Aelurostrongylus abstrusus (the ‘cat lungworm’), Troglostrongylus brevior and Capillaria aerophila are the three most important nematodes inhabiting the airways of cats. In the last decade they have attracted the attention from academics, veterinary practitioners and nurses from many countries in Europe and overseas (Traversa et al, 2021).
The adult life stages of A. abstrusus take place in the bronchioles, alveolar ducts and alveoli of domestic cats and, seldom, some wild felids. Adults of T. brevior usually affect the bronchi and bronchioles of the European wildcat but in some regions, it has spread to populations of domestic cats as well. Their life cycles are indirect and involve terrestrial gastropods (slugs and snails) as intermediate hosts. While A. abstrusus is endemic throughout Europe, including the UK, T. brevior is prevalent in countries of the Mediterranean basin and eastern Europe (Traversa et al, 2021).
First-stage larvae (L1), passed from the respiratory system to the intestinal tract, are shed in the environment via the faeces. In the intermediate host, L1 develop to second (L2) and third-stage larvae (L3), also known as the infective stage. Cats become infected by preying and ingesting paratenic hosts, including rodents, birds, small reptiles, and less oft en by ingesting slugs and snails (Traversa and Di Cesare, 2013; 2016). Moreover, T. brevior can be transmitted from infected cats to kittens, probably via lactation (Traversa et al, 2018).
C. aerophila lives embedded underneath the mucosa of bronchi and trachea of various species of carnivores, including dogs and cats. Aft er mating, the females lay eggs that reach the upper airways via mucociliary clearance, up to the pharynx, and are then swallowed and shed with faeces into the environment, where they mature. Animals become infected by the inadvertent ingestion of larvated eggs, although earthworms have been incriminated as possible intermediate or paratenic hosts (Traversa and Di Cesare, 2013, 2016; Stepanović et al, 2020).
While A. abstrusus and C. aerophila have a worldwide distribution, T. brevior has thus far only been described in southern and eastern Europe, and the Middle East. In terms of infection rates in the northern hemisphere, A. abstrusus is the most geographically distributed, followed by T. brevior and C. aerophila (Rehbein et al, 2014; Giannelli et al, 2017; Traversa et al, 2021).
Free-roaming cats are at risk of infection by these parasites, because of the increased opportunities for predating paratenic hosts. The presence of wildlife acting as natural reservoirs of C. aerophila (such as foxes) or T. brevior (in the European wildcat) may further increase the chances of infection for domestic cats (Stepanović et al, 2020; Traversa et al, 2021). Owing to the vertical nature of transmission, cats of less than 1 year of age are more commonly infected by T. brevior (Traversa et al, 2019a).
A growing awareness of these respiratory nematodes has stimulated the scientific community to acquire novel data on their expanding distribution and pathogenicity (Traversa et al, 2021). This article reviews and discusses novel knowledge in terms of clinical features, treatment and control of feline aelurostrongylosis, troglostrongylosis and capillariosis.
Clinical knowledge
Clinical signs
Signs and alterations caused by feline lungworms are non-specific and a clinical diagnosis is challenging. Clinical signs overlap those of other feline respiratory diseases, such as bronchial disease or asthma, bacterial and mycotic infections, airway foreign bodies and lung tumours (Traversa and Di Cesare, 2016). Although A. abstrusus, T. brevior and C. aerophila have different localisation within the respiratory system, both upper and lower respiratory tract signs may occur.
Eggs and larvae of A. abstrusus elicit an inflammatory response causing a verminous pneumonia with different degrees of severity, depending on worm burden, health status, immune response and age of the infected cat. Clinical pictures may range from subclinical to a potentially life-threatening disease (Elsheikha et al, 2016; Traversa and Di Cesare, 2016). When the disease is clinically evident, upper respiratory tract signs (such as sneezing and ocular or nasal discharge), and lower respiratory tract signs (including dry or productive cough, dyspnoea, tachypnoea, abdominal, laboured and open-mouth breathing) are commonly described, along with wheezing and end-respiratory crackles detectable at lung auscultation. General and non-specific clinical signs such as anorexia, weight loss, lethargy and fever have also been recorded (Traversa et al, 2008; Schnyder et al, 2014). Bacterial superinfections may cause severe pneumothorax and pleural effusion, leading to potentially life-threatening respiratory failure (Morelli et al, 2021). Although rare pulmonary hypertension with congestive heart failure and death may occur (Dirven et al, 2012; Vezzosi et al, 2020). Laboratory alterations are inconstant and non-specific, including mild anaemia, leukocytosis with eosinophilia, occasional lymphocytosis, monocytosis and basophilia (Schnyder et al, 2014; Elsheikha et al, 2016). Cats with concomitant retroviral coinfection have been traditionally considered more at risk of developing a clinical disease. Nonetheless, it has been shown that the occurrence of A. abstrusus in feline immunodeficiency virus-infected cats does not show significant differences in terms of clinical severity, blood parameters, larval count and radiological findings, compared with cats with A. abstrusus only (Murad et al, 2019; Morelli et al, 2021).
Clinical presentations of troglostrongylosis are similar to those of aelurostrongylosis, although clinical signs are more severe and dangerous in young animals. In fact, adult parasites obstruct small bronchi and bronchioles causing catarrhal bronchitis, pulmonary haemorrhages, lung congestion with oedema and parenchyma hepatization, dangerously decreasing the surface available for respiratory exchange. If not promptly diagnosed and treated, troglostrongylosis is often deadly in kittens and young cats (Traversa and Di Cesare, 2013, 2016; Giannelli et al, 2014; Crisi et al, 2018). The common occurrence of upper respiratory signs (including sneezing and ocular-nasal discharge) underlines the importance of possible misdiagnosis with aelurostrongylosis or with upper respiratory tract diseases, characterised by overlapping clinical pictures and frequently observed in kittens and young cats (Crisi et al, 2018; Morelli et al, 2021). Moreover, damage caused by T. brevior to the heart and pulmonary vessels can cause irreversible pulmonary hypertension (Crisi et al, 2015). As in A. abstrusus-infected cats, haemato-biochemical alterations are non-specific and eosinophilia is only seldom reported (Crisi et al, 2018).
Lung capillariosis causes a chronic bronchitis with different degrees of severity ranging from mild to a life-threatening disease (Conboy, 2009; Stepanović et al, 2020). Sneezing with nasal discharge and dry or productive cough, dyspnoea, tachypnoea, wheezing end-inspiration crackling and increased respiratory sounds are the most common signs. Severe conditions as lung oedema, pleural effusion, interstitial emphysema and secondary bacterial infection are described in cases of high parasitic burden (Traversa et al, 2009a; Traversa and Di Cesare, 2016). Again, some non-specific laboratory findings (leukocytosis with eosinophilia and monocytosis) are described (Elhamiani Khatat et al, 2016; Stepanović et al, 2020).
Mixed infections by two or more lungworms frequently occur in endemic areas, resulting in more severe clinical presentation than in mono-infections (Febo et al, 2019). This means that cats with respiratory signs and living in endemic areas should be examined by both floatation and the Baermann's methods (Morelli et al, 2021), for the detection of C. aerophila eggs and A. abstrusus and T. brevior larvae, respectively.
Diagnostic imaging
Thoracic radiography is usually one of the first diagnostic investigations in referred animals presenting with a respiratory disease. In general, changes are not directly correlated with the severity of the clinical picture, and it is hard to attribute specific alterations to a given lungworm species. The most common alterations, regardless of the nematode(s) harboured, vary from bronchial, nodular and unstructured interstitial patterns with multifocal distributions, to a generalised alveolar pattern (Morelli et al, 2021).
In A. abstrusus-infected cats, an alveolar pattern occurs in the early phases of the infection and coincides with overt clinical signs and a high grade of lung damage. The alveolar pattern is followed by an interstitial pattern, either nodular or, most commonly, diffused and unstructured. Bronchial pattern is also frequently described and can be associated to alveolar, interstitial and/or vascular patterns; the enlargement of the cardiac silhouette caused by pulmonary hypertension has been also reported (Dirven et al, 2012; Dennler et al, 2013; Lacava et al, 2017; Febo et al, 2019; Vezzosi et al, 2020). A mild to severe bronchial pattern is the main radiographic finding in cats with troglostrongylosis. Sometimes it is associated with alveolar and/or interstitial patterns, although unstructured or nodular interstitial patterns have also been reported as a single finding. As for aelurostrongylosis, an alveolar pattern is often associated with more severe clinical pictures and with early disease stages, and pulmonary hypertension can lead to the detection of an enlarged cardiac silhouette (Crisi et al, 2015; Traversa et al, 2018; Febo et al, 2019).
There are few data on known radiographic alterations caused by C. aerophila. Studies indicated that thoracic X-rays of infected cats can show no alterations to bronchial patterns, associated with interstitial and/or alveolar patterns (Elhamiani Khatat et al, 2016; Febo et al, 2019; Stepanović et al, 2020).
Sometimes, verminous bronchopneumonia by A. abstrusus and T. brevior may secondarily cause a cardiovascular disease. Cases of fatal aelurostrongylosis leading to severe pulmonary hypertension and congestive heart failure are known (Vezzosi et al, 2020), as T. brevior may also induce severe right-sided cardiac enlargement, systolic tricuspid regurgitation and irreversible pulmonary hypertension (Crisi et al, 2015). These findings underline the importance of faecal examinations in cats presenting with cardiovascular and respiratory signs and, vice versa, to perform echocardiography in animals diagnosed with lungworms and showing heart murmur and pleural, pericardial and/or abdominal effusions.
Computed tomography (CT) scans are less commonly used than conventional radiology in the diagnostic evaluation of respiratory parasitosis of cats, despite their higher accuracy in characterising lower airway diseases. CT may reveal multiple nodules distributed throughout the lungs, associated with interstitial-alveolar infiltrate, peri-bronchial changes, generalised lymphadenopathy, subpleural thickening and multiple small hyperattenuating areas in the case of aelurostrongylosis (Payo-Puente et al, 2005; Dennler et al, 2013; Lacava et al, 2017). Very few data are available for T. brevior infected cats (such as interstitial ground glass), while there is no information on CT findings in cats with pulmonary capillariosis (Febo et al, 2019).
Parasitological diagnosis
Microscopic methods
Faeces can be examined to detect A. abstrusus and T. brevior L1, although intermittent larval shedding or low numbers can cause false negative results. Thus, faecal examinations should be performed on three consecutive defecations via the Baermann's test – the gold standard technique (Elsheikha et al, 2016). It is important to use fresh faeces, to reduce the risk of larval death and contamination with free living nematodes from the environment.
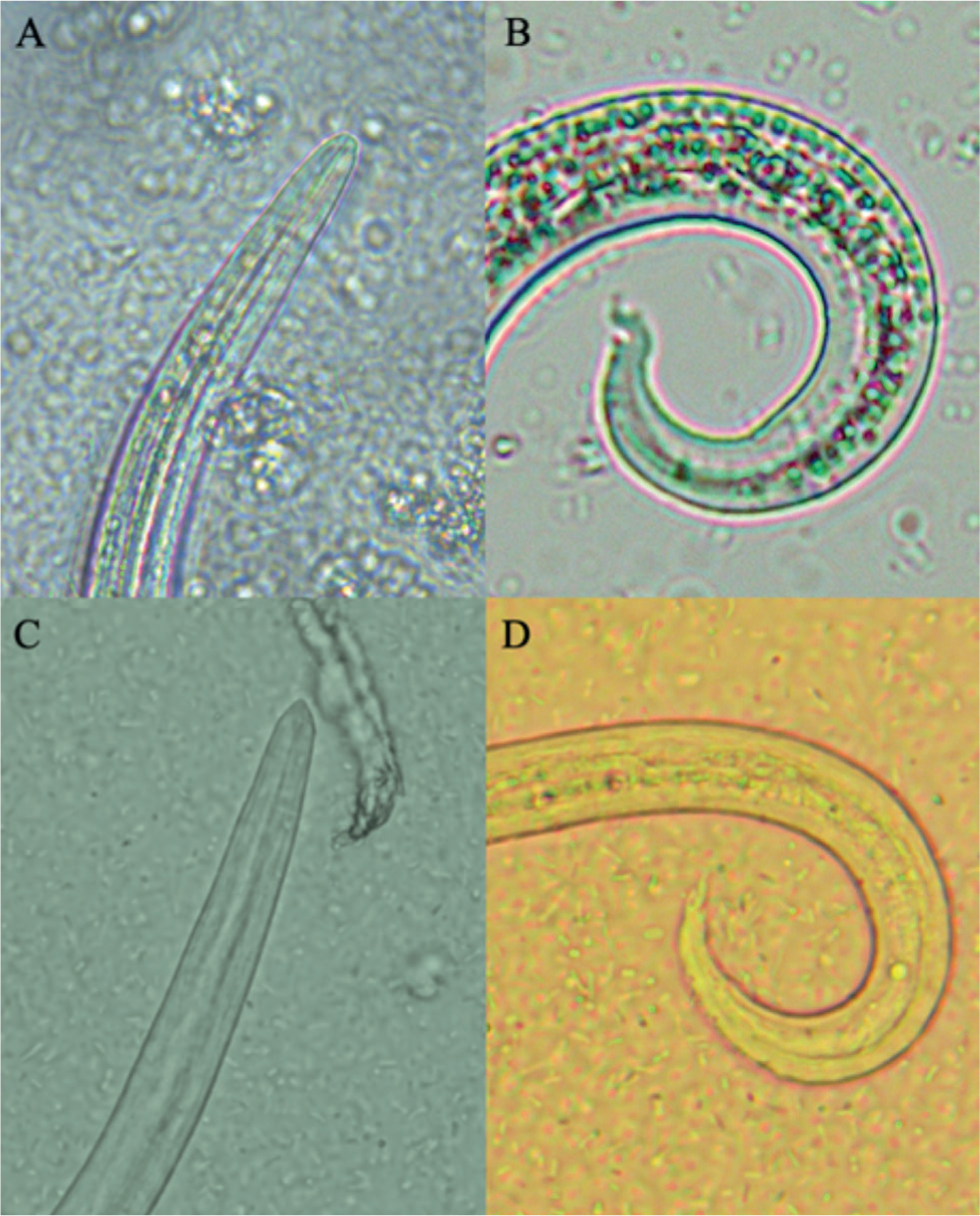
This test can be easily conducted by leaving the sample in water at room temperature to allow L1 migrate from faeces to the bottom of the Baermann apparatus. The liquid is then collected after 12–24 hours and, after centrifugation, L1 can be collected from the sediment. Although L1 of A. abstrusus and T. brevior may overlap in length and width, the latter is generally shorter than the former (Morelli et al, 2021). The extremities of larvae allow their differentiation (Figure 1): A. abstrusus presents a simple anterior end with a plateau and a terminal opening, while T. brevior has a subterminal oral opening and a clearly visible apical prominence. The posterior end of A. abstrusus is characterised by a sigmoid end with a prominent ventral kink, deep dorsal and ventral incisures and a terminal knob-like ending, while that of T. brevior has a shallow or absent dorsal kink, a deep dorsal and a shallow ventral incisure and a slender terminal appendix (Traversa and Di Cesare, 2013; Giannelli et al, 2017).
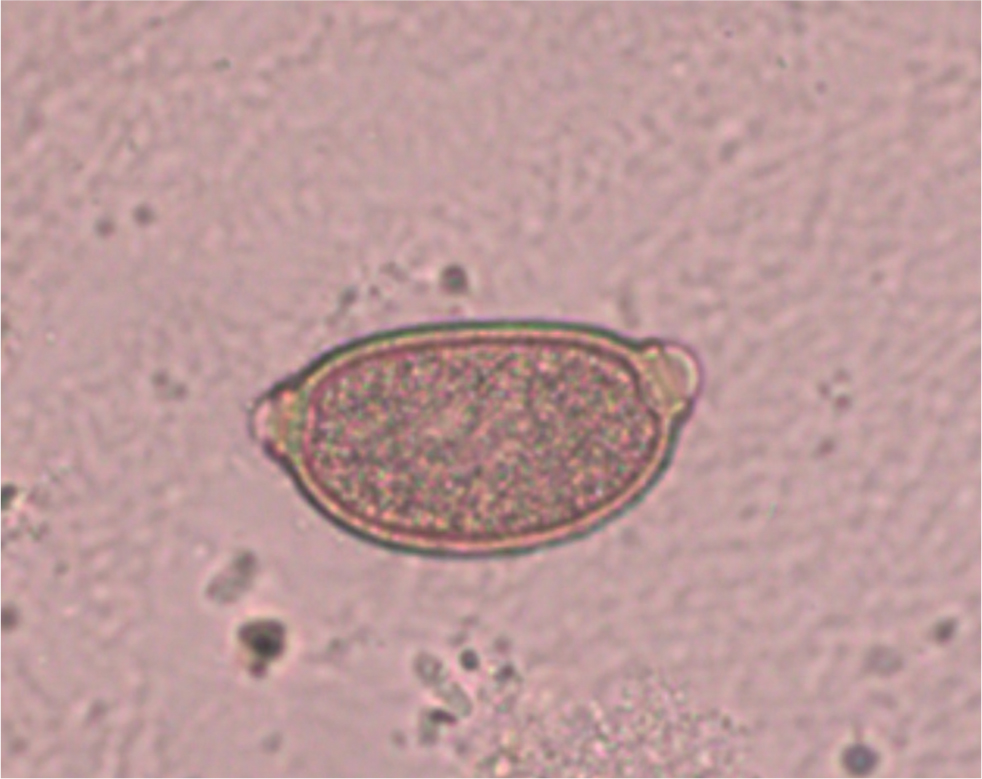
C. aerophila eggs can be detected both by floatation and sedimentation methods (Traversa and Di Cesare, 2016). Their morphological characteristics, including their lemon-like shape, bipolar appearance and yellowish colour (Traversa et al, 2011), can lead to a misdiagnosis as Trichuris eggs. C. aerophila ova must be discriminated from whipworm (Trichuris muris) or capillariid ova, which can be present in the faeces of cats preying on infected small rodents or birds (pseudoparasitism). It is important to remember that cats are not parasitised by the canid whipworm Trichuris vulpis, and that felid whipworms (Trichuris campanula and Trichuris serrata) are rare parasites distributed in limited tropical geographies (Morelli et al, 2021). In cat faeces, eggs of C. aerophila can be recognised by the network of anastomosing ridges on the wall surface, asymmetrical polar plugs and absence of a thickening with ringed appearance at the base of the polar plugs (Figure 2) (Traversa and Di Cesare, 2016).
Serology
An enzyme-linked immunosorbent assay has been recently developed for the detection of antibodies vs A. abstrusus, with satisfactory levels of sensitivity (88.2%) and specificity (68.1–98.5%) (Zottler et al, 2017). This test is not used in clinical routine yet, and needs some refinements.
Studies have shown higher prevalence rates using this serological method, compared to the positivity obtained using copromicroscopic examinations (Gueldner et al, 2019; Morelli et al, 2020). This discrepancy could be caused by:
- Intermittent shedding of L1
- Occult infections as a result of prepatency (for example, cats seroconvert before patency)
- Chronic or repeated infections
- Antibodies which may circulate until 6 weeks after the death of adult worms (Morelli et al, 2021).
False negative results should also be considered, because this method reaches its highest sensitivity at 10 weeks post-infection or, alternatively, cats may have an antibody titre below the threshold. Potential cross-reactions with T. brevior also need further evaluations (Zottler et al, 2017). Overall, other studies are needed for the development of a commercially available laboratory and in-clinic rapid test for the serological diagnosis of aelurostrongylosis and possibly troglostrongylosis.
Endoscopy and bronchoalveolar washing
Bronchoscopy and bronchoalveolar lavage are not used to diagnose feline respiratory parasites, as they are invasive and require general anaesthesia, that can be hazardous in cats infected by lungworms (Gerdin et al, 2011). Moreover, bronchial alterations and cytological findings in infected cats are non-specific and overlap with those of other feline airway diseases (Lacorcia et al, 2009).
DNA-based assays
Polymerase chain reaction assays allow genetic identification, with high sensitivity and specificity, of A. abstrusus, T. brevior and C. aerophila in different biological samples, including faeces, flotation supernatant, Baermann sediment and pharyngeal swabs (Annoscia et al, 2014; Di Cesare et al, 2014; 2015a; Traversa et al, 2021). These tools proved to be highly efficacious in many studies and clinical settings, being able to detect lungworms DNA also in copromicroscopically negative samples. Pharyngeal swabs proved also useful for the molecular diagnosis of A. abstrusus and T. brevior in experimental settings, and this opens new routine diagnostic perspectives when faecal collection is difficult (for example, when cats are allowed to go outdoors) (Di Cesare et al, 2014; 2015b; Crisi et al, 2020).
Treatment and control
Aelurostrongylosis
Formulations containing emodepside 2.1%, moxidectin 1% or eprinomectin 0.4% are licensed in the European Union for treating aelurostrongylosis (Table 1). Also, oral products containing fenbendazole are labelled in some countries. In most cases, these formulations guarantee the reduction or prevention of larval shedding and the resolution of clinical signs in infected cats (Traversa et al, 2009b, 2009c; Böhm et al, 2015; Crisi et al, 2017, 2018, 2020).
Table 1. Formulations labelled for the prevention and/or treatment of aelurostrongylosis, troglostrongylosis and capillariosis in cats in Europe
| Formulations | Aelurostrongylus abstrusus | Troglostrongylus brevior | Capillaria aerophila |
|---|---|---|---|
| Emodepside (2.1%) and praziquantel (8.6%) | Yes (for treatment - two administrations, 2 weeks apart)) | No | No |
| Moxidectin (1%) and imidacloprid (10%) | Yes (for prevention and treatment - monthly administration for 3 months consecutively)) | No | Yes (treatment) |
| Eprinomectin (0.4%), praziquantel (8.3%), fipronil (8.3%) and (S)-methoprene (10%) | Y (for prevention and treatment - a second administration 1 month after the initial treatment may be recommended) | Yes (treatment and prevention) | |
| Fenbendazole | Yes (for treatment - labelled in some markets for treatment with a 3-day regimen of 50 mg/kg)) | No | No |
Products containing selamectin and milbemycine oxime are ‘off label’ for treating cat aelurostrongylosis, although promising results have been shown in single clinical cases (Dirven et al, 2012; Iannino et al, 2013).
The spot-on containing moxidectin 1% is licensed for the prevention of aelurostrongylosis by once-a-month administrations, able to prevent lung damage and patent aelurostrongylosis (Heuer et al, 2020). The formulation containing eprinomectin 0.4% is also registered for the prevention of aelurostrongylosis with monthly applications (Knaus et al, 2014).
Troglostrongylosis
To date, only the topical product containing eprinomectin 0.4% is labelled to treat this disease, with high efficacy in stopping larval shedding and assuring a clinical recovery (Giannelli et al, 2015; 2017; Knaus et al, 2020).
The spot-on medications containing moxidectin 1% and emodepside 2.1% are not labelled for use in treating troglostrongylosis, though they were highly efficacious in reducing or stopping larval shedding. Specifically, the moxidectin 1% formulation was proven to have 100% efficacy even after a single administration, while those containing emodepside 2.1% showed 87–100% efficacy after one or two topical topical administrations two weeks apart. Furthermore, the administration of emodepside proved useful to the clinical recovery of infected cats, while further clinical studies are warranted to investigate the efficacy of moxidectin 1% in clinically affected cats (Diakou et al, 2019; Traversa et al, 2019b).
No data are available on the efficacy of milbemycin oxime or selamectin in treating cats infected by T. brevior.
The formulation containing eprinomectin 0.4% is labelled also for the prevention of troglostrongylosis with monthly dosing (Knaus et al, 2020).
Capillariosis
Only the topical formulation containing moxidectin 1% is labelled for treating C. aerophila in cats. This product showed 100% efficacy in reducing egg output and clinically curing the vast majority of infected cats (Di Cesare et al, 2017).
Studies on topical formulations containing eprinomectin 0.4% and emodepside 2.1% showed promising results, while no sound data are available on the efficacy of milbemycin oxime and selamectin (Rehbein et al, 2014; Knaus et al, 2015; Rohdich et al, 2018).
Conclusions
The cat lungworm A. abstrusus remains the most important respiratory nematode affecting cats, although T. brevior and C. aerophila are gaining attention in feline clinical practice.
Clinical and radiographic signs caused by these parasites overlap each other, and with those of other respiratory diseases.
A. abstrusus is more frequent in chronically and subclinically infected adult cats, while T. brevior is more common in kittens or young animals showing severe clinical signs. Cats living in the same areas as foxes may often suffer trachea-bronchitis caused by C. aerophila. Both A. abstrusus and T. brevior may cause cardiovascular damage more often than previously thought. This means that cats presenting with heart murmur, pulmonary hypertension and/or right-sided congestive failure should be examined to detect these nematodes, especially in cases of kittens or young cats.
A Baermann's test and faecal flotation should always be performed on samples from cats with respiratory signs, which are also recommended for routine application in healthy cats living in endemic areas, or if they should be subjected to general anaesthesia, as aelurostrongylosis is one of the main causes of anaesthesia-related deaths in cats. The microscopic identification of larvae and eggs remains the gold standard of diagnosis of feline lungworms, although there are some drawbacks that can be overcome with the use of molecular and serological tools.
New treatment and prevention options have been generated in the past few years, especially for aelurostrongylosis, and further studies are encouraged to implement control methods for troglostrongylosis and capillariosis.
KEY POINTS
- The parasitic nematodes Aelurostrongylus abstrusus, Troglostrongylus brevior and Capillaria aerophila affect the respiratory system of cats and are a primary cause of respiratory disease in cats.
- The clinical picture can be extremely variable in lungworm infections: both upper and lower respiratory tract signs may occur, along with non-specific clinical signs. Moreover, the severity of the disease ranges from subclinical pictures to potentially life-threatening conditions.
- The gold standard technique to diagnose Aelurostrongylus abstrusus and Troglostrongylus brevior infections is the detection of larvae using the Baermann test. The diagnosis of Capillaria aerophila infections relies on the detection of eggs at floatation.
- The aetiological diagnosis is pivotal to choose the most appropriate treatment, as different formulations are labelled to treat feline aelurostrongylosis, troglostrongylosis and capillariosis.


