Birds have been kept in captivity since the beginning of records. The Sumerians (c 4100–1750 BC), the oldest civilisation known to have kept written records, had a word for bird cage: subura (Dennis, 2014). Birds are kept for many reasons across the globe, including: food (both as meat and to hunt with), research, companionship, beauty, entertainment and in zoological collections.
In the UK alone, the Pet Food Manufacturers Association (2020) estimated that there were 1.1 million indoor birds kept as pets in 2019. Given that many birds are not kept indoors—including aviary birds, birds of prey, pigeons, pet poultry and zoological collections—it is fair to assume that this is a gross underestimate.
Owners can form strong bonds with their birds and avian pets presenting to the veterinary practice is becoming more common. Blood sampling, just as in more familiar species, such as cats and dogs, can be an important part of a wellness exam and for disease investigations. It can also be used to develop a baseline personalised ‘reference range’ for an apparently healthy patient. Many species are not sexually dimorphic and owners may wish to have their bird DNA sexed, although this is not possible for all species and the laboratory would have to be contacted beforehand. If an owner wished to add a bird to a collection, viral and chlamydia screening should also be considered for biosecurity purposes.
This article intends to give the clinician the anatomy, equipment required and importantly the confidence to perform venipuncture in their avian patients.
Sedation and anaesthetic
The decision of whether to sedate or anaesthetise the patient before taking blood depends on the condition of the patient, the nature (personality and risk to health and safety) of the patient, and the experience of the phlebotomist and handler. Anaesthesia techniques are outside the scope of this article, but can be found in Speer (2018) and Mann (2014).
There are three commonly used sites for venipuncture:
- Jugular vein (v. jugularis dextra)
- Basilic (ulnar or wing) vein (v. cutanea ulnaris superficialis)
- Medial metatarsal vein (v. metatarsalis plantaris superficialis).
The choice of vein is dictated by the bird's species and condition, as well as the clinician's experience and preference (Table 1).
Table 1. Advantages and disadvantages of the three main venipuncture sites used in avian patients
| Vein choice | Advantage | Disadvantage |
|---|---|---|
| Jugular (v. jugularis dextra) |
|
|
| Basilic (ulnar or wing) vein (v. cutanea ulnaris superficialis) |
|
|
| Medial metatarsal vein (v. metatarsalis plantaris superficialis) |
|
|
The equipment required for avian venipuncture can also depend on the species, but it is important to have everything immediately to hand before the procedure is started.
For most species of bird, the equipment list is as follows:
- Scales accurate to 1 g (accurate to 0.5 g for tiny species)
- Towel (varies on size of patient, may use a facecloth in small birds or fleece blanket in feather plucking parrots to prevent trauma to skin)
- Several needles, with size ranging from 23–27 g depending on size of bird (Table 2)
- 1 ml and 2 ml syringes
- Heparin blood tube (1 ml and 0.5 ml) (Table 2)
- Microhaematocrit tubes
- Surgical spirit swab, or preferred skin disinfectant
- Dry cotton wool ball
- Surgical tape, such as micropore
- Microscope slides, frosted
- Pencil.
Table 2. Guide suggesting needle size and sample volume for a few commonly kept species
| Weight of bird | Needle size | Maximum sample size |
|---|---|---|
| 25 g, eg. canary (Serinus canaria forma domestica) | 27–30 g | 0.25 ml |
| 35–60 g, eg. show budgie (Melopsittacus undulates) | 26 g–27 g | 0.35–0.6 ml |
| 120 g, eg. cockatiel (Nymphicus hollandicus) | 25–26 g | 1.2 ml |
| 500 g, eg. Congo African grey parrot (Psittacus erithacus erithacus) | 23–25 g | 5 ml |
| 2 kg, eg. chicken (Gallus gallus domesticus) | 23 g | 20 ml |
Anticoagulants
Anticoagulant choice can be a contentious issue. The author was taught to always use heparin, as ethylenediaminetetraacetic acid (EDTA) can cause cell lysis, erythrolysis in corvids and ratites, and altered leukocyte morphology with prolonged exposure in any avian species (Latimer and Beinzle, 2000).
However, heparin can cause inadequate staining of leukocytes leading to erroneous counts, and also result in leukocyte and thrombocyte clumping, further leading to inadequate counts with prolonged exposure (Campbell and Ellis, 2007). Harr et al (2005) established that there is no statistical difference to haematology parameters in EDTA or heparin samples in macaws (the genus Ara) as long as they are both analysed within 12 hours of sampling and kept at 4 °C.
It is the author's opinion that, as clinicians are often faced with a small animal size, the blood sample may not be enough to fill two tubes adequately. It is better to use heparin, but make a smear from fresh blood at the time of sampling and label the smear with a pencil. It should be noted that avian erythrocytes are relatively fragile and rough smearing techniques can result in damaged or unidentifiable cells (Jennings, 1996; Fudge, 2000) (Figure 1).
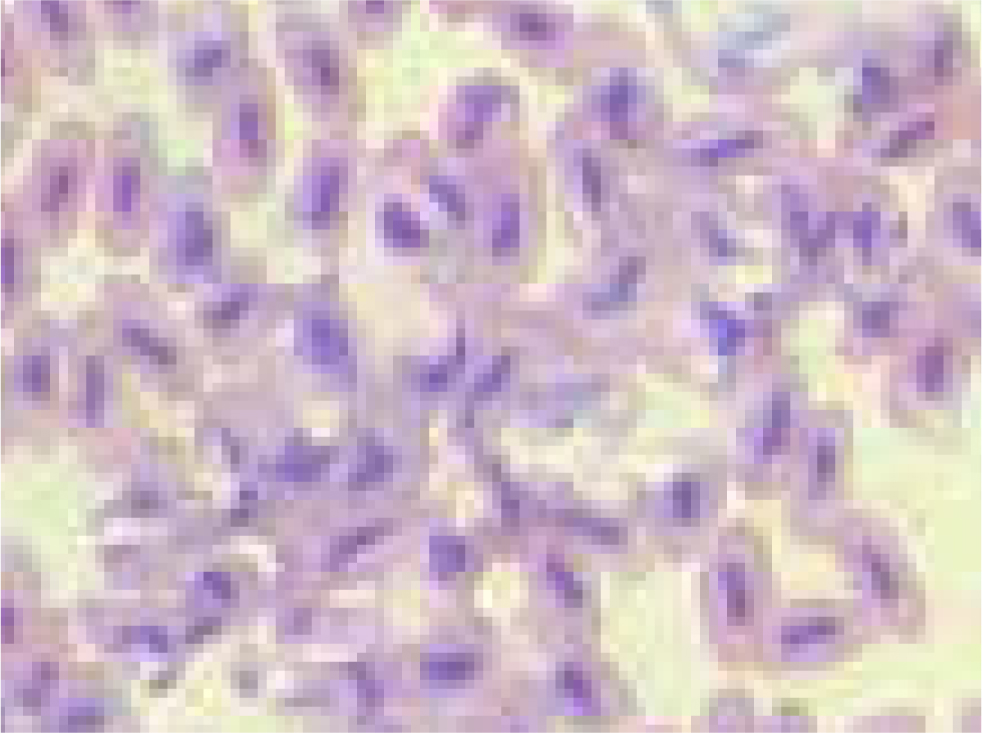
It is also for this reason that the needle should always be removed from the syringe before filling the sample tube (Fudge, 2000; Pollock, 2015). Using a fan or hairdryer on a cold setting to orientate air flow in the direction of the smear on the slide ensures that the smear dries quickly (Campbell and Ellis, 2007). Slow drying of a slide can result in issues such as perinuclear ring formation, caused by nuclear shrinkage, or the formation of clear irregular, refractile spaces in the erythrocyte cytoplasm, which could be confused with parasites (Campbell and Ellis, 2007).
Weighing the patient accurately is important, both for medication and to calculate what volume of blood can be safely drawn. The recommended maximum volume of blood that can be taken is 1% of the animal's bodyweight or, put differently, 1 ml per 100 g bodyweight. If the patient is ill, has lost blood or has had a blood draw within the last 28–45 days—which is the usual avian erythrocyte lifespan (Rodnan et al, 1957)—then the volume should be decreased to 0.5 ml per 100 g. It should be noted that any haematoma formation should also be considered as part of the total blood loss (Campbell, 1988; Fudge, 2000; Harr et al, 2005).
If liver disease is a concern then coagulopathies may also be an issue, which would influence the choice of venipuncture site. The skin covering the jugular has an easily distensible subcutaneous space, thus choosing this site could result in large, life-threatening haematomas (Dyer and Cervasio, 2008). Species dependent, if the bird is large enough, the medial metatarsal vein can be used in this situation, as there is a lower risk of haematoma because of the surrounding soft tissue and non-expansile skin (Fudge, 2000; Kramer and Harris, 2010; Pollock, 2015). In the author's experience, the jugular site forms haematomas less often than the basilic vein. However, haematoma formation at the basilic vein site is unlikely to become life threatening.
Risks to handlers
Different species have different defensive strategies. Some, such as passerines, pose no real risk to the handler, whereas large psittacines or raptors can pose a very real risk to the handler and the phlebotomist (Table 3).
Table 3. Species specific risks to the handler and preventative measures
| Species | Risk to handler | Precaution |
|---|---|---|
| Psittacines | Beak. Severe bite | Control head first |
| Birds of prey (not vultures) | Feet. Sharp talons, strong grip | Control feet first |
| Vultures | Beak designed for tearing flesh | Control head first |
| Large waterfowl | Large powerful wings | Control wings first |
| Seabirds eg. cormorants | Sharp pointed beak, will aim to stab eyes | Control head first |
| Seabirds eg. puffins | Sharp claws and painful bite | Control feet and head |
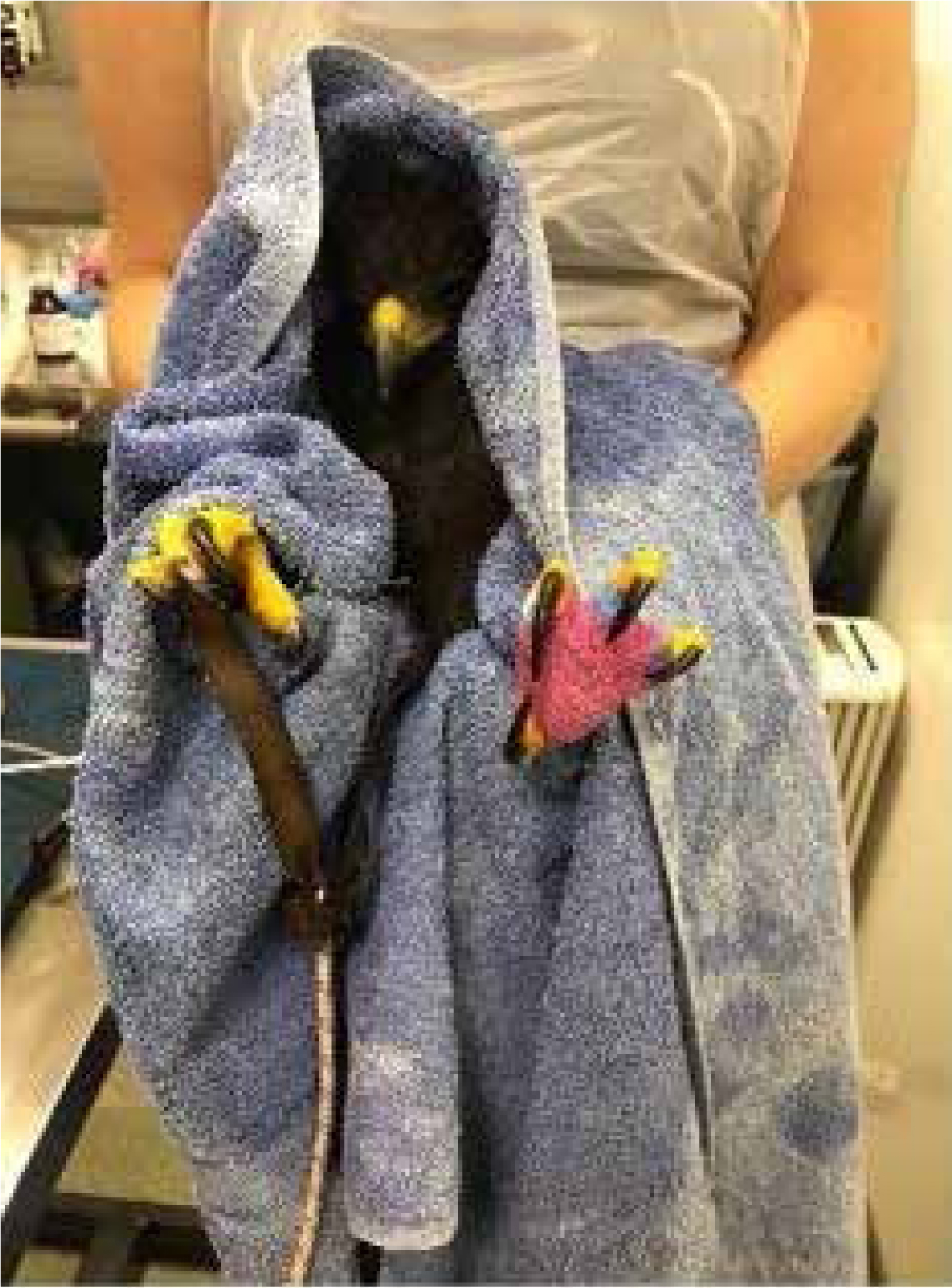
Psittacines can do the most damage with their beak, which has an incredibly strong bite because of their unique anatomy (Buhler, 1981; Tokita, 2003). It is important to restrain the head first and then the wings to prevent them damaging themselves (Figure 2).

With most raptors (vultures being an exception) it is the feet with talons that can do the damage, so their legs should be controlled first, then the head and wings next (Figure 3). Vultures have a beak designed for tearing flesh, so in those species it is vital to control the head first (Hertel, 1995). Vultures may also defensively vomit if stressed, as do many gulls.
Large waterfowl, such as geese and swans, have very powerful wings; a hit from the wing of a mute swan can feel like a punch (personal communication with Dr Iain Livingstone, British Trust for Ornithology bird ringer). In these species, controlling the wings is the first priority (Figure 4).

Many sea birds, such as cormorants, will aim for the eyes with their very sharp beak, so control of the head is vital. Meanwhile, while they may look comical, puffins have exceedingly sharp claws for excavating their burrows and are able to give a painful bite (Figure 5).

Once the patient is restrained the sample can be taken. As birds do not have a diaphragm, movement of the keel should not be restricted or suffocation could ensue. If the patient is fractious or it is a warm day, care should be taken not to cause the patient to overheat. A desk top fan may be used to circulate air.
Venipuncture
As with any species, the venipuncture site should be aseptically prepared (Fudge, 2000). Care should be taken not to over-wet the bird (especially if alcohol is used) to prevent excessive heat loss (Curro, 1998).
Sometimes aseptically bending the needle to approximately 20 degrees can make entry easier (Figure 6). Breaking the seal on the plunger by moving it several times can ensure smoother handling (Pollock, 2015).
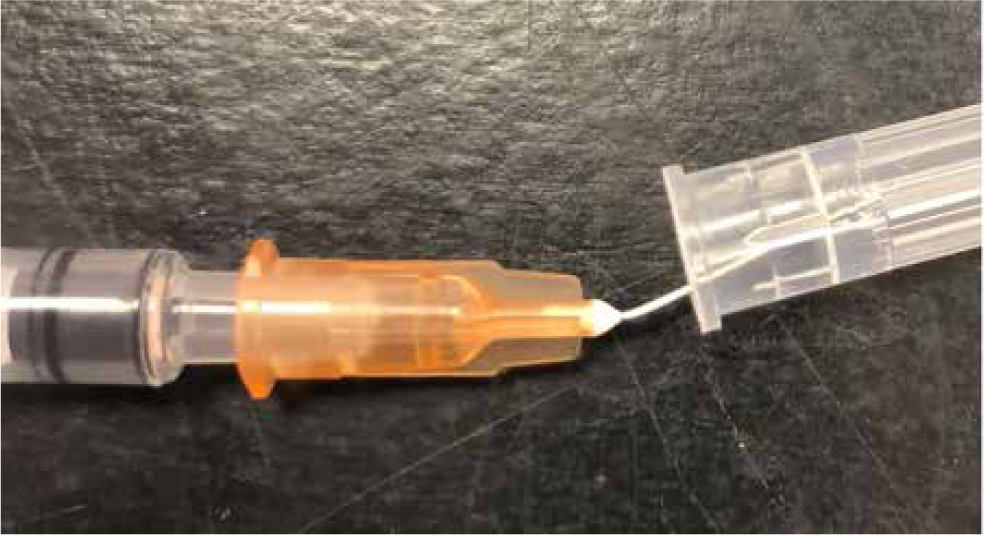
Birds are often sampled with the bevel of the needle facing up. However, Kramer and Harris (2010) make a good argument for taking blood with the bevel of the needle facing down, demonstrating that this results in less of the needle in the vein, which may reduce the risk of lacerating the other side of the vessel and may reduce the risk of collapsing the vein. Ultimately the technique used will come down to the clinician's preference and experience. Excessive negative pressure should not be employed, as this will collapse the vein.
Putting pressure on the site of venipuncture for between 30 seconds and 2 minutes is recommended (Kramer and Harris, 2010). The author maintains pressure for 2 minutes with a ball of dry cotton wool. The bird should not be released until it is certain that there is no continued bleeding. The author recommends timing the 2 minutes; it is important to exercise patience and refrain from removing the cotton wool too early to check the site, as this can lead to haematoma formation.
Jugular vein
Traditionally, the right jugular is used for venipuncture in birds, as it is the biggest vein. However, either jugular vein can be used and will often come down to personal preference, or which hand is dominant.
Many species (not waterfowl) have a featherless tract over the jugular called the apterium. Eclectus (Eclectus roratus) and caiques (Pionites) have less distinct apterylae because of their down feathers in this region (Kramer and Harris, 2010). Members of the Columbiformes family also lack an apterium. The jugular can still be visualised (Kramer and Harris, 2010), although some clinicians may find the medial metatarsal vein more accessible in these species.
Depending which jugular is to be sampled, the bird is held in lateral recumbency with the desired jugular uppermost. The head and neck should be extended towards the phlebotomist. The skin can be wiped with alcohol, which will also help visualise the jugular. The handler can raise the jugular by putting pressure on it at the thoracic inlet while the phlebotomist takes control of the head (Figures 7and8). The vein should be approached from a cranial caudal direction. This technique means that, if there is any sudden movement of the birds' head, all the equipment will move with the bird and so reduce the risk of laceration and haematoma formation (Pollock, 2015).
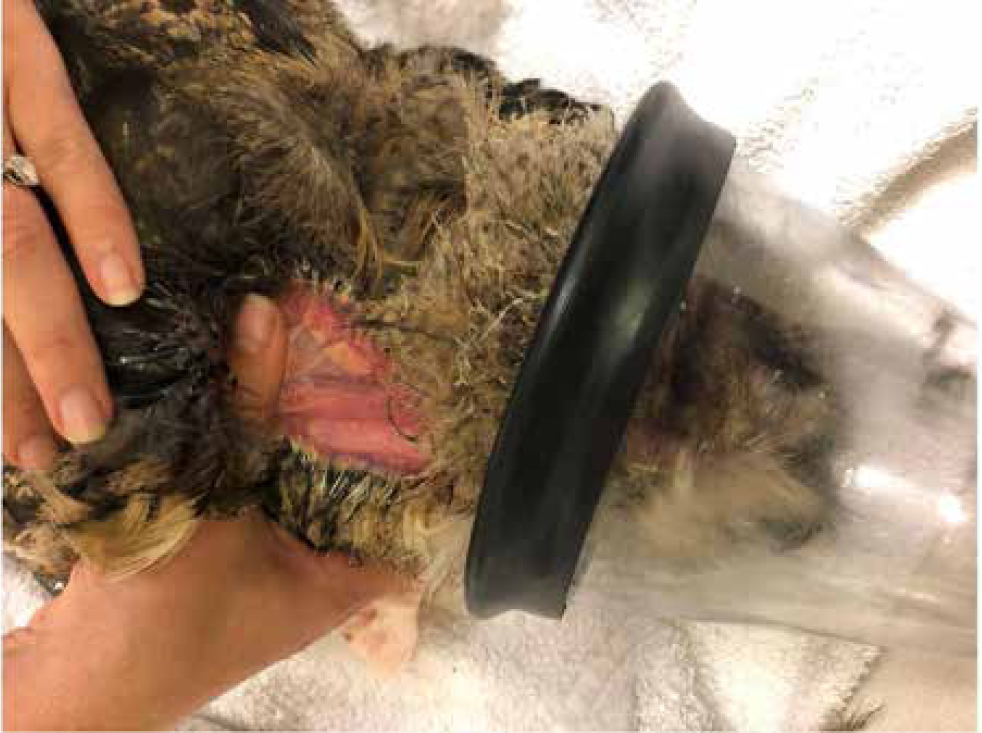

With smaller birds, such as cockatiels (Nymphicus hollandicus), an experienced vet can take blood without an assistant. For right-handed individuals the bird should be held in the left hand with the head held between the first finger and middle finger (the ‘British bird ringers grip’), the neck should be extended and the thumb can be used to occlude the vein. The syringe should be used with the right hand to draw blood (Figure 9).
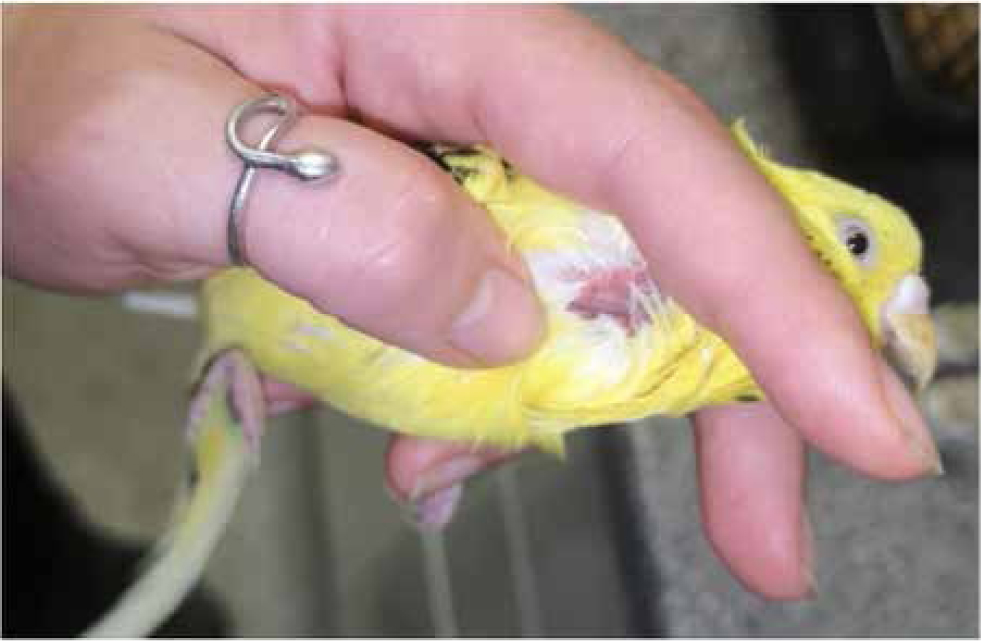
There is a risk of puncturing the cervicocephalic air sac, especially if the patient is fractious. This can lead to coughing up of blood and could cause respiratory distress (Kramer and Harris, 2010). Fractious patients may also lead to the laceration of the jugular vein, which could result in fatal haemorrhage. As previously mentioned, general anaesthesia should be considered on a case by case risk-assessed basis.
Basilic wing vein
The Basilic vein runs between the biceps and triceps over the medial aspect of the humerus and bifurcates just proximal to the elbow (Figure 10). With the bird restrained in lateral recumbency, the ventral wing can be extended. If necessary, the bird can be partially moved into dorsal recumbency. The area over the vein often has no feathers, but if obscured the area can be gently plucked. The area can be swabbed with surgical spirit, or the preferred skin disinfectant. The author often uses the cap of the needle to bend it by approximately 20 degrees. At this site, the vein does not need to be raised and gentle traction on the syringe plunger will result in a steady sample (Figure 11).

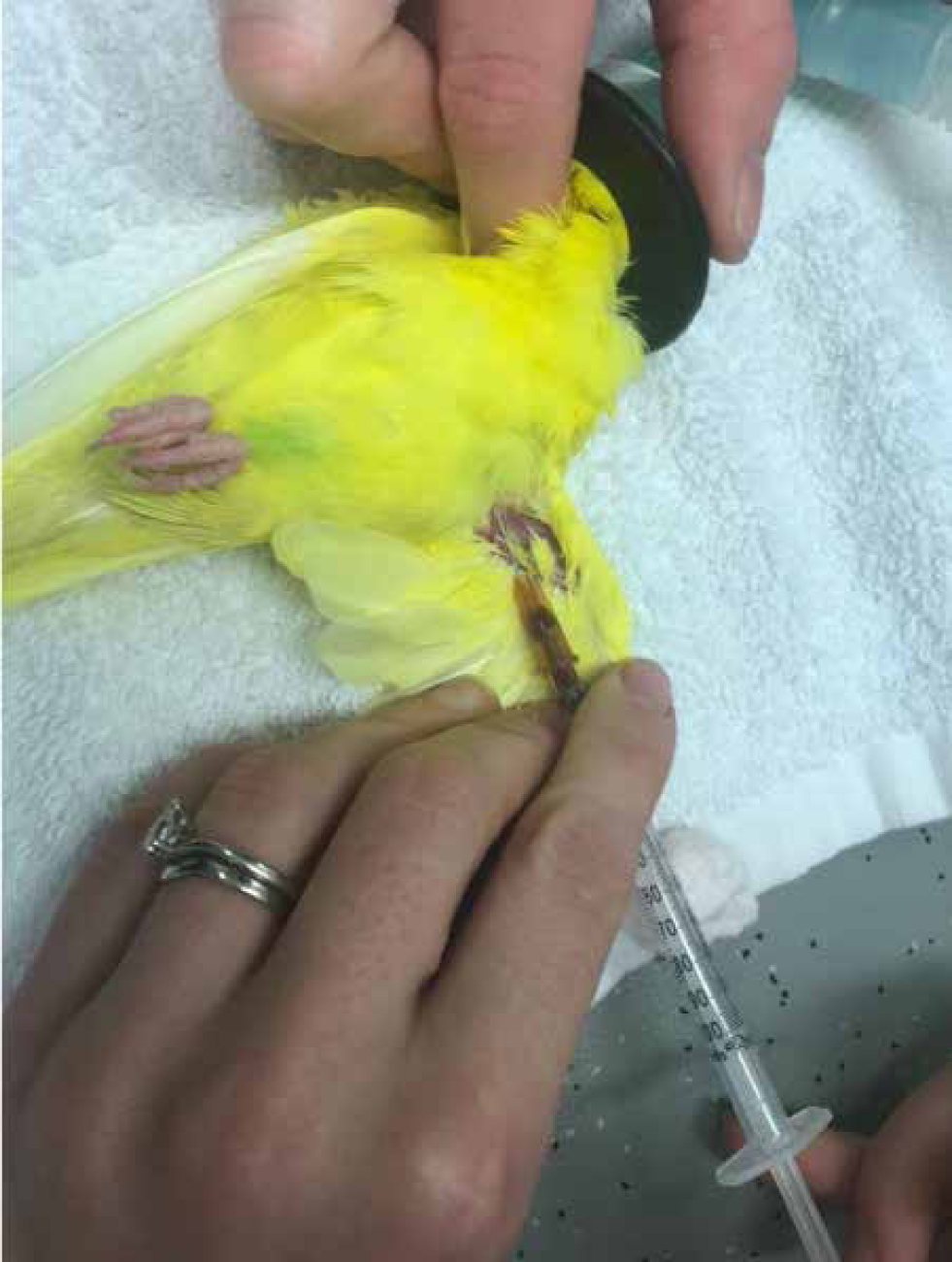
The Basilic vein is fragile and gentle technique is required to obtain an adequate sample. Kramer and Harris (2010) recommended the use of this vein in medium to large birds because of its small size. The author would recommend using the jugular in small birds unless there is a medical reason prohibiting it. Use of this vein can result in a wing ‘droop’ for a couple of days because of haematoma formation, thus the owners should be counselled of this possibility.
Medial metatarsal vein
Use of the medial metatarsal vein is most suited to waterfowl, larger parrots, raptors, and pigeons, although this is not an exhaustive list.
The bird should be held in ventral recumbency either on a table or in the arms of the handler. The leg to be sampled should be extended to allow medial access. Circumferential pressure is then applied distal to the stifle to raise the vein. The vein can be readily visualised at the junction between the feathered and scaled skin (Figures 12and13).

Other uses for these veins
Although this article is primarily focused on venipuncture it should be noted that all of these veins can also be used for catheterisation (Figures 14and15). The author's personal vein of choice for intravenous catheterisation is the basilic vein, therefore the author will often choose to sample the jugular vein to preserve the basilic veins if a catheter is likely to be required. It should also be noted that venipuncture can be used to administer fluid therapy bolus and also to administer intravenous medication.

Pedal and digital veins
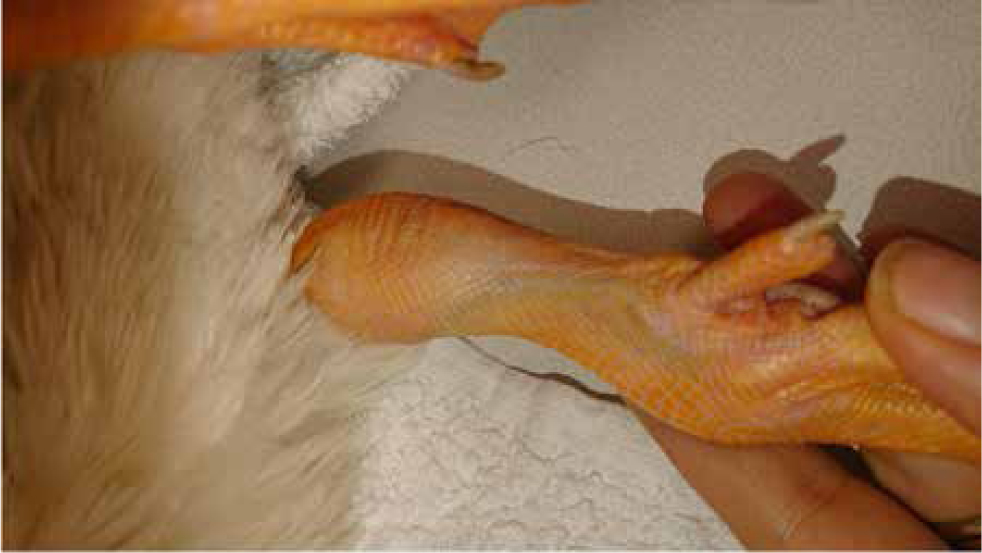
Another site used for venipuncture is the pedal or digital veins in species such as penguins, ducks and geese. However, this is a heavily contaminated site, thus time needs to be taken to aseptically prepare the site (Figure 16).

Blood vessels in the foot web run lateral to the digits. The vein may be raised by putting pressure proximally. The needle should then be introduced parallel to the vein. Pressure applied to the site for 30 seconds is adequate for haemostasis. This vein is suitable for small volume samples, although in cold conditions this can be challenging as the veins will be constricted.
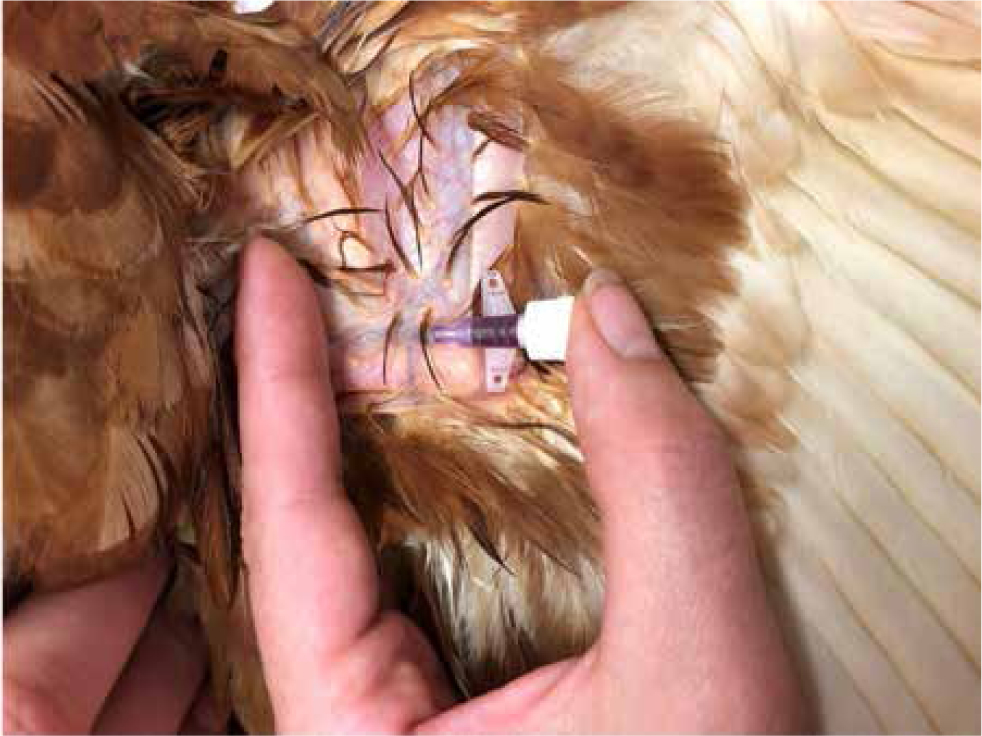
Redrobe (2000) and Sayers (2015) described taking jugular blood samples from penguins (Figure 17). The bird should be restrained by the handler holding the body and wings under their arm. The head and neck are extended straight. Pressure is applied at the thoracic inlet to raise the vein. The jugular may be palpable or, with knowledge of the anatomy, a ‘blind stick’ lateral to the trachea may be required. With practice, this technique is fast, has less chance of bacterial contamination and yields a good sample size.

Historically, many texts will discuss using a toe nail clip to obtain blood; this technique should be avoided as it is considered more painful than venipuncture (Kramer and Harris, 2010). Furthermore, the high level of contamination makes it unsuitable for biochemistry and also increases the risk of introducing infection to the patient. Campbell et al (1994) also pointed out that a toe nail clip means the sample is from capillary blood, which may yield different results from peripheral blood. The ease of performing venipuncture in the avian patient has resulted in this technique being consigned to history.
Conclusions
Avian patients are being presented to general practice much more commonly. Owners understandably expect (and will pay for) just as high a level of care for their feathered pets as for their dogs and cats. Avian venipuncture is commonly required, easy to perform and not as intimidating as it first appears. Clinicians should contact their preferred laboratory to ensure that they are used to handling avian samples and which sample type they require. It is crucial to remember to make a smear from fresh blood at the time of sampling and to label samples appropriately.
KEY POINTS:
- Avian patients are being presented to general veterinary practice with increasing frequency and often require venipuncture.
- The maximum recommended volume of blood to be taken is 1ml per 100 g of bodyweight. Less should be taken in ill individuals.
- The same veins can be used for catheterisation as venipuncture.
- Venipuncture can be used to administer fluid therapy boluses and medication.

