there are financial concerns or the cat becomes stressed or resistant to diet change. This may also be true if the cat presents with a poor muscle condition score, where any further potential reduction in dietary protein content, as might occur if moved onto an early renal diet, could have adverse effects on muscle mass. In such cases, regular monitoring with transition to a therapeutic renal diet when azotaemia develops would be a reasonable course of action. Part of the rationale for introduction of a renal diet early enough in the course of disease is to gain acceptance before nausea or significant inappetance develops, but a cat is unlikely to suffer from these to a clinically relevant degree at IRIS stage 1–2 (R Geddes, unpublished observations, 2019). If a cat is not on an age-appropriate diet then recommendations to move onto one should be made. A number of different options are available, including several from Royal Canin, Purina Pro Plan, and Hills.
Advice on introduction of the new diet
There can be challenges with acceptance of any new diet in cats. This is particularly true in renal patients later in the course of disease once azotaemia has developed to any significant degree, but may also be experienced earlier in the disease. Protein and sodium are key contributors to palatability, and reduced levels in renal diets can result in a potential reduction in palatability and poorer acceptance. Owing to the higher levels of protein found in diets designed for early CKD, palatability of these may be slightly higher but resistance could still be met. Persistence is critical when transitioning to the diet: cats may take 1 month or more to accept the new diet, and setting owner expectations is important. Points to consider are detailed in Box 3.
Box 3.Advice for successful transition to a new diet for cats at any stage of chronic kidney disease
- Introduce diet in a non-stressful environment (for example, a quiet area at home). Avoid initial introduction at the veterinary practice
- Ensure cat is not clinically unwell, for example, severely nauseous. Try to adequately treat this first, for example with anti-emetics
- Consider diet introduction in a new bowl (next to bowl with old food), or occasionally by mixing the new and old foods
- The amount of old food should gradually be reduced and the amount of new food increased over up to 4 weeks (rather than the standard 1 week recommended for most diet transitions)
- Most cats prefer two separate food bowls, rather than mixing the food
- Avoid food aversions by administering any medications in a different, highly palatable food, at a separate time to the main meals
- If feeding wet food, gentle heating to increase aroma may help
- Consider any dietary preferences of the patient
- Where possible a wet renal diet is preferable, since it contains 70–85% moisture and will thus improve overall fluid intake. Encouraging adequate hydration is a key goal for any cat suffering from chronic kidney disease
- However, for patients that have only ever eaten dry foods a dry renal diet could be a better initial option to try. Soaking dry food to increase overall fluid intake and adequate hydration may be advisable.
- Consider renal diets from different brands — textures and flavours differ between brands
- Consider any comorbidities, for example for cats suffering with osteoarthritis, easy access to the food bowl and slight elevation may be helpful
- Palatability enhancers may be helpful, for example Pro Plan Fortiflora (a probiotic supplement which many cats also find highly palatable)
In most renal patients, successful transition to the new diet is possible, but maintaining adequate dietary intake to ensure caloric and nutrient intake is sufficient for the cat’s needs is critical. Endogenous protein is used for energy if the body is placed in a negative energy balance (Witzel, 2018) with adverse consequences. If there is complete refusal of all renal diets tried — including a home-prepared one where feasible — then an age-appropriate senior, mildly phosphate-restricted diet should be selected (ideally a wet diet), and an intestinal phosphate binder added to it (Sparkes et al, 2016). This will not provide the other advantageous elements that renal diets offer, including protein restriction, increased omega 3 fatty acids and B vitamins, and reduced sodium content, and phosphate binders themselves can reduce food palatability. However, this approach is more preferable to ongoing inappetance and weight loss. IRIS provides guidelines for recommended target blood phosphate levels in CKD stage 2–4 and doses of phosphate binder should be adjusted accordingly (Sparkes et al, 2016). A phosphate binder is rarely required at IRIS stage 1 CKD. As CKD progresses, serum phosphate levels tend to increase and may become refractory to control with dietary phosphate restriction alone, rising above IRIS’s therapeutic targets for blood phosphate levels. In this case, phosphate binders may also need to be added to the renal diet to control serum phosphate levels, although in many cats use of a phosphate-restricted renal diet alone can be sufficient to control blood phosphate levels and prevent a rise in blood parathyroid hormone levels (Barber et al, 1999). If a phosphate binder is required, care must be taken to avoid a calcium-containing binder if the patient has any hypercalcaemia (seen more commonly in advanced CKD (Sparkes et al, 2016) but possible at any stage (Geddes et al, 2016)).
Conclusions
There is a good evidence base to the use of renal diets to increase longevity and quality of life in cats with IRIS stage 2 CKD onwards (Sparkes et al, 2016). However, the optimal nutritional plan for non-azotaemic cats with early CKD is still unknown, particularly since, until recently, as was discussed in the previous article, veterinarians have not been able to identify cats with early CKD. Commercially available early renal diets offer a further choice for clinicians or owners keen to intervene earlier, but significant evidence for using this as a blanket approach for all IRIS stage 1 patients is still lacking.
There are currently no studies specifically evaluating the impact of phosphorus restriction in early feline CKD, and there are concerns about whether early protein restriction, rather than being helpful, could actually prove harmful to patients by decreasing palatability and potentially resulting in premature muscle mass loss (Elliott et al, 2000; Ross et al, 2006). At this stage, therefore, the main nutritional approach in early CKD should be to ensure the patient is on an age-appropriate complete and balanced diet and that muscle wastage and weight loss is minimised. However, cases should be assessed individually and in some instances preference for an ‘early’ renal diet with phosphorus restriction and mild protein restriction may be warranted. All patients should be monitored regularly, and, if and when disease progresses with development of azotaemia, protein reduction to minimise the development of uraemia from protein catabolism becomes a greater focus and a renal diet with greater protein and phosphorus restriction should be started.
KEY POINTS
- Clinicians are increasingly commonly diagnosing ‘early’ feline chronic kidney (CKD) disease at IRIS stage 1 or early IRIS stage 2, before onset of azotaemia.
- There is a strong evidence base for the use of protein and phosphorus restricted therapeutic renal diets from IRIS stage 2 of disease onwards to increase longevity and quality of life.
- There is less evidence for dietary management of patients with early CKD. The optimal nutritional plan, particularly with regards to the degree and timing of protein and phosphorus restriction implementation, is still unknown.
- There are currently no studies specifically evaluating the impact of phosphorus restriction in early feline CKD. Protein restriction later in CKD can help to reduce nitrogenous waste. However, restriction too early may cause a loss of lean muscle mass and reduce diet palatability, and could impact negatively on long-term survival.
- Commercially available early renal diets offer a choice for clinicians or owners keen to intervene as early as possible after disease diagnosis, but significant evidence for using this as a blanket approach for all IRIS stage 1 patients is still lacking. In many cases the main nutritional approach may be to ensure the patient is on an age-appropriate complete and balanced diet and that muscle wastage and weight loss is minimised.
- All feline patients with CKD should be monitored regularly, and, if disease progresses with development of azotaemia, a renal diet with greater protein and phosphorus restriction should be started.


