Histopathology is the study of alterations in tissue caused by disease, including inflammatory, infectious and neoplastic processes. It encompasses examination at a microscopic level, by a trained veterinary pathologist, of tissues (including biopsies and post-mortem samples) that have been retrieved by a veterinary practitioner. These tissues must be fixed in formalin, processed and stained before examination. Histopathology can be a key tool for practitioners, providing a diagnosis or assisting with treatment (Stidworthy and Priestnall, 2011).
In some cases, inadequate sampling, submission or iatrogenically induced artefacts can invalidate microscopic interpretation. An incomplete or incorrect clinical history can also lead to incorrect dissection or microscopic interpretation of the specimen. Furthermore, there will always be a small number of cases with non-specific or equivocal findings, even with excellent quality samples and thorough accompanying history, and it can be helpful to warn owners of this possibility before proceeding.
The following recommendations are general outlines and it is always recommended that the specific laboratory is contacted to discuss their sample submission requirements.
Complete history and signalment
Providing a complete, concise and accurate clinical history is key for accurate processing and interpretation of histology samples. Most laboratories provide a submission form that should be sent along with the formalin fixed tissue. Fill in all the blanks if possible.
The form will generally include:
- Signalment: Knowing the sex, age and breed are key to providing accurate differential diagnoses, as there are some pathological processes that are more common in certain breeds, age ranges and genders. For example, cutaneous mucinosis is relatively common in the Chinese Shar Pei but rare in other breeds of dogs. Similarly, although lesions such as cutaneous histiocytomas and viral papillomas can be seen at any age, they are more common in younger dogs (Gross et al, 2005)
- Location: Be as precise as possible and try not to use general terms, such as mouth or eye. It can be very difficult to differentiate histologically between the different segments of the small intestine and large intestine, so careful labelling of all the containers or cassettes is recommended. Also, it can be impossible to determine the location histologically if no normal tissue is present, for example epithelial polyps can look similar independent of their location
- Type of sample/biopsy: Indicate whether the sample is an incisional (partially excised) or excisional (fully excised) biopsy, and if all the sample has been submitted or only a portion. This information is vital for interpretation and for establishing surgical margins of the lesion
- Brief history: Provide a brief patient history, including clinical signs, diagnostic imaging and/or laboratory findings and relevant treatments. Include differential diagnoses, but avoid sending the patient's entire clinical history; only submit clearly relevant information
- Photographs: If submitting pictures, ensure that they are sent in an email, or send a good quality colour print, as low-quality black and white pictures are often of limited help.
Once a sample reaches a histopathology laboratory, it is trimmed and processed into glass slides that are then read by the anatomic pathologist. The sample should arrive at the laboratory with the submission form. A qualified histology technician or pathologist will trim and oversee the processing of the sample. Not all of the submitted tissue is trimmed, so the technician and pathologist may require detailed information to enable them to examine the areas of interest. This information should be included in the submission form and, if needed, the lesion and/or margins can be marked with sutures or ink (Figure 1).
Correct sample submission
Different laboratories have different requirements regarding sample submission, which is why it is important to contact the laboratory of choice for specific information and requirements.
Detailing all of the different types of sample would be outside the scope of this article. Instead, key information regarding the most commonly submitted samples is summarised.
Containers
Remember to label all containers, including patient information and site of sample collection. The formalin to tissue ratio should ideally be 10:1; at the very least, the sample must be fully covered.
An appropriate container should be used to submit samples: glass containers, containers with narrow necks or containers that are prone to leakage should not be used. Relying on features such as colour or size for sample distinction should be avoided, as fixation markedly distorts tissue and samples often cannot be differentiated when received in the lab. If submitting multiple samples, it is recommended to submit the samples in separately labelled containers; if it is absolutely necessary to send samples in the same container, sutures should be used to tag individual tissues.
What if I have a very large sample?
Samples such as spleens or limbs are routinely submitted for histopathological examination. If the sample is too large to put in a container, there are other options to consider:
- Pre-fix the sample in clinic, ensuring that health and safety procedures are followed. Once fixed, wrap the fixed sample in formalin-soaked gauzes and double bag it. Incising the sample in parallel slices with a fresh, sharp blade, leaving a common base, facilitates formalin penetration. However, this may not be possible with every sample.
- Submit portions of the mass or masses only. This is an acceptable option when the lesion/s can be clearly identified, although laboratories usually prefer to receive the whole organ or tissue. If submitting portions of the mass/masses only, multiple sections should be sent, including mass and interface between normal and affected tissue. Areas of clear necrosis or haemorrhage should be avoided. If possible, the rest of the tissue should be saved until the final report is received from the laboratory.
What if I have a very small sample?
If the sample is very small, the smallest container available should be used. Placing the sample on porous material, such as cardboard or gauze, should be avoided. Ideally, a cassette should be used. If multiple cassettes are submitted, they can be labelled with a pencil (Figure 2).

Specific organ or sample submissions
The following sections give specific information about organs and samples that are commonly submitted to histopathology laboratories.
Spleen
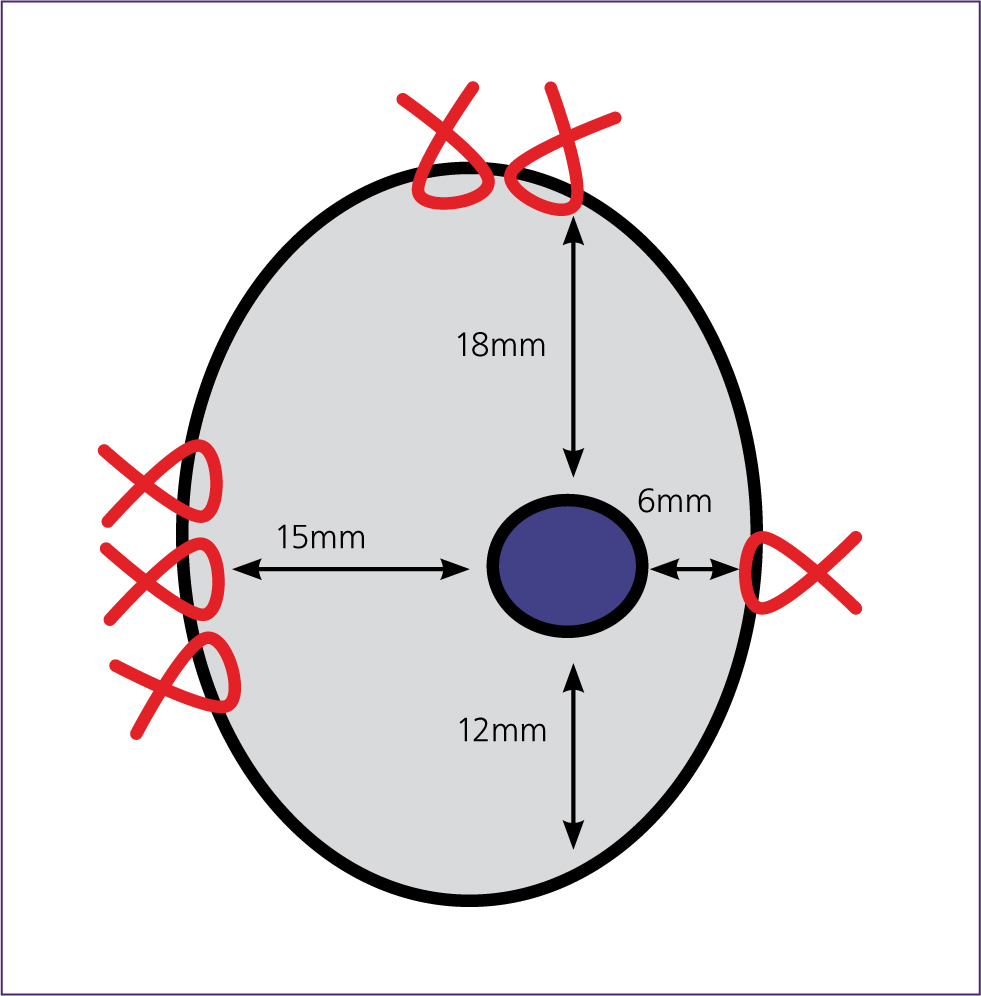
Splenic nodular lesions can look very similar on macroscopic or gross examination, thus histology is needed to further characterise them. This is because areas of nodular hyperplasia and tumours often rupture, causing severe haemorrhage and tissue congestion. It is therefore recommended that the entire spleen is submitted if possible. If not, portions of the spleen should be submitted, as discussed above. Areas of haemorrhage or necrosis should be avoided, as those areas will not provide any useful information on histopathology (Figure 3).
Integument
Samples from the integument include cutaneous and subcutaneous masses, skin biopsies, ear and/or digit samples.
Cutaneous and subcutaneous masses
Cutaneous and subcutaneous masses are the most common samples received in laboratories. Knowing the exact location that the mass was taken from is crucial to be able to provide a specific diagnosis. For example, in the authors' experience, poorly differentiated carcinomas can look alike no matter the tissue of origin, so providing the exact location can help with reaching a final diagnosis.
Location is also important in terms of the prognosis. For example, the prognosis of some tumours, such as melanomas, depends on their specific location (Goldschmidt and Goldschmidt, 2017). It is also very important to specify if the sample is an excisional or incisional biopsy, and whether the whole tumour was submitted, or only a portion. This is important for margin assessment and for grading, as grading of incisional biopsies can sometimes be less reliable than grading of excisional biopsies.
Dermatopathology cases
Dermatology cases can be challenging. Providing a detailed history, distribution of the lesions, breed, sex and age are key in reaching a definitive diagnosis. The patient's clinical history information including prior failed or successful treatments should also be included. If clinically possible, the animal should be off steroids for at least 21 days before the sample is collected.
The sample should be taken from affected areas and the interface between affected and non-affected areas. Ideally, more than one sample should be provided, as some may not be representative. Generally, submitting at least six punch biopsies is optimal when multiple lesions are present. The possibility that there may be two or more concurrent processes (for example, atrophic dermatopathy and pyoderma) should also be considered, making sampling the different type of lesions important. Biopsy of cases where there is pyoderma as the main presentation should be avoided, but this should be reconsidered if signs are still present after management of the infection.
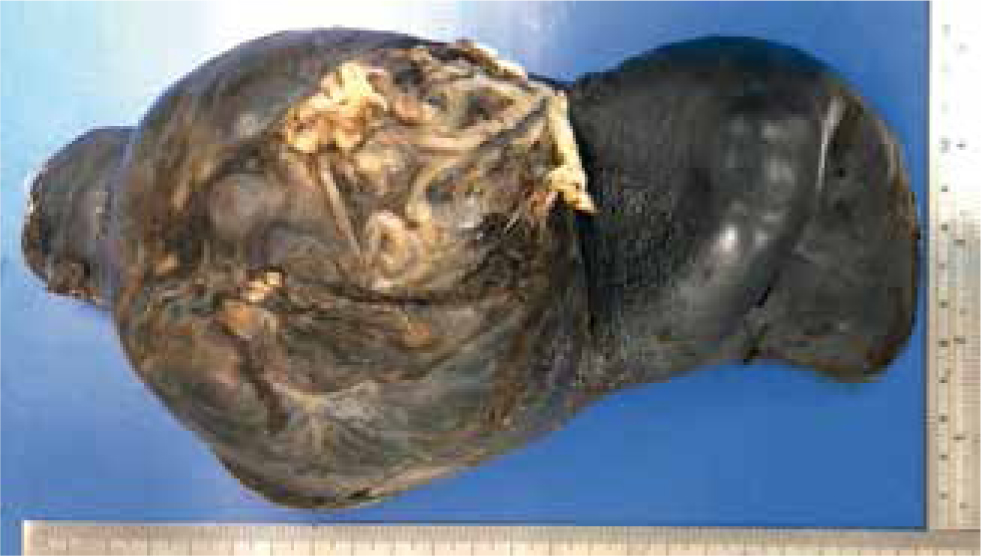
The biopsy site should not be surgically prepared or scrubbed, as this can remove superficial crusts. Examination of superficial crusts is important in diseases such as pemphigus foliaceus or some types of dermatophytosis to be able to achieve a definitive diagnosis (Figure 4). Injecting local anaesthetic into the dermis at the site of biopsy should also be avoided, as this creates artefact. Instead, anaesthetic should be injected into the subcutis if possible.
Cases where there are no active primary lesions should not be biopsied. The cause of pruritic skin disease often cannot be differentiated on histology, particularly in cases of atopy and flea allergy. Consider biopsying pruritic patients only where clinical signs cannot be explained by classic allergic skin disease.
Ear masses or lesions
A variety of neoplastic and non-neoplastic lesions can occur in the ear canal or periaural skin. Masses that arise from or are present in the ear canal include:
- Ceruminous gland lesions, which are relatively common in dogs and cats. Distinction of ceruminous gland hyperplasia, adenoma and adenocarcinoma often relies on assessment of the margins of the lesion and may be difficult in incisional biopsies
- Nasopharyngeal polyps are relatively common in young cats
- Inflammatory polyps can be seen in dogs and cats.
Ulceration and secondary infection are common in these types of lesions and that can sometimes hinder interpretation. In some cases, samples are taken from chronically inflamed external ear canals. In such cases, including fresh samples for microbiology or parasitology is ideal.
Digits
Amputated digits are also frequently sent to diagnostic labs, usually because of the presence of a mass or non-healing nail bed infection.
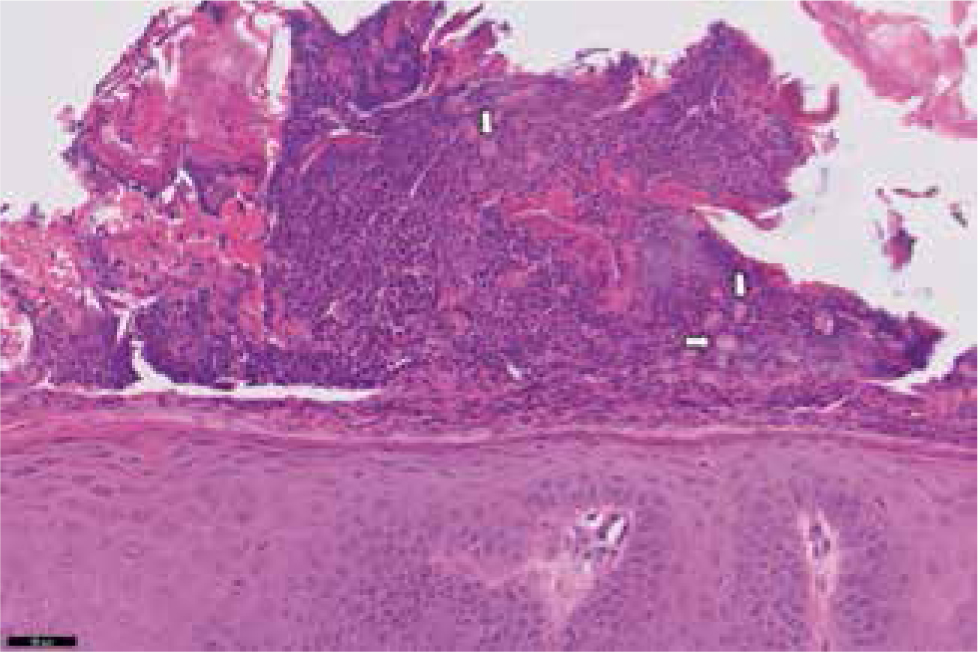
In cases in which symmetrical lupoid onychodystrophy is suspected or there is nail pathology, the nail bed epithelium needs to be included as part of the biopsy. Traditionally, biopsy has been via the amputation of a third phalanx (P3). If affected, a dewclaw may be sacrificed. Many patients will have secondary bacterial infection, but it is helpful to rule out fungal disease with culture. Leishmania can also cause nail bed pathology, so adjunctive testing may be needed (Figure 5).
Oral samples
Oral neoplasia and inflammatory conditions are common in dogs and cats. Some points that need to be considered include:
- Knowing the exact location of the lesion can be of vital importance for making an accurate diagnosis and for the prognosis. For example, canine oral and tonsillar squamous cell carcinomas have different prognoses (Munday et al, 2017)
- Correlation with clinical findings (such as diagnostic imaging) may be needed, as some odontogenic tumours may share histological findings but have a different behaviour (infiltrative vs expansile mass) (Figure 6)
- Cautery artefacts can interfere with diagnosis or assessment of margins. Coagulation of the tissue is a consequence of the use of diathermy or electrocautery for excision, which causes loss of tissue architecture and cellular detail. This artefact can lead to some samples being non-diagnostic. Consequently, sharp surgical excision is generally recommended for cases where histopathological examination of the excised tissue is required.

Note that if squamous cell carcinoma is suspected, superficial samples of inflamed or ulcerated lesions may not give a definitive diagnosis because of the presence of epithelial hyperplasia or dysplasia.
Digestive
Endoscopic and surgical (full-thickness) biopsies are commonly submitted as diagnostic samples. With the former, careful sample handling is required to avoid introducing further artefacts. An adequate number of samples, ideally eight to ten, should be collected from each location. Containers should be clearly labelled with the patient's name and the site of biopsy. Samples should be submitted in cassettes or floating in formalin.
Full-thickness samples are generally superior to endoscopic biopsies in quality. There is evidence that full-thickness biopsies are preferable to differentiate inflammation from small-cell lymphoma in cats (Evans et al, 2006; Kleinschmidt et al, 2010). Full thickness biopsies are needed if the lesion is suspected to be in the submucosa, muscularis or adventitia/serosa, rather than in the mucosa.
It is important to submit samples from adequate numbers of sites. For example, submission of samples from an endoscopic biopsy from the stomach for vomiting may not be informative as intestinal pathology may be causing the clinical signs.
Lymph nodes
Excisional biopsy is preferred over incisional biopsy for lymph nodes, as this allows for proper evaluation of architecture. Many lymph nodes that contain neoplasia also have areas of reactive lymphoid hyperplasia, thus partial sampling of a lymph node can result in an incomplete diagnosis. Sub-classification of lymphoma often requires immunohistochemistry.
Biopsy of mesenteric lymph nodes without intestinal tract samples to look for a cause of inflammatory intestinal disease is rarely diagnostic, as they provide no specific information about pathology in the gastrointestinal tract.
Nasal cavity
A complete clinical history and diagnostic imaging results are often of great help when interpreting nasal biopsies. If a mass is present, several samples from different areas should be submitted, ideally including deep biopsies. If infection is suspected, fresh samples for culture should be submitted along with the biopsy.
Reproductive tissue
In male patients, testicular and prostatic pathology includes inflammatory and neoplastic processes. Ideally both testicles should be submitted whole, even if only one looks abnormal. It may be difficult to distinguish prostatic hyperplasia and neoplasia in small samples, so histopathology may need to be correlated with findings on ultrasound.
In female patients, ovarian and uterine pathology includes inflammatory and neoplastic processes. The ovaries and uterus should be submitted whole, as this helps with orientation when sampling.
Mammary tissue
Incisional biopsies of mammary tissue can be inconclusive, as low-grade carcinomas and adenomas are often impossible to differentiate without marginal tissue, and invasion, if present, may be focal. In addition, the grading of mammary carcinomas in incisional biopsies may not be accurate and may change when the entire mass is examined. When submitting multiple mass lesions, locations should be clearly indicated, as many patients have a mixture of benign and malignant tumours. If possible, strips should be orientated. Areas of concern can also be marked with sutures if required. Please note that smaller mass lesions, not visible clinically, may be found at dissection.
Liver
When submitting liver samples, ideally liver values and macroscopic and ultrasound descriptions should be provided, if possible. It is crucial for the pathologist to know if the change is diffuse or nodular, or if there is a localised mass.
It is important to note that it can be very difficult to differentiate between nodular hyperplasia, hepatocellular adenoma and well-differentiated hepatocellular carcinoma in incisional biopsies. In a diffuse change, sampling of more than one lobe is likely to provide more information. Distribution of lesions and copper is usually not uniform in the liver, so if a single lobe or area has been sampled, the absence of a lesion on histopathology does not necessarily rule it out (Kempt et al, 2015). Biopsy is generally not recommended for a single abnormal raised alanine aminotransferase (ALT) level on preanaesthetic bloods; the change needs to be repeatable and chronic.
Bone
When submitting bone samples, it is useful to provide a complete clinical history and diagnostic imaging findings where possible. It is very helpful to know if the lesion is lytic or proliferative, if it's affecting a specific area and if the joints are affected. It should be noted that differentiating between reactive and neoplastic lesions on histopathology alone can be extremely difficult. Furthermore, reactive lesions can be around inflammation or neoplasia, thus if the samples submitted are not deep enough, they may not be representative.
Postmortem samples
Sample submission for postmortem cases will depend on the specific findings, therefore the laboratory of choice should be contacted directly to discuss any queries about this type of sample submission. If there are no visible gross findings, samples from all major organs should ideally be submitted. These include the liver, heart, brain, lungs, kidneys and representative sections of the gastrointestinal tract.
Conclusions
Histopathology can be a key tool for practitioners, helping to provide a specific diagnosis or assisting with treatment. Correct submission of good quality samples accompanied by a thorough clinical history will help to maximise results. Ultimately, good communication between the clinician and the pathologist/laboratory is key. Contacting the laboratory or pathologist with any questions regarding sample submission, or if the histopathological results do not correlate with the clinical findings, is very much encouraged.
KEY POINTS
- A complete clinical history and signalment should be provided with any sample sent to a histopathology laboratory.
- Appropriate containers that are labelled with the patient's name, identifying information and samples should be used.
- Contacting the laboratory directly for specific queries is highly encouraged.


