Chronic kidney disease (CKD) is a highly prevalent disease and a common cause of morbidity and mortality in older cats. It is estimated to affect up to 30–40% of cats over the age of 10 years (Lulich, 1992) and is the cause of death of over 13% of cats (O’Neill et al, 2015). Th ere are thought to be a number of potential underlying aetiologies contributing to disease, but the risk factors, other than age (Sparkes et al, 2016), and causes associated with the development of CKD are still not well elucidated, and most cases are classified as idiopathic in origin. However, there is an increasing awareness that early diagnosis and management of CKD can slow the progression of disease and have a significant impact on longevity as well as quality of life. Th is has a number of challenges and controversies associated with it.
Dietary management, ideally with a therapeutic renal diet, is arguably the most important aspect of treatment for cats diagnosed with CKD in International Renal Interest Society (IRIS) stage 2 onwards (Polzin and Churchill, 2016); this is generally accepted by most veterinarians. However, given that until relatively recently veterinarians have not been able to identify early CKD (IRIS stage 1 disease), and that there are a limited number of studies looking at management of cats at this early stage of disease, the best diet for these cats is still unknown. Th is article explores the diagnostics currently available to identify early renal disease. A second article looks at current understanding of therapeutic strategies and the impact this can have on progression of the disease, with a particular focus on dietary management.
CKD can be defined as an alteration in kidney structure and/or function, present for a sustained duration (for example over 1–3 months) (Sparkes et al, 2016). This has superseded the previous terminology of ‘chronic renal failure’, since it is now recognised that renal insufficiency as well as renal failure can be classified as CKD ( Box 1).
Box 1. The term ‘chronic kidney disease’ incorporates a spectrum of disease (International Renal Interest Society, 2019)
- Sustained alteration in kidney structure and/or function, e.g. present for over 1–3 months
- Renal insufficiency: a loss of concentrating ability (urine specific gravity <1.035)
- — occurs when 67% of functional nephrons are lost
- Renal failure: glomerular filtration rate no longer adequate to maintain normal excretory function (azotaemia + urine specific gravity <1.035)
- — occurs when >75% functioning nephrons are lost
Diagnosis of chronic kidney disease
CKD is primarily diagnosed by a history, physical examination and clinical signs (Box 2, Figure 1) compatible with CKD, alongside identification of azotaemia and a urine specific gravity <1.035. Note that in IRIS stage 1 and early IRIS stage 2 there may be no clinical signs present, although some cats may have one or more signs present. CKD can then be staged, based on blood creatinine concentrations, according to the IRIS staging system (Table 1). Substaging according to blood pressure and degree of proteinuria should be done. With the advent of symmetric dimethylarginine measurement (discussed later in the article), blood concentrations of symmetric dimethylarginine have also been incorporated into the IRIS staging system. Staging influences treatment recommendations and prognosis, and can be used to assess the success of treatment interventions. All of these diagnostics can present challenges for early diagnosis, some of which are discussed below.

Box 2. Clinical signs associated with chronic kidney disease
- Polyuria
- Polydipsia
- Weight loss In one study, cats lost a median of 8.9% body weight in the 12 months before diagnosis, but weight loss was already present 3 years before diagnosis and accelerated after diagnosis of chronic kidney disease (Freeman et al, 2016)
- Poor muscle condition score
- Inappetance
- Lethargy
- Dehydration
- Halitosis
- Vomiting
- Signs resulting from complications of kidney disease, such as retinal detachment as a result of hypertension
- Changes in kidney size or shape on palpation
Table 1 International Renal Interest Society (IRIS) staging of chronic kidney disease, based on fasting blood creatinine levels
| IRIS stage | Description | Fasting blood creatinine level (μmol/litre) | Blood [SDMA] (μg/dl) |
|---|---|---|---|
| 1 | Non-azotaemic | <140 | <18 |
| 2 | Mild renal azotaemia | 140–250 | 18–25 |
| 3 | Moderate renal azotaemia | 251–440 | 26–38 |
| 4 | Severe renal azotaemia | >440 | >38 |
SDMA = symmetric dimethylarginine. See www.iris-kidney.com for further information. From International Renal Interest Society (2019)
Since CKD is a progressive disease, clinical signs become increasingly evident as disease advances. IRIS stage 1 disease may offen present with no or few identifiable clinical signs. Further challenges in recognition of clinical signs include the fact that subtle changes such as a decrease in coat quality or appetite may be missed or interpreted to be ‘normal’ signs of ageing by owners, rather than arousing concern of disease, and polyuria and polydipsia may not be noticed until quite advanced if a cat has outdoor access.
Direct measurement of glomerular filtration rate (GFR) is the most accurate way to evaluate functional renal mass, but no practical method to assess this in companion animals currently exists (Polzin and Churchill, 2016). No single biomarker correlates with GFR, but biomarkers can be taken as an indirect indicator of it. Urea and creatinine are the conventional markers for renal function on blood tests, but they are insensitive markers of GFR and do not start to rise above the reference range until 75% of nephron function has been lost (Polzin and Churchill, 2016). They can also be affected by other factors (Table 2). Creatinine is preferred to urea as a marker of GFR, but its exponential relationship with GFR means substantial early declines in GFR may be accompanied by only small changes in creatinine levels, whereas later in disease a large change in creatinine levels may represent only a small change in GFR (Sparkes et al, 2016) (Figure 2).
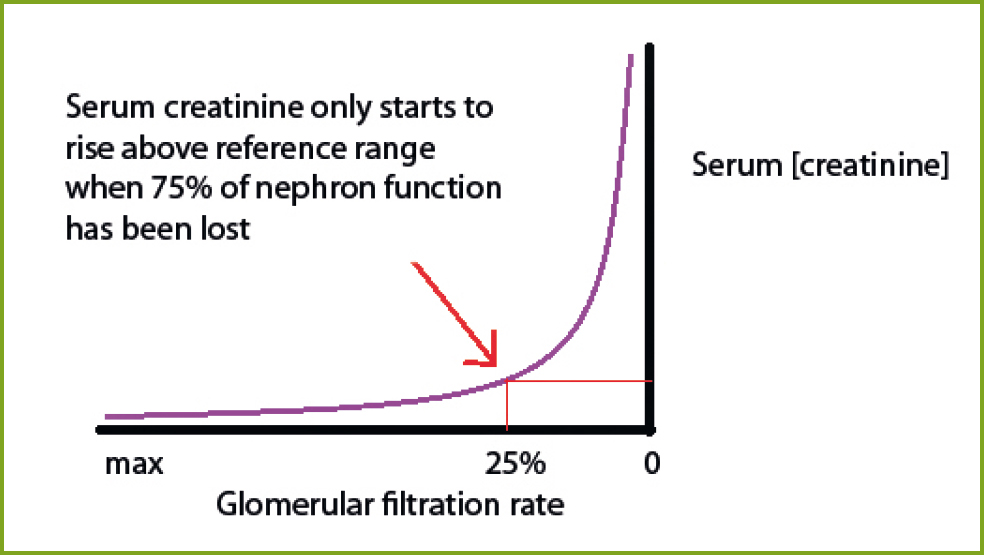
Table 2 A number of extra-renal factors can influence urea and creatinine, making them relatively insensitive markers of renal function
| Creatinine | Filtered in the kidney but not reabsorbed | |
| Constant rate of excretion | ||
| Levels inversely proportional to glomerular filtration rate | ||
| Extra-renal factors affecting creatinine include: | Hydration | |
Muscle mass
|
||
| Urea | Filtered in the kidney and passively reabsorbed | |
| Variable rate of excretion | ||
| Levels can rise disproportionately in dehydrated cats as a result of reduced glomerular filtration rate | ||
| Extra-renal factors affecting urea include: | Increases: post-prandial, gastrointestinal haemorrhage, high protein intake (polyphagic cats), catabolic conditions | |
| Decreases: liver disease, low protein diet, non-renal polyuria, anabolic conditions | ||
Adapted from Sparkes et al (2016)
Urinalysis plays an important role in diagnosis, with urine specific gravity being the chief measurement of significance. Urine samples can be obtained by cystocentesis, free catch or a catheter, and free catch is adequate for measurement of urine specific gravity (Figure 3) (S Caney, unpublished observations, 2019). Urine specific gravity must be assessed by a refractometer. Healthy cats have highly concentrated urine, although factors such as water intake and the water content of their diet means a relatively wide range exists. In cats with CKD, a persistently reduced urine specific gravity below 1.035 is seen (Figure 4), and this can assist in diagnosis of CKD, particularly in the early stages since urine specific gravity typically falls below 1.035 before azotaemia is measurable (Sparkes et al, 2016). Cats in IRIS stage 1 and 2 disease tend to maintain a degree of concentrating ability so urine specific gravity remains above 1.015 but it may fall into the isosthenuric range later in disease. Urine specific gravity is also important to consider in conjunction with any azotaemia found, to determine whether any azotaemia found is pre-renal in origin (in which case urine specific gravity will be highly concentrated) or renal (in which case urine specific gravity <1.035).


Cats with IRIS stage 1 kidney disease, however, do not have measurable azotaemia or many or any clinical signs of disease, and have thus previously been challenging to diagnose. However, more clinicians are now starting to identify non-azotaemic cats with early CKD (IRIS stage 1 or early stage 2 disease) as a result of several factors. CKD is more common is older cats, so increased monitoring of these patients to detect early signs of disease is warranted (Sparkes et al, 2016) and senior clinics can have an important role to play in this. Increased screening of cats in senior clinics and suspicious clinical signs or urine samples can prompt further investigation and result in diagnosis of early disease (Table 3). For example, reduction in urine specific gravity (<1.035) — which may be picked up incidentally on a screening urinalysis — may be an important early indicator of CKD preceding any blood abnormalities.
Table 3. Recommended routine screening for healthy older cats
| Age (years) | Recommended screening | |
|---|---|---|
| Frequency | Parameters | |
| Up to 7 | Annual | Health check: physical examination, weight check |
| 7–10 | Annual | Health check: physical examination, weight check |
| Annual | Blood pressure | |
| Annual | Urinalysis | |
| 11–14 | Every 6–12 months | Health check: physical examination, weight check |
| Every 6–12 months | Blood pressure | |
| Annual | Urinalysis | |
| Annual | Bloodwork | |
| >15 | Every 3–6 months | Health check: physical examination, weight check |
| Every 6 months | Blood pressure | |
| Every 6 months | Urinalysis | |
| Annual | Bloodwork | |
From Sparkes et al (2016)
Veterinarians also now have access to a greater number of diagnostic tests, most significantly the advent of a commercially available symmetric dimethylarginine (SDMA) assay. SDMA is a more sensitive marker than creatinine for early renal disease (Hall et al, 2014). Importantly, SDMA levels are not affected by muscle mass. SDMA is primarily eliminated by renal clearance, and plasma concentrations correlate with GFR (Hall et al, 2014). SDMA levels increase as a result of reduced renal function: they can rise as early as once 25% of renal function is lost and on average once 40% of renal function has been lost (Hall et al, 2014). SDMA is a useful biomarker to identify early CKD in non-azotaemic cats (Hall et al, 2014), particularly in those with marked muscle loss. In one study, SDMA increased above the reference range and helped in detecting CKD a mean of 14.6 months before serum creatinine concentrations increased above the reference range, and up to 48 months before serum creatinine concentrations increased above the reference range in one case (Hall et al, 2014). These findings also showed that non-azotaemic cats with SDMA concentrations >14 #x03BC;g/dl eventually progressed to azotaemic CKD (Hall et al, 2014).
Blood SDMA concentrations have now been incorporated into the IRIS staging system and can be helpful in guiding accurate staging and management, both in early and later CKD (Tables 1 and 4). SDMA should not be used to diagnose CKD on the basis of a single elevated reading and should always be interpreted in the context of the patient. If the SDMA reading is the only finding suggesting the presence of CKD, repeat testing is needed. SDMA levels can fluctuate from day to day and can be affected by non-renal factors including diet and medication use. In humans SDMA levels have been shown to be increased in non-renal diseases such as sepsis, cardiac disease and hepatic disease (Koch et al, 2013) but research into this in cats has not been conducted. Further research is also needed to assess the specificity of SDMA (Hall et al, 2014), and to fully assess its accuracy in the clinical setting, but it is a promising biomarker of early renal disease (Hall et al, 2014) and is now being increasingly commonly used.
Table 4. Symmetric dimethylarginine (SDMA) interpretation in International Renal Interest Society (IRIS) staging system of chronic kidney disease (CKD)
| If blood SDMA concentrations are known, some modification to the guidelines may be considered | ||
|---|---|---|
| Patients with creatinine <140 μmol/litre | SDMA persistently increased over 14 μg/dl suggests reduced renal function | Consider classifying cat as IRIS CKD stage 1 |
| Patients with creatinine 140–250 μmol/litre | Patients with low body condition scores and SDMA ≥25 μg/dl may indicate the degree of renal dysfunction has been underestimated | Consider treating patient as IRIS CKD stage 3 |
| Patients with creatinine 251–4400 μmol/litre | Patients with low body condition scores and SDMA ≥45 μg/dl may indicate the degree of renal dysfunction has been underestimated | Consider treating patient as IRIS CKD stage 4 |
Clinical findings which may increase suspicion of kidney disease in non-azotaemic cats — including urine specific gravity and SDMA discussed above — are outlined in Box 3. Early diagnosis of CKD is important because early intervention, before clinical signs become evident, can significantly reduce the rate of progression of disease and increase longevity in affected cats (Hall et al, 2016). It may also help to identify complications before they impact on the patient, helping to support their quality of life. However, when early CKD is detected, it can present a challenge to the form that these interventions should take, and at what point they should be implemented. Note that with the exception of genetic testing, all findings should be repeatable over several weeks — a single reading is not sufficient to diagnose early CKD.
Box 3. Clinical findings arousing suspicion of kidney disease in non-azotaemic cats
- Documentation of a persistent elevation in symmetric dimethylarginine levels on at least two occasions, 3 weeks apart (>14 μg/dl)
- Imaging to examine kidney structure (reduced kidney size, reduced corticomedullary definition, renal infarcts or renal cysts)
- Genetic testing for polycystic kidney disease mutations (particularly in Persian cats)
- Persistent proteinuria (urine protein:creatinine ratio >0.4) with no urinary tract infection
- Hypertension
- Serial monitoring of creatinine over time with an upward trend, even if creatinine remains within the reference range (particularly true if cat is poorly muscled)
- Urine specific gravity <1.035
Recommended initial approach for International Renal Interest Society stage 1 feline patients — blood pressure and urinalysis
In confirmed cases of feline IRIS stage 1 kidney disease, urine protein: creatinine ratio and blood pressure measurements should be obtained if not already performed during diagnostic work up (Figure 5). If both are within normal limits, current advice recommends monitoring the patient, at least every 6–12 months dependent on case, to check for azotaemia or development of proteinuria or hypertension (Sparkes et al, 2016). These are also important considerations for IRIS sub-staging.
Proteinuria can develop as a consequence of CKD and renal proteinuria is associated with more rapid progression of disease and significantly shorter survival time (Syme et al, 2006), so screening of all cats with CKD for renal proteinuria is recommended. Since even small increases in levels of urine protein can be significant, a sensitive test should be used, with urine protein:creatinine measured, rather than urine dipsticks. When measuring urine protein:creatinine, consideration of factors which increase proteinuria, including lower urinary tract inflammation and/or bacterial infection, is required. Thus, evaluation of urine sediment in conjunction with urine protein:creatinine is needed. The method of urine collection (free catch from a clean tray or by cystocentesis) does not have a significant effect on protein content. If urine protein:creatinine >0.4, treatment with telmisartan or benazepril as anti-proteinuric therapies should be started.
If systolic blood pressure is persistently over 160 mmHg then anti-hypertensive treatment should be commenced with amlodipine or telmisartan to minimise the risk of end target organ damage (Sparkes et al, 2016). If treated, the presence of systemic hypertension in itself does not suggest a worse prognosis (Syme et al, 2006) and one study found that hypertensive cats with CKD treated with amlodipine did not have a significantly different survival time to cats with CKD that were normotensive at presentation (Syme et al, 2006). The clinician is referred to the IRIS guidelines for further discussion of such treatments.
Conclusions
CKD is a very common disease in older cats. In the majority of cases the primary cause is unknown, and the kidney damage at the time of diagnosis will be irreversible. However, early diagnosis and appropriate treatment can slow down disease progression and have a very significant positive effect on both quality of life and longevity. Until relatively recently the majority of cases of CKD were not identified until at least IRIS stage 2 of disease. Advances in our understanding of the pathophysiology of CKD and access to a greater number of diagnostic tests, including a commercially available SDMA assay, are now enabling veterinarians to identify CKD at an earlier stage than was usually previously possible. However, because early CKD detection has previously been difficult, only a limited number of studies have been done exploring dietary management for IRIS stage 1. Thus, while dietary intervention is regarded as a cornerstone of management of CKD, there is controversy over the timing of renal diet introduction, and the optimal nutritional approach for non-azotaemic early feline CKD patients. The second of these articles will explore the approach to nutritional support of CKD patients and explore the current evidence supporting such recommendations.
Key Points
- Chronic kidney disease (CKD) is a common cause of morbidity and mortality in older cats, affecting up to 30–40% of cats over the age of 10 years and the cause of death of over 13% of cats.
- Early diagnosis and management of CKD can slow down disease progression and positively impact quality of life and longevity.
- Clinicians are increasingly commonly diagnosing ‘early’ feline CKD at IRIS stage 1 or early IRIS stage 2, before onset of azotaemia and clinical signs.
- A commercially available symmetric dimethylarginine (SDMA) assay is available, which is a more sensitive marker than creatinine for early renal disease and a useful biomarker to identify early CKD. Blood SDMA concentrations have been incorporated into the IRIS staging system and can help guide accurate staging and management.
- Reduction in urine specific gravity (<1.035) usually precedes azotaemia and is an important early indicator of CKD.
- Once CKD has been identified, further investigations including blood pressure and urinalysis are required to identify the treatment needs of the individual cat.


