Feline infectious peritonitis is a devastating disease caused by virulent mutations of an ubiquitous feline coronavirus. With the perfect storm of exposure, genetic predisposition and random virulent mutations, feline coronavirus is transformed from a mild gastrointestinal infection into a significant systemic disease with historically high mortality rates of ≥95% (Kornya, 2020). Until recently, cats developing feline infectious peritonitis succumbed to this disease quickly or had to be euthanised (Figures 1–3), with attempts at treatment being largely futile (Addie et al, 2009). However, with the nucleoside analogues, hope is at hand and, while these drugs are still expensive, they can save lives. This new focus on feline infectious peritonitis has revived interest in repurposing other therapeutics. This article discusses all of the current options and describes the treatment protocols. However, how best to use these drugs is still being determined, so therapeutic regimens are still being optimised.

Specific treatment options: remdesivir and oral GS-441524
The two drugs discussed in this first section are injectable remdesivir and oral GS-441524, both nucleoside analogues. These treatments are legal in the UK, although they are not licensed – therefore, off-licence consent forms should be obtained before treatment is initiated. The recommended treatment protocol is a 5–14 day course of remdesivir, followed by 10–11 weeks of oral GS-441524, resulting in a total 12-week treatment course. Alternatives include a 12-week course of injectable remdesivir (although this is rarely used because of the discomfort and cost of daily injections), or a 12-week course of oral GS-441524 (which is recommended in less severe cases). A flowchart summarising the recommended treatment protocols has been attached at the end of the article (Appendix 1).
Remdesivir
Remdesivir is the prodrug of GS-441524, a nucleoside analogue. Remdesivir is a broad-spectrum antiviral drug that was originally developed to treat hepatitis C and Ebola virus in humans; it is also effective against many other RNA viruses. It has also been used during the pandemic to treat COVID-19 (Frediansyah et al, 2021). Remdesivir and GS-441524 are the two antiviral drugs that are now legally available for the treatment of feline infectious peritonitis in the UK. These special formulations are also available to veterinarians in Australia, and since the earlier launch of remdesivir in both countries more than 1500 cats have been treated, with up to 80% having a successful outcome. In small-scale veterinary trials, remdesivir has been shown to be rapidly effective in treating feline infectious peritonitis (Taylor and Barker, 2021). It has been available in the UK since August 2021 (Figure 4), and can be obtained through a Veterinary Specials manufacturer (contact: FIPAdvice@gmail.com).

Dosage and duration of treatment
The dose of remdesivir differs depending on the type of feline infectious peritonitis present (Table 1). A treatment duration of 5–14 days is recommended, before changing to oral GS-441524 for another 10–11 weeks (12 weeks of treatment in total). This change can be made directly, with remdesivir being given 1 day and oral GS-441524 given the next. The swap to GS-441524 can be made when the cat is stable clinically and is able to take oral GS-441524, although at least 5 days of injectable remdesivir is recommended. Treatment with remdesivir or GS-441524 should be stopped only when the patient is clinically normal and all laboratory parameters have normalised aft er the total minimum 12 weeks of treatment. Extended treatment courses may be required if full resolution of clinical signs and/or laboratory parameters have not been achieved by the 12-week time point. It is recommended treatment should only be ceased 2–4 weeks aft er the cat is clinically normal, as well as on haematology and biochemistry.
Table 1. Dose rates for remdesivir for feline infectious peritonitis (FIP)
| Type of FIP | Likely clinical signs | Dose | Frequency | Route |
|---|---|---|---|---|
| Effusive (wet)FIP/dry FIP, with no ocular or neurological signs | Ascites and/or pleural effusions without ocular or neurological signs | 10–12 mg/kg | Every 24 hours | Oral (per os) |
| Dry FIP with ocular signs | Uveitis, chorioretinitis, hyphaema, hypopyon | 15mg/kg | Every 24 hours | |
| Dry FIP with neurological signs | Ataxia, hyperaesthesia, nystagmus, seizures, dullness | 10mg/kg (or 20mg/kg in divided doses) | Every 12 hours | |
| Higher doses may be required if there is poor response to treatment (sustained clinical signs and biochemical abnormalities such as hyperglobulinaemia persisting after 6–8 weeks of treatment) – increasing the dose rate by an additional 3–5mg/kg per day is then recommended. For example, if a cat is diagnosed with effusive feline infectious peritonitis and is currently on a dose of 12mg/kg, increase the dose to 15–17mg/kg and continue this dose for the rest of the treatment period. The maximum dosage that the treatment can be increased to is 20mg/kg. This dose should be divided into two 10mg/kg doses, as used in dry feline infectious peritonitis with neurological signs. If this is not effective, adjunct treatment will be necessary, using mefloquine and/or interferon (Box 3). | ||||
Remdesivir is usually given by subcutaneous injection into the loose skin of the intrascapular region of the cat, but any areas with loose skin can be used. Injection with remdesivir can cause transient local discomfort, and the steps detailed in Box 1 can help mitigate this when advising owners on how to administer subcutaneous remdesivir (Taylor et al, 2022).
Box 1.Steps to help mitigate discomfort when administering subcutaneous injections of remdesivirDiscomfort on injection is very variable, some cats do not appear to notice the injection, while others do appear uncomfortable. Many can be injected with nothing more than owner reassurance, cuddles and treats.
- Wipe the reusable seal of the bottle with a 70% alcohol swab, then use a new needle each time to draw up the drug from the bottle. This helps reduce bacterial contamination of the bottle. Change to another new needle before injecting the drug into the cat.
- Either a 21G (green) or 23G (blue) needle is recommended, but this is also dependent on patient factors and owner preference. A 21G needle may reduce injection time and work better in cats that are stressed or fractious to reduce handling time, whereas a 23G needle is finer and may reduce local discomfort. It is worth trying both sizes and seeing which the owner and cat prefers.
- In cats that are stressed and fractious, administering oral gabapentin (50–100 mg) 60 minutes before the injection can help make the whole process smoother.
- The area of skin to be injected can be clipped and topical EMLA cream applied 30–40 minutes before the injection. However, it is usually the presence of remdesivir under the skin that causes discomfort. Advise owners to use gloves when applying EMLA to prevent potential lidocaine toxicity.
- Try not to use the same injection site each time and rotate between sites of loose skin every day.
- Making the injection process as positive as possible will help to preserve the cat–owner bond. Offering treats, and brushing or playing with the cat before and after the time of the injection is recommended.
In very sick or inappetent cats, remdesivir can also be given intravenously. To do so, 10mg/kg of the drug should be diluted to 10ml in saline and administered over 10–20 minutes. This should achieve a rapid antiviral effect and can be then changed to subcutaenous injections aft er 3–4 days, when the cat is well enough to leave the clinic.
Monitoring
Parameters to be monitored regularly during treatment with remdesivir or GS-441524 are shown below and in Appendix 1. It is important that these cats are monitored regularly, especially during hospitalisation in acute cases. These parameters should ideally be re-assessed aft er 2 weeks of treatment, then aft er a month, then every 3–6 months for a year aft er treatment has stopped, as relapses can occur.
Clinical parameters to monitor:
- Body temperature (axillary temperature can also be assessed by the owner once the cat goes home)
- Pulse, respiratory rate and effort (note; pleural effusion can worsen for 1–2 days, especially aft er intravenous treatment with remdesivir, so repeat thoracocentesis may be needed)
- Appetite
- Activity
- Stool volume and consistency
- Urination
- Monitor effusions and abdominal lymphadenopathy by point of care ultrasound
- Changes in body weight and percentage weight change.
Note, it is very important to weigh cats regularly during treatment (ideally weekly), as necessary weight gain, and growth in kittens, will occur with successful treatment. However, this means the dose given will need to increase in order to ensure that the actual dose (in mg/kg) is still appropriate for the type of feline infectious peritonitis being treated. It is recommended that kittens are weighed at least once weekly, using accurate scales. Try to use the same scale for the rest of the treatment course (Figure 4). The authors also advise that the weight used in cases of wet feline infectious peritonitis is the weight of the cat minus estimated effusion.
Haemotology and serum biochemisty parameters to monitor:
- Packed cell volume
- Total white blood cell count
- Absolute lymphocyte, neutrophil, monocyte and eosinophil count
- Total serum protein, globulin, and albumin concentrations, and albumin:globulin ratio
- Alpha 1 acid glycoprotein concentration
- Bilirubin
Assess these parameters during hospitalisation, 2 weeks aft er starting treatment, a month aft er starting treatment and every 3–6 months for a year aft er completing treatment.
If cost is a concern, focus on checking clinical parameters, packed cell volume, total proteins, colour of plasma, bilirubin and serum globulins.
Response
Improvement in clinical signs should be rapid, typically over the first few days. An increase in weight should be seen as well, followed by normalisation of laboratory parameters.
Effusions, especially pleural effusions, can worsen for 1–2 days, particularly aft er intravenous administration, so thoracocentesis may need to be performed. The thoracic and abdominal cavity should be monitored with point of care ultrasound. If at home, owners should be advised to measure resting respiratory rate and effort. Effusion typically resolves within 2 weeks, but if an effusion is still present at 2 weeks, despite interventions, consider increasing the dosage currently being used. Neurological signs may appear or worsen during the first few days of treatment. Seizures may also occur and may necessitate the use of anti-seizure medication, such as levetiracetam (20–30 mg/kg every 8 hours per os or intravenous). A short course of corticosteroids at an immunosuppressive dose (2–4 mg/kg per os every 24 hours or 0.2–0.4 mg/kg intravenously) has been anecdotally reported to help improve clinical signs as well, even though there are no controlled studies proving any effects (Hartmann and Ritz, 2008). It is recommended to only administer prednisolone if there is an ongoing immune-mediated disease such as haemolytic anaemia, which should be tapered and stopped as soon as possible. If anaemia or lymphopenia is present, the packed cell volume and lymphocyte numbers usually return to normal levels before 6 weeks of treatment. Abnormal serum protein values usually improve progressively and reach normal levels by 6 weeks of treatment. There may be a transient increase in serum globulin levels as the globulins in the body fluids are reabsorbed, but this should resolve before 6 weeks of treatment. A mild peripheral eosinophilia may also be noted and may be a favourable marker for resolution of clinical signs, as noted in patients with COVID-19 (Nair et al, 2020). Peripheral lymphadenopathy resolves over a course of few weeks as well.
If no response, or only a partial response to treatment is seen (such as if cat is not improving clinically, or hyperglobulinaemia persists after 6 weeks), re-confirming the diagnosis of feline infectious peritonitis is important – reviewing history and clinical signs, repeating sampling and cytology of fluid and additional biopsies are indicated. Ruling out other infectious processes such as feline immunodeficiency virus, feline leukaemia virus, toxoplasmosis, mycobacteriosis and haemoplasmosis is important as well. If you are still confident of a diagnosis of feline infectious peritonitis, increase the dose by 3–5 mg/kg per day.
Side effects
Transient stinging and/or injection site reactions, and even injection site sores have been seen with the illegal versions of injectable GS-441524. This type of reaction appears to be much less common with legal remdesivir, probably because this formulation is water soluble. Cats may also appear depressed or show signs of nausea (restlessness, lip licking, hypersalivation) for a few hours after intravenous administration of remdesivir. If sustained, mirtazapine (see non-specific treatment options) can be given. Raised renal parameters (such as symmetric dimethylarginine) have been reported occasionally and in cases of azotaemia, intravenous fluids can be given. Raised liver enzymes (such as alanine transaminase) have also been reported, which usually normalise when treatment is stopped. If needed, adenosylmethionine can be given; it is unclear if this is more typically because of feline infectious peritonitis or is a side effect of these drugs. All side effects occurring during treatment with remdesivir or GS-441524 should be reported to BOVA Veterinary Specials and to FIPAdvice@gmail.com.
Cost
A 10ml bottle of 10mg/ml liquid is available to order from a Veterinary Specials company in the UK for around £250 (as of March 2022). Table 2 gives the doses and volumes of Remdesivir that will be required for 14 days of treatment.
Table 2. Doses and volumes of remdesivir required for the first 14 days of treatment*
| Body weight | Effusive 10–12 mg/kg q24h SC or IV | Bottles needed for 14 days | Ocular FIP 15 mg/kg q24h SC or IV | Bottles needed for 14 days | Neurological FIP 10 mg/kg q12h SC or IV | Bottles needed for 14 days |
|---|---|---|---|---|---|---|
| 1kg | 1–1.2 ml | 2 bottles | 1.5 ml | 3 bottles | 2ml | 3 bottles |
| 1.5kg | 1.5–1.8 ml | 3 bottles | 2.25 ml | 4 bottles | 3ml | 5 bottles |
| 2kg | 2–2.4 ml | 3–4 bottles | 3 ml | 5 bottles | 4ml | 6 bottles |
| 2.5kg | 2.5–3 ml | 4–5 bottles | 3.75 ml | 6 bottles | 5ml | 7 bottles |
| 3kg | 3–3.6 ml | 5 bottles | 4.5 ml | 7 bottles | 6ml | 9 bottles |
| 3.5kg | 3.5–4.2 ml | 5–6 bottles | 5.25 ml | 8 bottles | 7ml | 10 bottles |
| 4kg | 4–4.8 ml | 6–7 bottles | 6 ml | 9 bottles | 8ml | 12 bottles |
FIP= feline infectious peritonitis; q24/12h= every 24/12 hours, IV=intravenous; SC= subcutaneous.
* Please note, this is only for the first 5-14 days of treatment, this will be followed by 10-11 weeks of oral GS-441525. Each bottle of remdesivir costs approximately £250 as of March 2022, without VAT or veterinary mark-up (which will vary with each practice), and does not include costs of the equipment needed to give the drug, such as syringes, needles, giving sets and EMLA cream. Since kittens are expected to grow and sick cats to gain weight, note the number of bottles that will be needed for the cat's current weight, then note the number of bottles needed to treat the cat's likely weight in 6 months' time; the mean of these two numbers is likely to be the final number of bottles needed. Multiply that number by the current cost of a bottle of remdesivir to work out the cost of the drug. Please note that this is only an estimate, and the final total number of bottles is dependent on the cat's final weight during treatment. Once a bottle of Remdesivir has been opened, it should be disposed of after 30 days. That being said, most cats use a bottle within a week.Nucleoside analogue GS-441524
GS-441524 is the breakdown product of remdesivir, it is a nucleoside analogue that terminates the RNA chain of the viral RNA-dependent RNA polymerase. It acts as an alternative substrate for viral RNA synthesis, resulting in RNA chain termination during viral RNA transcription. It is legally available as 50mg tablets in the UK (Figure 5); it can be obtained through a Veterinary Specials manufacturer (contact FIPAdvice@gmail.com for more information).

Dose and duration of treatment
The dose of GS-441524 differs depending on the type of feline infectious peritonitis present (Table 3). Treatment is typically given after 5–14 days of remdesivir, with GS-441524 being given for 10–11 weeks. However, less severe cases can be treated with GS-441524 alone, in which case it needs to be given for 12 weeks total
Table 3. Dose rates for GS-441524. Higher doses may be required if there is a poor response to treatment
| Type of FIP | Likely clinical signs | Dose | Frequency | Route |
|---|---|---|---|---|
| Effusive (wet) FIP/dry FIP without ocular or neurological signs | Ascites and/or pleural effusions without ocular or neurological signs | 10–12 mg/kg | Every 24 hours | Oral (per os) |
| Dry FIP with ocular signs | Uveitis, chorioretinitis, hyphaema, hypopyon | 15mg/kg | Every 24 hours | |
| Dry FIP with neurological signs | Ataxia, hyperaesthesia, nystagmus, seizures, dullness | 10mg/kg (or 20mg/kg in divided doses) | Every 12 hours |
FIP= feline infectious peritonitis. Higher doses may be required if there is a poor response to treatment. Please note, these are the same doses as used with remdesivir. GS-441524 should be given for a minimum of 10–11 weeks if given after 5–14 days of remdesivir. However, if it is given on its own it should be given for a total of 12 weeks. As with Remdesivir, if no or a partial response is seen, increasing the dose by an additional 3-5mg/kg a day is recommended. GS-441524 should be given on an empty stomach (approximately 5ml of water should be given after immediately). Owners should wait 30 minutes before feeding their cat. Giving oral medications to cats can be challenging, direct clients to this resource on how to give oral medications to their cats if they need help - https://icatcare.org/advice/how-to-give-your-cat-a-tablet/
Monitoring
The same parameters should be monitored as stated above for remdesivir. Tracking changes in weight is important to ensure the dosages of the drug remain adequate.
A similar response to that of remdesivir is expected.
Side effects
In a small number of cases, increased hepatic and or renal parameters have been reported with GS-441524 (Pedersen et al, 2019). If these occur, add in supportive medications, such as nutraceuticals such as SAMe, and intravenous fluids for azotaemia cases. All side effects occurring during treatments should be reported to BOVA Veterinary Specials and to FIPAdvice@gmail.com.
Previous studies
In a study looking at 31 cats with spontaneous feline infectious peritonitis, GS-441524 was given by subcutaneous injection over 12–30 week and was shown to be remarkably safe (Pedersen et al, 2019). No long-term abnormalities were observed in haematology, and liver and kidney function remained normal. Of the 31 cats, five had to be euthanised as a result of severe feline infectious peritonitis at the start of the study, and 25 were classified as long-time survivors (one of these was subsequently euthanised with presumably unrelated heart disease). This left 24 cats remaining healthy at the time of publication (approximately 80% of cases had long-term resolution).
A further study by Dickinson et al (2020) looking at cats with neurological feline infectious peritonitis revealed similarly excellent response rates, with 3/4 cats being cured.
Cost
A pack of 10 (50mg) palatable tablets are around £350 plus VAT (as of March 2022). These are available through a Veterinary Specials manufacturer – please contact FIPAdvice@gmail.com.
Table 4 gives the doses and volumes of GS-441524 that will be required for the treatment of feline infectious peritonitis.
Table 4. Dose and number of GS-441524 tablets needed
| Body weight | Effusive 10–12 mg/kg q24h | Tablets required (minimum 10 weeks)* | Ocular 15 mg/kg q24h | Tablets required (minimum 10 weeks)* | Neurological FIP 10 mg/kg q12h | Tablets required (minimum 10 weeks)* |
|---|---|---|---|---|---|---|
| 1kg | ¼ tablet | 18; costs for 20 | ½ tablet | 35; costs for 40 | ¼ tablet q12h | 35; costs for 40 |
| 1.5kg | ½ tablet | 35; costs for 40 | ½ tablet | 35; costs for 40 | ½ tablet q12h | 70 |
| 2kg | ½ tablet | 35; costs for 40 | ¾ tablet | 53; costs for 60 | ½ tablet q12h | 70 |
| 2.5kg | ½ tablet | 35; costs for 40 | ¾ tablet | 53; costs for 60 | ½ tablet q12h | 70 |
| 3kg | ¾ tablet | 53; costs for 60 | 1 tablet | 70 | ¾ tablet q12h | 105; costs for 110 |
| 3.5kg | ¾ tablet | 53; costs for 60 | 1¼ tablet | 88; costs for 90 | ¾ tablet q12h | 105; costs for 110 |
| 4kg | 1 tablet | 70 | 1 ¼ tablet | 88; costs for 90 | 1 tablet q12h | 140 |
| If a 4kg cat needs to be treated for the full 12 weeks with GS-441524 (not including an initial remdesivir start): | ||||||
| 4kg | 1 tablet | 84; costs for 90 | 1 ¼ tablet | 105; costs for 110 | 1 tablet q12h | 168; costs for 170 |
FIP= feline infectious peritonitis; q24/12h= every 24/12 hours. Tablets are scored so they can be easily divided into halves or quarters. A packet of 10 GS-441524 tablets costs around £350, as of March 2022, without VAT or veterinary mark-up (which will vary with each practice). Since a full box of 10 tablets has to be purchased each time, the number of tablets needed has been rounded up to the nearest 10 tablets. Since kittens are expected to grow and cats to gain weight, note the number of boxes that will be needed for the current cat's weight, then note the number of boxes needed to treat the cat's likely weight in 6 months' time; the mean of these two numbers is likely to be the final number of boxes needed. Multiply that number by the current cost of a box of GS-441524 to work out the cost of the drug. Please note that this is only an estimate and the final total number of boxes is dependent on the cat's final weight during treatment.
Other specific treatment options
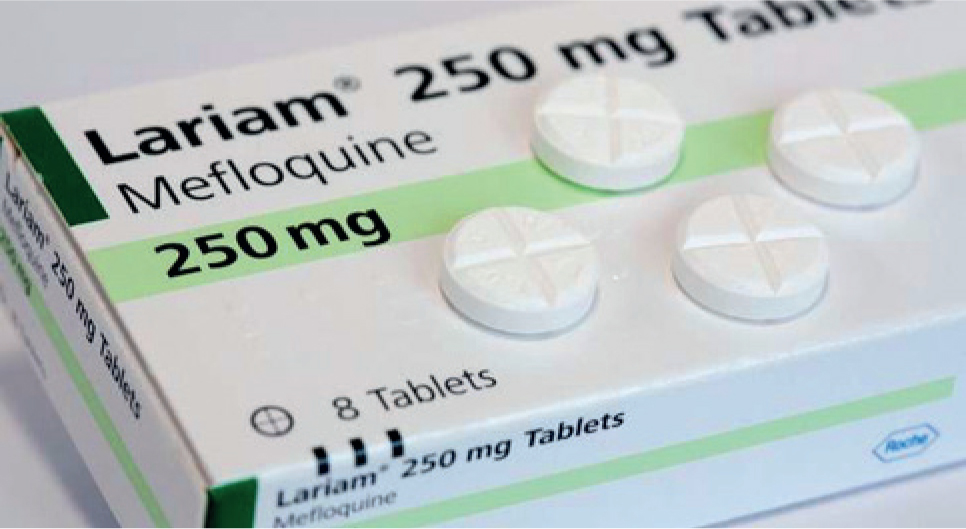
Mefloquine
Mefloquine is a human anti-malarial drug (Larium; Figure 6). However, through its repurposing for the treatment of COVID-19 (Shionoya et al, 2021), it is now gaining attention for its use in the treatment of feline infectious peritonitis. It can inhibit the viral load of feline coronavirus in experimentally infected Crandell feline kidney cells (McDonagh et al, 2014). Mefloquine is highly protein bound and has shown to be safe, when used in clinically normal cats, in a preliminary in-vivo study investigating its use as a potential treatment for feline infectious peritonitis (Yu et al, 2020). However, anecdotal cases report side effects including nausea, vomiting, dermatopathy, and raised symmetric dimethylarginine. Sally Coggins of the University of Sydney is undertaking a PhD study looking into the new treatments for feline infectious peritonitis, including the use of mefloquine; she recommends dosing at 62.5 mg/cat per os 2–3 times a week (three times for large cat) or 20–25 mg/kg per os every 24 hours. Vomiting can be seen as a side effect, but this can be lessened by giving mefloquine with food. Reformulation can be performed by PCCA Labs, but it can take a few weeks.
Polyprenyl immunostimulant
Th is veterinary biologic (Figure 7) is licenced by the USA Department of Agriculture (Food and Drugs Administration) for the treatment of the signs of feline herpesvirus and upper respiratory tract infection; it is safe for use in cats over 8 weeks of age.

It upregulates the Th-1 type pathway via toll-like receptors and may therefore be of benefit in diseases involving suppression of cellular immunity, such as feline infectious peritonitis, as cell-mediated immunity is required for the elimination of feline coronavirus. Cats with feline infectious peritonitis have severe depletion of the CD4 + and CD8 + T-lymphocytes necessary for mounting a cell-mediated immunity response, and polyprenyl immunostimulant enhances cell-mediated immunity through upregulation of Th1 cytokines. To date, polyprenyl immunostimulant has mainly shown success with the dry form of feline infectious peritonitis (Legendre et al, 2017), but there have been anecdotal reports of successful cases with wet feline infectious peritonitis.
Dose
The bottle contains 10ml of a 2mg/ml solution and it is dosed at 3mg/kg per os three times a week (cats above 5kg should be given a total 15mg per dose for each dose). Polyprenyl immunostimulant is mainly used in cases of dry feline infectious peritonitis, but it has successfully treated a small number of wet cases too (anecdotal reports). Please note that the dose of polyprenyl immunostimulant when treating feline herpesvirus-1 is 0.5mg/kg (or 0.25 ml/kg per os every 12 hours for 14 days).
Previous studies
A study by Legendre et al (2017) showed that 22% of 58 cats diagnosed with presumptive dry feline infectious peritonitis lived for at least 6 months, and about 5% were alive for at least a year when receiving polyprenyl immunostimulant. Cats receiving corticosteroids concurrently with polyprenyl immunostimulant had a significantly shorter median survival time (21.5 days) than cats not receiving corticosteroids (73.5 days). However, a definitive diagnosis of feline infectious peritonitis was not established in all cats in this study.
Cost
Polyprenyl immunostimulant is sold as packs of 6 × 10ml vials. It can be ordered via: http://vetimmune.com (USA) or http://vetimmune.cz (Czech Republic). A package of six vials costs around £500 plus handling and shipping costs. This will treat a 3kg cat for approximately a month.
Protease inhibitor GC376
GC376 is not currently commercially available, but work is underway in the USA to licence it. While only illegal formulations are available, the Veterinary Medicines Directorate has stated that vets should not recommend them or be involved in the use of them to treat feline infectious peritonitis.
GC376 is a protease inhibitor that acts as a reversible, competitive inhibitor of feline coronavirus protease, which cleaves viral polymerase from polyprotein 1 and is vital for viral replication. By inhibiting feline coronavirus protease, it inhibits viral replication.
Dose
Protease inhibitor GC376 is dosed at 10mg/kg every 12 hours by subcutaneous injection for a minum of 12 weeks for cats presenting with effusive feline inefctious peritonitis. The dose can be raised to 15mg/kg every 12 hours subcutaneously if the response is inadequate. As with all treatments for feline infectious peritonitis, it is important to recalculate the dose if the cat gains weight during treatment.
Response
Dramatic and progressive resolution of clinical signs can be seen in the first 4 weeks of treatment. Ocular disease usually starts to clear within the first 48 hours and resolves within 1 week, regardless of the presenting severity. Pyrexia typically resolves alongside an improvement in appetite, activity, growth and weight gain. Abdominal and thoracic effusions usually resolve within 2 weeks and become undetectable, as do enlarged lymph nodes. Icterus resolves slowly over 2 weeks or more, alongside decreasing hyperbilirubinemia. Significant weight gain is usually noted during the treatment course, and cats in sustained remission continue to gain weight aft er this treatment. The the clinical parameters that should be monitored when using GC376 are the same as for remdesivir. In cases of relapse, the authors advise following the steps detailed in Box 2.
Box 2.In the event of a relapse.A recurrence of clinical signs such as thoracic or abdominal effusion, pyrexia, ocular and/or neurological signs may indicate a relapse of feline infectious peritonitis. It is important to note that a relapse can occur without any significant changes to serum biochemistry and haematology. In the event of a relapse the following steps should be taken:
- Ensure that it is indeed a relapse of feline infectious peritonitis, rather than a separate disease process. Reviewing history and clinical signs, repeating sampling and cytology of fluid and additional biopsies are indicated. Ruling out other infectious processes such as feline infectious peritonitis, feline leukaemia virus, toxoplasmosis, mycobacteriosis and haemoplasmosis is important as well.
- If relapse occurs after completion of the full treatment course, restart treatment with either remdesivir for 5–14 days followed by GS-441524 for 10–11 weeks, or GS-441524 for 12 weeks. The dose should be for the type of feline infectious peritonitis present (effusive, non-effusive, ocular or neurological, with an additional 3–5mg/kg per day added. For example, if a cat previously treated for non-effusive feline infectious peritonitis now presents with signs of ocular feline infectious peritonitis, the dose used should be 15mg/kg per day + 3–5mg/kg per day = 18–20mg/kg per day, which should ideally be divided and given 12 hours apart.
- Some cats appear to achieve clinical resolution before 12 weeks of treatment; it is recommended that treatment should be continued to the full 12 weeks to limit the chances of a relapse.
- The maximum dose of remdesivir/GS-441524 is 20mg/kg per day; if this is insufficient, consider the use of adjunct treatments, like mefloquine and/or interferon while continuing to give remdesivir or GS-441524.
Side effects
Stinging on injection appears to occur in all cases, with swelling at the injection site if consecutive injections are given at the same site. In a study by Pedersen et al (2018), 1 out of 20 cats treated developed deep localised ulceration at the injection site, which then resolved rapidly. The most severe side effects were noted on juvenile dentition in cats treated before 16–18 weeks old. All four cats starting treatment within this age group showed delayed normal formation, growth and eruption of permanent teeth. No other defects were noted in any of the survivors in remission, or in cats that had a postmortem performed (Pedersen et al, 2018).
Previous studies
A study by Pedersen et al (2018) showed that seven cats out of the 20 achieved disease remission (around 35% of cases had long-term resolution). A dose of 15 mg/kg subcutaneously every 12 hours was administered and 19/20 cats presenting with naturally occurring feline infectious peritonitis treated with GC376 showed rapid resolution of clinical signs within 2 weeks of initial treatment. However, relapses occurred 1–7 weeks aft er treatment, so new cases were then treated for 12 weeks. Relapses that were no longer responsive to treatment occurred in 13/19 cats within 1–7 weeks of initial treatment. Neurological signs occurred eight out of these 13 cats, and five cats had recurrent abdominal lesions.
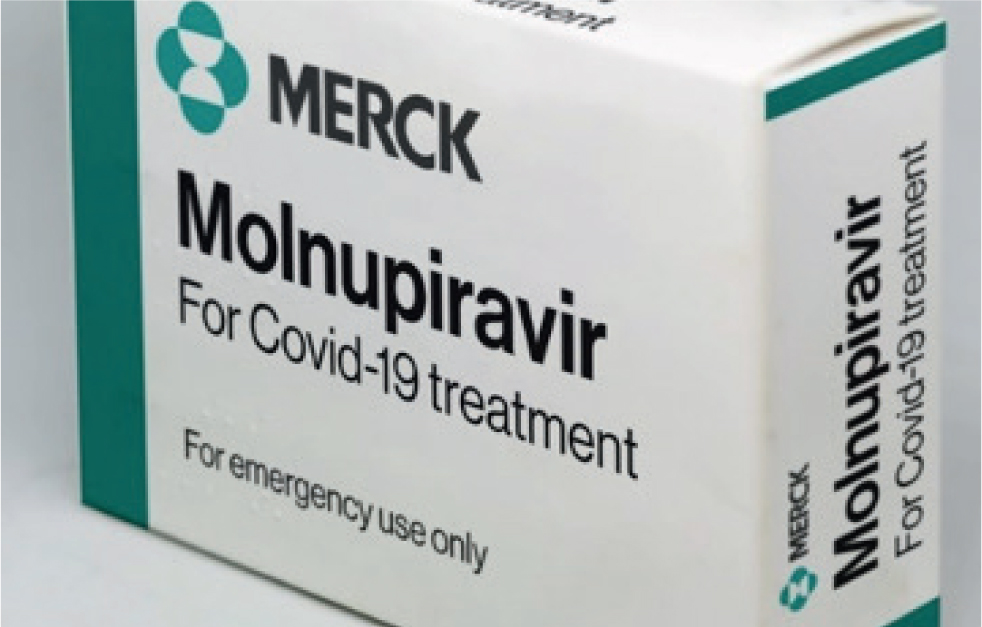
Molnupiravir (Lagevrio™)
Molnupiravir (Figure 8) is being used to treat people with COVID-19 and is currently illegal for use in cats. It is an oral antiviral nucleoside analogue (EIDD-2801). It has a cytidine where GS-441524 and remdesivir have an adenosine. It is being sold illegally by the Chinese company that supplies Hero (Hero is apparently GS-441524). In vitro data apparently look promising, and the company have run a field trial on ‘HERO Plus, 2801’ or illegal molnupiravir, that will hopefully be published soon. The results apparently look positive, with efficacy rates similar to those for oral GS-441424, but with a different drug resistance pattern, so it will be helpful in feline infectious peritonitis cases that become resistant to GS-441524.
Recombinant feline interferon omega
Feline interferon omega (IFNω; Virbagen Omega) is an immune-modulator licenced for subcutaneous and intravenous use in treatment of feline leukaemia virus and feline immunodeficiency virus. It has been shown to display antiviral, immuno-modulatory and antiproliferative actions when used in treatment of viral diseases.
Previous studies have shown significant clinical improvement and reduction of mortality in cats diagnosed with feline leukaemia virus and/or feline immunodeficiency virus (de Mari et al, 2004; Gil et al, 2014). There have been limited reports of the use of feline interferon omega in feline infectious peritonitis, acute feline coronavirus and feline chronic gingivostomatitis.
Doses
Virbagen Omega is sold in vials of 10 million units and one vial should be reconstituted with 1ml of saline diluent; 0.1ml of the diluted stock solution then constitutes 1 million units of feline interferon omega.
Once diluted, feline interferon omega maintains its potency in the fridge for up to 3 weeks, so the rest of stock solution should be frozen if not in use, where it can be kept frozen for up to 6 months.
- Dose for effusive infectious peritonitis: 1 million units/kg subcutaneously or per os every other day for up to five doses, and then twice a week until recovery is obtained or until death occurs (Allerton et al, 2020; Addie, 2022).
- Dose for effusive infectious peritonitis: 100 000 units per cat subcutaneously or per os per day until recovery is obtained or until death occurs; 0.1ml of previously diluted stock solution containing 1 million units of feline interferon omega should be diluted again with 4.9ml of saline dilutent, hence, 0.5ml of the total 5ml of stock solution now yields 100 000 units (Addie et al, 2020; Addie, 2022).
Adverse effects
Previously reported side effects include fatigue, hyperthermia, vomiting and mild diarrhoea. A slight decrease in neutrophils, platelets and red blood cells, alongside an increase in liver enzymes have also been reported.
Previous studies
One placebo-controlled, double blinded study that looked into using feline interferon omega as the primary therapeutic option for cats diagnosed with feline infectious peritonitis found no statistically significant differences in survival time of groups treated and not treated with feline interferon omega (Ritz et al, 2007). Reasons postulated for the lack of efficacy include the inability of feline interferon omega to reach adequate tissue concentrations to demonstrate an antiviral effect, or treatment with feline interferon omega being initiated too late.
Although feline interferon omega has been deemed not efficacious enough as a sole treatment modality for cats diagnosed with feline infectious peritonitis, it can be considered as an adjunctive treatment modality in conjunction with remdesivir or GS-441524 as seen with a case report that administered a low dose feline interferon omega for 13 weeks (100 000U daily), in the time period following the full course of illegal GS-441524 nucleoside analogue (Addie et al, 2020). Further studies are needed to understand their combined efficacy in treating feline infectious peritonitis.
Cost
Virbagen Omega is available in either packs of two or five. A pack of two 10 million unit vials costs around £250, whereas a pack of five 10 million unit vials cost £550 including shipping and handling costs. A pack of five 10 million unit vials can yield 16 doses for a 3 kg cat, which lasts approximately 45 days.
Black market drugs
There are currently many products available on the black market claiming to be GS-analogues (such as GS-441524, or EIDD-2801), although the purity and safety of these products is unknown and contamination has been reported. As of January 2022 the Veterinary Medicines Directorate stated that any veterinarian obtaining these drugs for the treatment of feline infectious peritonitis in the UK will be acting illegally, as will any owners who administers these drugs to their cat. Every effort should be made to only use legally available treatments. In a previous study, which looked at almost 400 cats treated with illegal GS-analogues, only 8.7% of owners reported receiving significant help from their veterinarian as to how to safely administer these drugs, nor did they receive enough information on how to monitor response to treatment or care for their critically ill cats (Jones et al, 2021). If an owner insists on giving these drugs to their cat and asks you to monitor and support the cat, you can consider doing so. However, if you do, you must make it very clear in the cat's notes that you did not recommend the use of these drugs (not even directing the owner to any websites), that you told the owner that these drugs are illegal and that they may not contain what they are supposed to.
Combination treatments
Combinations of an antiviral drug and an immunostimulant are usually used when treating most severe long-term viral diseases in people. Further controlled studies are needed to see what combinations work best for cats with feline infectious peritonitis, such as remdesivir, GS-441524 or molnupiravir plus polyprenyl immunostimulant, feline interferon omega, or mefloquine. Box 3 gives options for owners with financial constraints.
Box 3.Options for owners with financial constraintsAlthough treatment protocols for feline infectious peritonitis should be given in accordance with guidelines to maximise chances of a cure, there are options that can be offered to clients with financial constraints. These options should only be taken if deemed absolutely necessary, and it should be made clear to clients that treating at a dose below that which is recommended may necessitate a longer treatment course and/or future relapses, which may incur more costs in the future.
- Give only oral GS-441524 for the full course of treatment instead of initially starting with remdesivir then transitioning to GS-441524
- Starting on injectable remdesivir for as long as possible, before swapping over to oral mefloquine at 62.5mg 2–3 times weekly (give three times for a large cat) or 20–25 mg orally once daily.
- If an increase in remdesivir dosage becomes necessary (because of the development of neurological signs), but this increase cannot be afforded, mefloquine treatment can be added as an adjunctive treatment, although further studies are needed to judge the efficacy of this combination.
Non-specific supportive therapy
Mirtazapine
Mirtazapine is an appetite stimulant that is thought to work by antagonising the 5HT2c H1 receptor. It is also thought to have an antiemetic effect by antagonising the 5-HT3 receptor. There is a licenced transdermal formulation available (Mirataz, Dechra) for unintended weight loss, and it is used at the dose rate of 2mg/cat every 24 hours.
Vitamin B12 (cobalamin)
Vitamin B12 (cobalamin) supplementation is thought to be important in cats with feline infectious peritonitis as the infection tends to deplete the liver's B12 stores via its inflammatory pathways. Vitamin B12 deficiency can lead to anaemia, hyporexia, and further weakening of the immune system via malabsorption of nutrients. It can be supplemented with 0.02 mg/kg given subcutanesouly weekly or 0.25mg/per os every 24 hours.
Gabapentin
Gabapentin is a non-opioid analgesic used to treat chronic pain, seizures and anxiety in cats. The exact mechanism of action of gabapentin is unknown, but it appears to result in a decreased release of excitatory neurotransmitters. As discussed previously, a 50–100mg dose of gabapentin before daily injections of remdesivir can help make the injection process smoother, ensuring the cat is less stressed or painful during the injections.
Thoracocentesis
Thoracocentesis is indicated for the diagnosis of wet feline infectious peritonitis cases and is needed therapeutically when dyspnoea is caused by the accumulation significant quantities of pleural fluid. There can be a transient increase in pleural fluid after starting remdesivir therapy, especially when it is given intravenously; as such, patients should be closely monitored, and respiratory rate/effort checked regularly because repeat therapeutic thoracocentesis may be required.
Abdominocentesis
Abdominocentesis can be very useful when trying to obtain a diagnosis of feline infectious peritonitis. However, therapeutic abdominocentesis is controversial as it can exacerbate dehydration if large volumes are removed, resulting in hypoproteinaemia. Moreover, these effusions also tend to reform quickly.
Intra-cavitary (pleural and/or abdominal cavity) corticosteroid administration
Ritz et al (2008) described this procedure following fluid drainage at a dosage of 1mg/kg of dexamethasone every 24 hours for the management of cases of feline infectious peritonitis. However, while effusions resolved temporarily in 6 of 36 cats, all eventually died of complications related to feline infectious peritonitis.
Antibiotics
Antibiotics are not required in the vast majority of cases. However, if there is significant immunosuppression resulting in bacterial infections, such as pyelonephritis or concurrent diseases such as feline haemoplasma, then appropriate antibiotics may be indicated. Ideally, any antibiotic should only be used for confirmed cases of bacterial infection, based on culture and sensitivity results, to reduce inappropriate use of antibiotics and safeguard these drugs use in the future.
Corticosteroids
Corticosteroids are frequently administered to try to improve some of the clinical signs of feline infectious peritonitis, which makes sense, as it is an immune-mediated disease. As mentioned earlier, it is thought best to only administer corticosteroids if there is an ongoing immune-mediated disease such as a secondary immune-mediated haemolytic anaemia, and this should be tapered and stopped as soon as possible. There are no clinical trials to conclusively support their use, although their combination with interferon omega proved unsuccessful (Ritz et al, 2007). In addition, it is important to note that corticosteroids may provide supportive relief at best and are not curative. In cats with ocular signs (chorioretinitis, uveitis) ophthalmic solutions containing corticosteroids can be used.
A starting dose for systemic treatment is 0.5mg/kg per os every 12 hours; which can go up to 2mg/kg per os every 12 hours, if needed. Doses should be tapered rapidly, if possible, unless there is a specific indication, such as secondary immune-mediated haemolytic anaemia or feline haemoplasma, in which case a longer course of prednisolone may be indicated. As corticosteroids cause suppression of cell-mediated and humoral immunity, their longer-term use can seriously dampen the cat's immune response to secondary bacterial infections. Longer term use also increases the risk of diabetes mellitus and iatrogenic hyperadrenocorticism.
Supportive therapeutics alongside fluid therapy and analgesics such as opioids or gabapentin can be given with remdesivir or GS-441524 as needed, to ensure the cat is kept comfortable during the treatment process. During hospitalisation, opioid analgesics such as butorphanol, buprenorphine and methadone are good options, while non-steroidal anti-inflammatories, such as meloxicam, should be carefully used and care should be taken to ensure that the cat is not azotaemic (as a result of feline infectious peritonitis or to treatment), or on concurrent corticosteroids.
Conclusions
This review summarises the current treatment options for feline infectious peritonitis. There is still much research to be done and it is likely that treatment regimens will change further over time, as veterinary professionals gain more experience with these new drugs. If you require any further information or advice, research abstracts or input on specific cases, or you need to report any side effects of these new drugs, then please contact the feline infectious peritonitis advice team on FIPAdvice@gmail.com.


