Surgical site infections are common in small animal veterinary practice and will have been encountered by most, if not all, practitioners. Well-established surveillance programmes in human medicine estimate the incidence of surgical site infections to be about 2.0–2.8% (Barie, 2002; de Lissovoy et al, 2009). Comprehensive surveillance is lacking in veterinary medicine; various studies report incidences ranging from 2.5–18.1% (Vasseur et al, 1988; Eugster et al, 2004). The significant variation is attributable to differences in surveillance methodology, difference in procedures undertaken, and large variation in degree of wound contamination (Turk et al, 2014).
Surgical site infections result in significant patient morbidity and mortality, including but not limited to pain, prolonged wound management, the need for revision surgery, increased antimicrobial use, patient death or euthanasia (Stetter et al, 2021). From an economic standpoint, surgical site infections can double the cost of hospital stays in human patients (Broex et al, 2009). A single-centre veterinary study observed an increase in total and postsurgical costs of 74.4% and 142.2% respectively in cases of surgical site infections (Espinel-Rupérez et al, 2019). In veterinary medicine, which is largely privately-funded, the financial implications of surgical site infections on clients should not be overlooked.
Definitions and classification of surgical site infections
A surgical site infection is a nosocomial infection temporally associated with a particular surgical procedure. A bacterial wound infection can be defined as the presence of more than 105 bacteria per gram of tissue (Fossum, 2013). Although objective and quantitative, this has limited clinical applicability. The Centers for Disease Control and Prevention (CDC, 1991) have established the National Nosocomial Infections Surveillance system to standardise surgical site infection definitions for surveillance purposes. Using a combination of clinical and microbiological information, surgical site infections can be classified as superficial, deep, or organ/space infections (Table 1, Figures 1–3).
Table 1. Surgical site infection criteria
| Type of surgical site infection | Must meet the following criteria: | And at least one of the following criteria: |
|---|---|---|
| Superficial incisional |
|
|
| Deep incisional |
|
|
| Organ/space |
|
|
Adapted from National Healthcare Safety Network (2021)
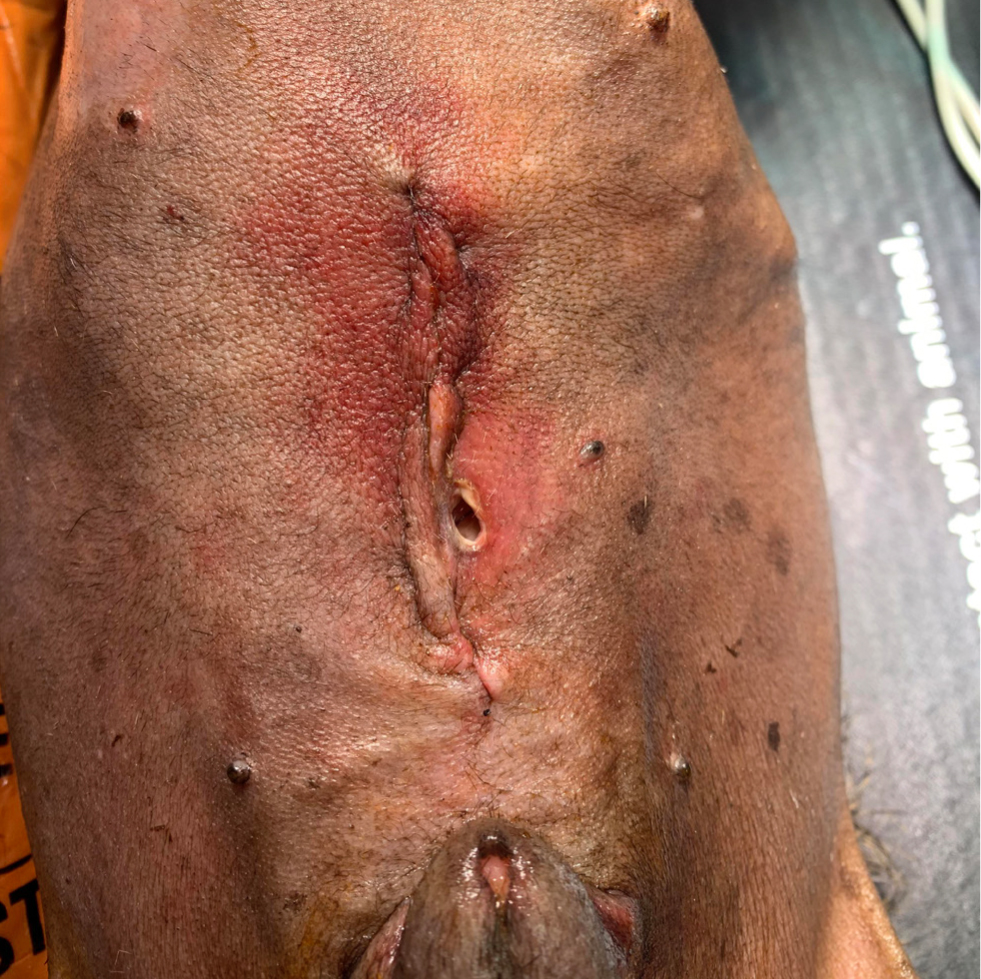
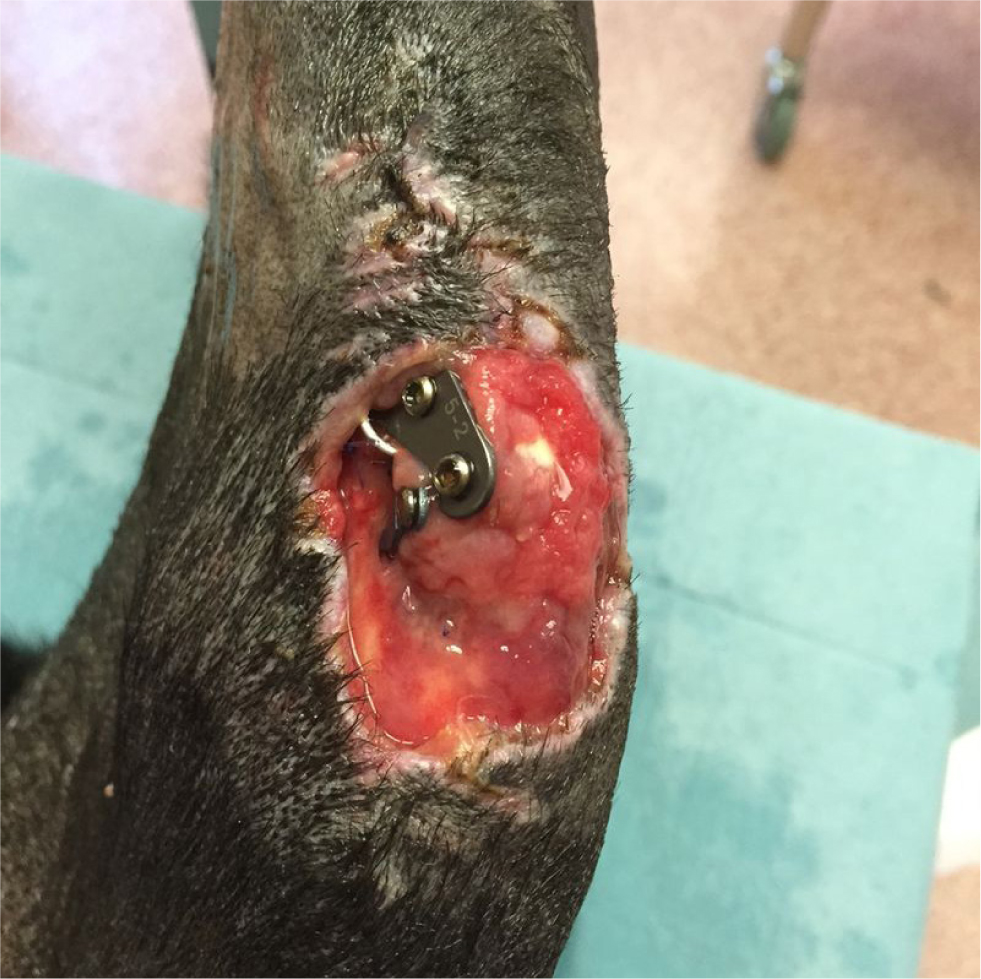
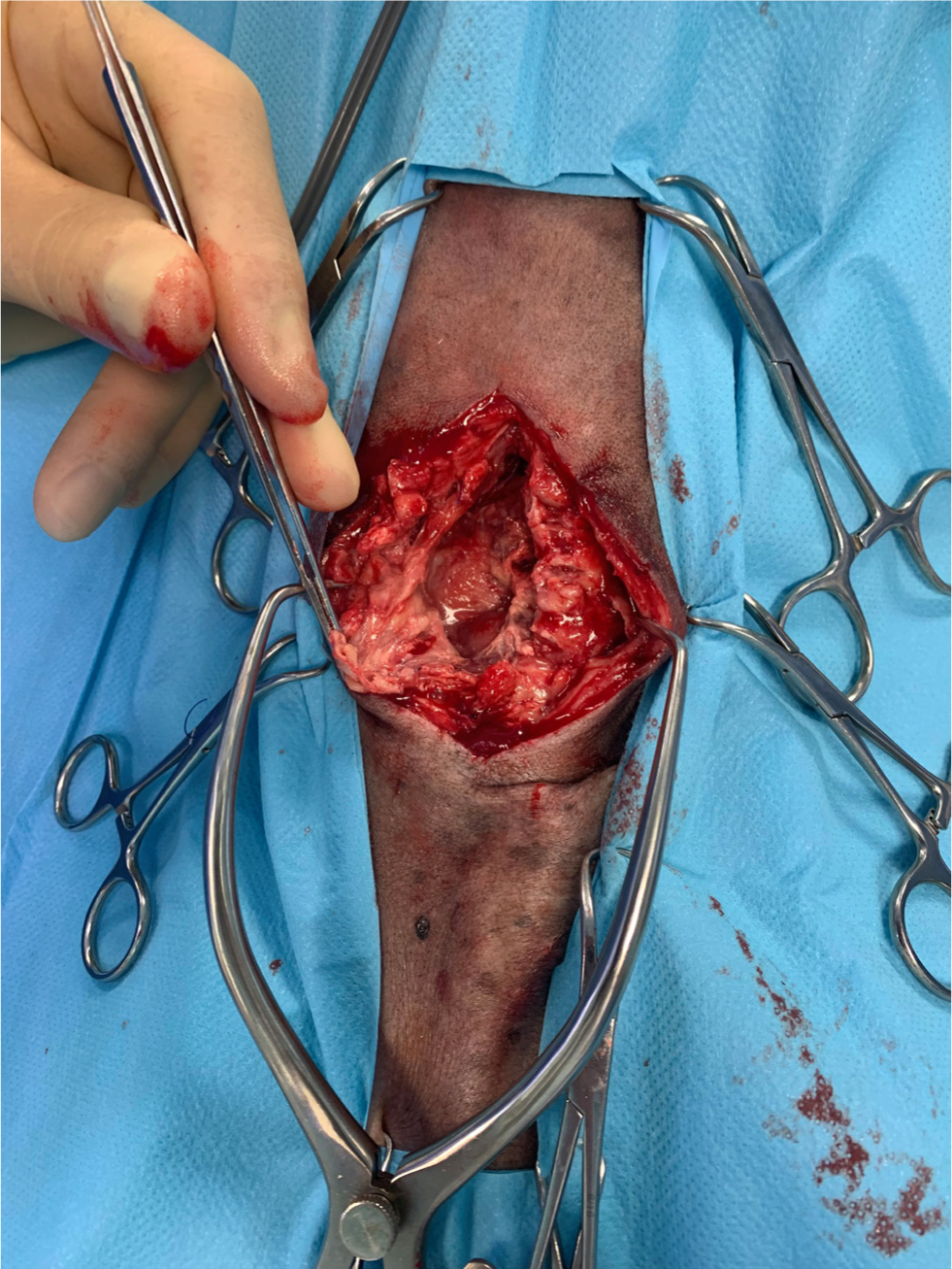
These criteria are useful for surveillance in terms of standardisation, but have certain limitations when applied in the context of small animal surgery (Nelson, 2011). For example, the clinical criteria for diagnosis can be applied subjectively, and there can be a degree of variation between observers and hospitals (CDC, 1991). The system also fails to differentiate simple inflammation from true infection (Turk et al, 2014).
Biofilms and surgical site infections
Bacteria exist in both their natural free-floating planktonic forms and in a sessile form within a biofilm (Costerton et al, 1999). A biofilm is a matrix of proteins and polysaccharides produced by bacteria, and they are ubiquitous in nature as well as in the body (Vestby et al, 2020).
Biofilms predate modern medicine and are thought to be a way microbes adapt to their environment (Yin et al, 2019). Clinically, biofilms confer advantages to bacterial colonies, which their free-floating counterparts lack. The mechanism by which this happens is complex and multifactorial and includes physical, chemical and immunological means of protection (Costerton, 1995). The significance of biofilms in surgical site infections is becoming increasingly clear in human nosocomial infections (Mah and O'Toole, 2001), and this is likely the case in veterinary medicine as well.
In the healthy patient, an intact epidermal or mucosal surface resists the establishment of a biofilm (Moser et al, 2017). The presence of foreign bodies also acts as a scaffold for planktonic bacteria to become sessile, and biofilms have been associated with a wide range of medical and surgical implants (Wu et al, 2015). Clearly, the act of surgery in itself creates favourable conditions for the formation of bacterial biofilms.
Once a biofilm-associated surgical site infection has been established, eradication may prove extremely difficult. Bacteria within biofilms can have a 10–1000-fold increase in antibiotic minimum inhibitory concentrations compared to planktonic bacteria (Sharma et al, 2019). Achieving therapeutic serum levels is either impossible, or would expose the patient to unacceptable side effects.
Diagnosis of surgical site infections
Surgical site infections can be diagnosed on the basis of available clinical and microbiological information, according to the aforementioned CDC guidelines (1991).
Clinical examination of a surgical wound postoperatively is usually when the clinician first becomes suspicious that a surgical site infection is developing, based on the presence of some or all of the five cardinal signs of inflammation (redness, swelling, heat, pain, and loss of function).
A superficial swab for culture and sensitivity testing is usually the most straightforward way to achieve a microbiological diagnosis of a surgical site infection. While a superficial swab is easily obtained, the results must be interpreted with caution. For example, in the presence of biofilms, the adherence of bacteria to their matrix leads to a low sample yield (Høiby et al, 2015). Additionally, sessile bacteria within a biofilm are metabolically dormant and do not grow on standard agar culture in the same fashion as their planktonic counterparts (Trampuz and Zimmerli, 2008). Essentially, culturing a swab obtained from a biofilm may lead to false negative culture results and can mislead the clinician. Next, the clinician must consider that the reported in vitro antibiotic sensitivity results do not necessarily reflect drug susceptibility in vivo (Giuliano et al, 2019). In other words, an isolate may be susceptible to a drug in the laboratory but not in the clinical setting, and vice versa. A tissue culture is more sensitive and may be considered as an alternative means of diagnosis (Aggarwal et al, 2013), while bearing in mind the increased invasiveness and risk of morbidity to the patient.
Ultimately, the gold standard of demonstrating the presence of a biofilm is direct visualisation of bacteria and their extracellular matrix through scanning electron microscopy (Davis et al, 2008), which is not a modality accessible to practitioners. There are certain clinical features that may suggest the presence of biofilms, such as a pale wound bed, discharge, necrotic tissue, and a putrid smell (Gardner et al, 2001). These signs are not specific to biofilms only, and again can be applied with a degree of subjectivity (Percival et al, 2012).
Management of surgical site infections
The optimal treatment of a surgical site infection differs from case to case and is guided by wound factors, type of infection, drug susceptibility, patient factors and client factors.
Evidence-based guidelines for the treatment of surgical site infections in veterinary medicine are lacking, and management relies heavily on first principles (Nelson, 2011). The general principles of surgical site infection management are outlined in Box 1.
Box 1.Principles of surgical site infection management
- Obtain tissue or superficial samples for microbiology
- Correlate results with clinical findings and to guide antimicrobial therapy
- Address any ongoing contamination (such as a dehisced enterotomy)
- Debride devitalised and necrotic tissue
- Employ judicious wound lavage to reduce microbial load
- Consider implant removal to remove focus of infection
- Reconstruct wounds only when a healthy bed of tissue is present
Adapted from Nelson (2011).
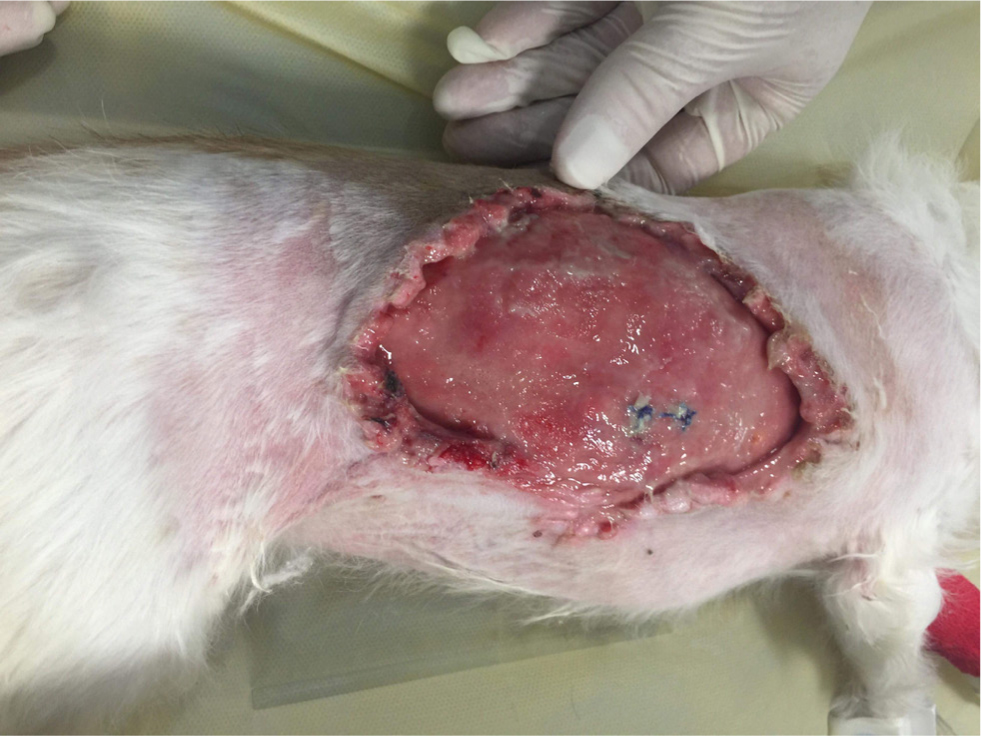
Figures 4 –8 illustrate management of a surgical site infection after removal of a lipoma.
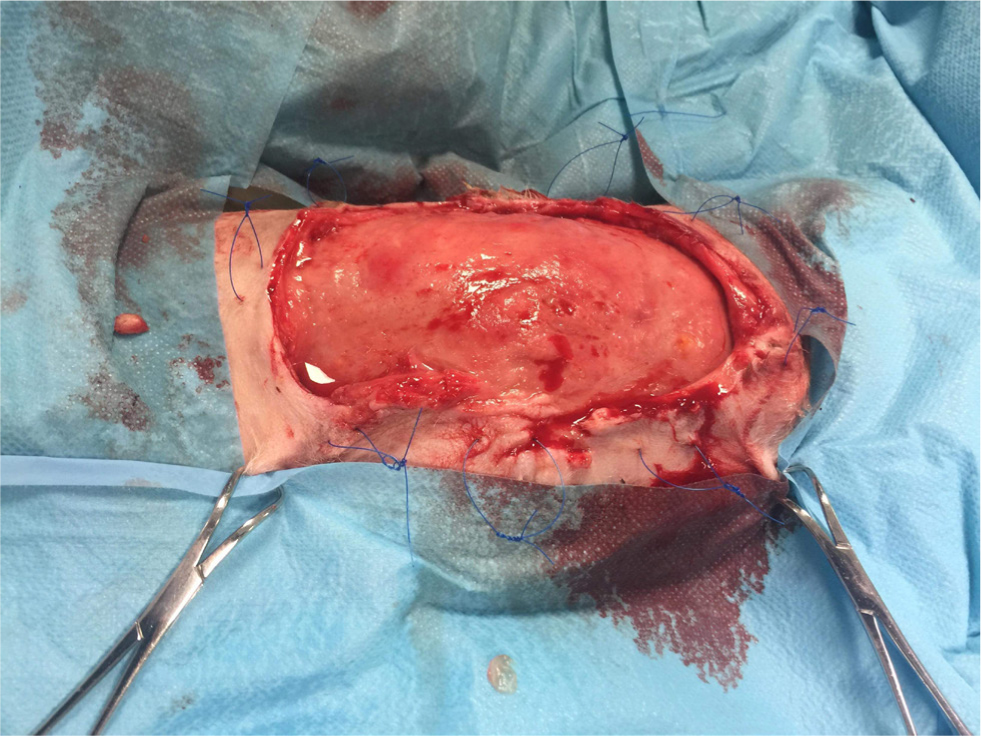
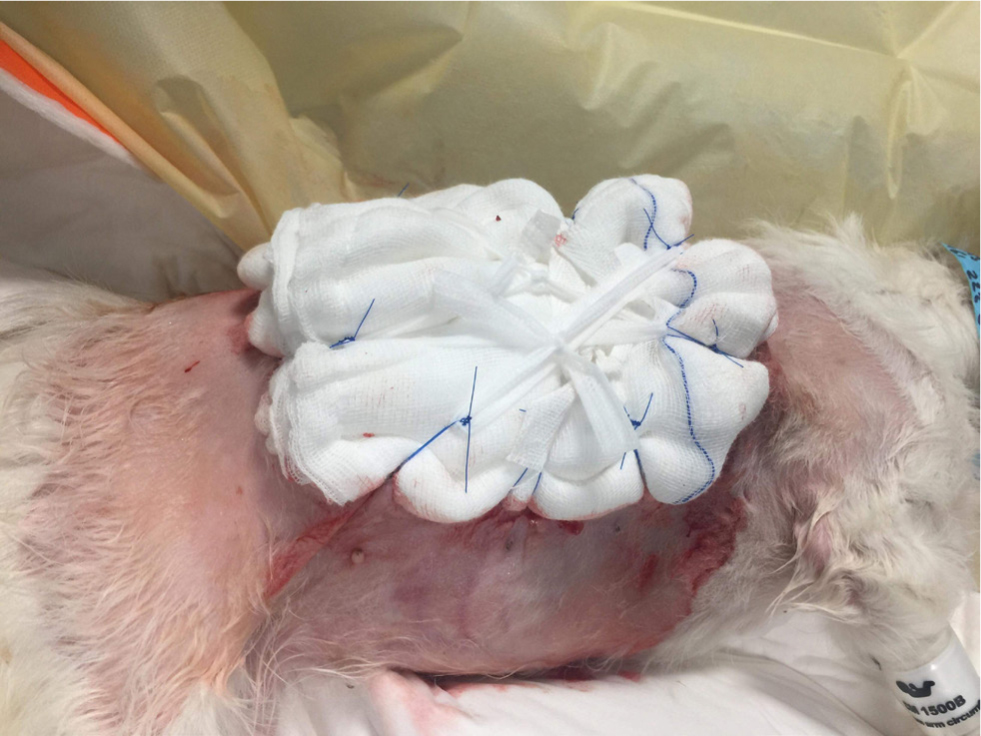
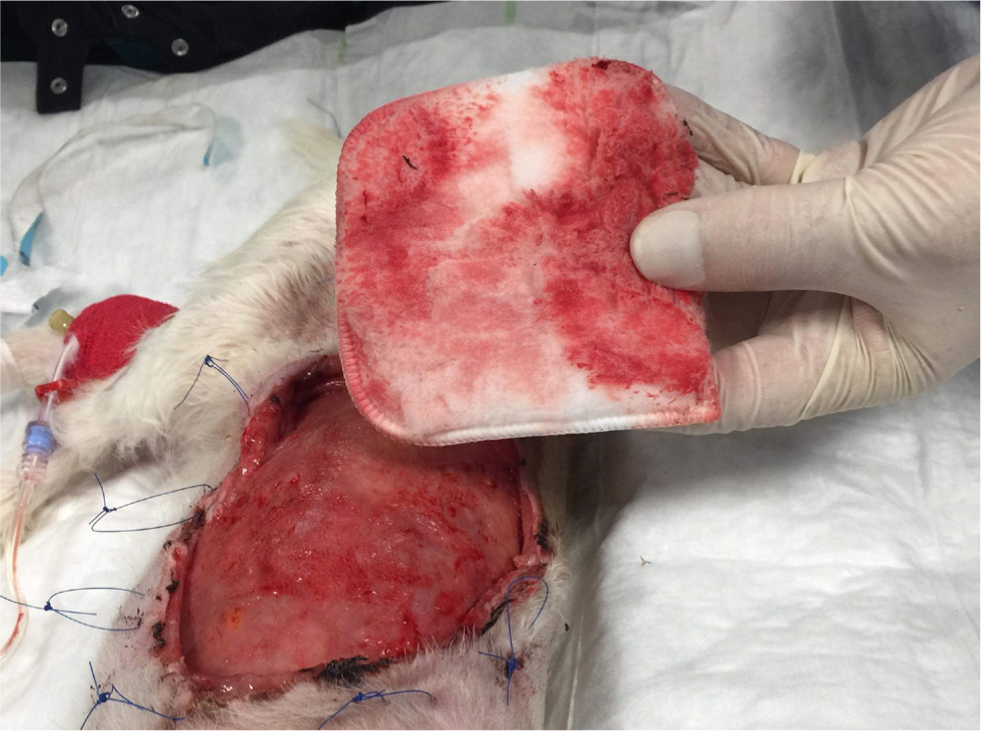
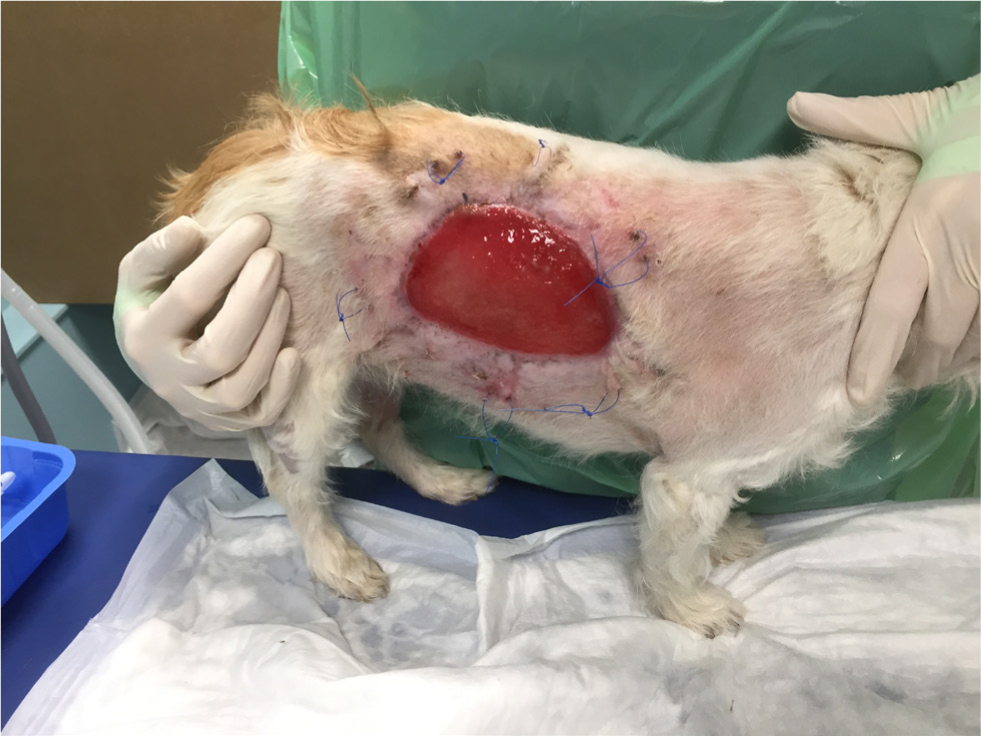
When dealing with a surgical site infection that is suspected to be associated with a biofilm, oral antibiotic therapy may not suffice, for reasons previously discussed. Specific veterinary evidence is lacking and to the author's knowledge, only isolated case reports exist (Swanson et al, 2014). In the absence of strong evidence, practitioners may apply first principles, and consider extrapolating findings from human medicine on a case-by-case basis.
The general principles of source control, wound decontamination, wound debridement and explantation will be familiar to all small animal surgeons and can be applied to the management of biofilm-infected wounds (Paterson, 2017). It has been suggested that explantation be reserved for chronically infected prostheses, but debridement, lavage and antibiosis may be sufficient for acute infections (Li et al, 2018).
More specifically, a variety of agents have been investigated to target biofilms. The antibiofilm agent with the largest evidence base is silver, used in silver-impregnated dressings (Thomas and McCubbin, 2003). Their use can be considered alongside other techniques to manage superficial biofilm-infected wounds. In cases of implant-associated biofilm infections, a 10% povidone-iodine lavage solution has been shown to be effective in reducing the bacterial load (Premkumar et al, 2021).
Prevention of surgical site infections
The patient's natural defensive mechanisms are compromised whenever any surgery is undertaken, hence surgical site infections are an intrinsic risk in any surgical procedure. Broadly speaking, surgical site infection prevention can be approached from three aspects: pre-operatively, intra-operatively and postoperatively (Table 2).
Table 2. Surgical site infection prevention
| Pre-operative measures | Intra-operative measures | Postoperative measures |
|---|---|---|
| Patient selection | Surgical technique | Postoperative antimicrobial administration |
| Preparation of surgical site | Operating room discipline | Active and passive surveillance |
| Preparation of surgical team | Patient care | |
| Antimicrobial prophylaxis |
However, this article is not a comprehensive discussion on surgical site infection prevention and the reader is referred to other surgical texts for further reading.
Preoperative measures
Certain comorbidities are known to be risk factors for the development of surgical site infections. Local and distant infection can predispose the development of surgical site infections (David and Vrahas, 2000), so should be addressed preoperatively. Systemic diseases such as obesity, diabetes mellitus, and endocrinopathies have been correlated with surgical site infection development (Mangram et al, 1999). After considering the risks and benefits to the patient, elective surgery should be postponed where possible, and comorbidities addressed.
Surgical site preparation should be performed after anaesthetic induction, just before surgery, and in a room separate from the operating theatre (Brown et al, 1997). Following hair clipping, the surgical site should be aseptically prepared with an appropriate antiseptic protocol.
The surgeon should adopt appropriate hand hygiene measures by keeping fingernails short and maintaining appropriate hand asepsis before surgery. Alcohol-based handrubs have been shown to be superior to aqueous-based products (Widmer et al, 2010), and should be considered as an alternative where feasible. The surgical team should be dressed appropriately in the operating theatre. Scrubbed personnel should wear surgical masks and caps, and all theatre personnel should be dressed in clean, laundered theatre scrubs. Although clear evidence supporting this recommendation is lacking (Lipp and Edwards, 2014), correct attire likely contributes to surgical site infection prevention and can help promote a sense of theatre discipline (Verwilghen and Singh, 2015).
Indications for prophylactic antimicrobials include, but are not limited to, contaminated surgery, prolonged surgical procedures, and in situations where the consequences of surgical site infections would be catastrophic (Välkki et al, 2020). Antimicrobials, if given, should be given intravenously 30–60 minutes before first incision, and repeated every 60–90 minutes during surgery (Classen et al, 1992). The choice of antibiotic should be based on the planned procedure and anticipated pathogens (Nelson, 2011). Strong evidence-based guidelines are lacking, but common antimicrobials used include potentiated amoxicillin, cefuroxime, and metronidazole, either alone or in combination.
Intraoperative measures
Strict adherence to Halsted's principles is believed to be a great contributor to reducing the incidence of surgical site infections. Surgical technique is somewhat correlated with surgeon experience, and a veterinary study demonstrated a lower incidence of surgical site infections with increasing surgeon experience (Wormstrand et al, 2014).
Operating room discipline refers to the behaviour of the surgical team within the theatre environment. Operating room discipline not only streamlines procedures and enhances efficiency but is also recommended to reduce the incidence of surgical site infections (National Institute for Health and Care Excellence, 2021). Operating room discipline is often overlooked in veterinary medicine (Verwilghen and Singh, 2015). Lapses in discipline of the surgical team have been shown to increase the likelihood of surgical site infections (Beldi et al, 2009). Increased personnel movement in theatre has also been suggested to increase levels of environmental contamination (Pryor and Messmer, 1998) and increased talking within the operating room has been shown to be associated with increasing surgical site infection rates (Kurmann et al, 2011). The effect of talking is likely both direct and indirect; increased noise levels may directly lead to more aerosolised pathogens and indirectly and excessive talking can be considered to be an indicator of poor operating room discipline, contributing to reduced concentration and interfering with communication within the surgical team (Fritsch et al, 2010).
Extended surgical time has been associated with increased development of surgical site infections, even after having allowed for other factors such as surgeon experience (Espinel-Rupérez et al, 2019). Sound presurgical planning and coordination within the surgical team can improve workflow within the operating theatre and reduce the patient's time under anaesthesia. Perioperative hypothermia has been shown to predispose surgical site infections in human medicine (Bu et al, 2019). Evidence in veterinary medicine is conflicting, but it is nonetheless prudent to maintain normothermia for other reasons such as tissue oxygenation, homeostasis and patient comfort (Beal et al, 2000).
Postoperative measures
Continuation of antimicrobials into the postoperative period has not been shown to reduce the incidence of surgical site infections (Aiken et al, 2015), and current recommendations are to administer antimicrobials before surgery, as previously discussed. In veterinary medicine, the exception to this is the tibial plateau levelling osteotomy, as several studies have shown the protective effect of postoperative antimicrobials (Nazarali et al, 2014; Solano et al, 2015), although the retrospective nature of these studies does not allow firm recommendations to be made.
Surgical site infections and implant biofilm prevention
Triclosan is a phenol compound with antibacterial properties through inhibition of bacterial fatty acid biosynthesis (Levy et al, 1999). The use of triclosan-coated suture material, in theory, should minimise surgical site infection and the establishment of biofilms. High-quality clinical trials have demonstrated the protective effect of triclosan-coated suture material, and their use in human surgery is now strongly recommended (World Health Organization, 2018). Veterinary studies have failed to demonstrate a clear benefit thus far (Etter et al, 2013; Thieman Mankin and Cohen, 2020), but their use can be considered in select cases.
Elemental silver-coated orthopaedic implants have been developed and are commercially available for common veterinary procedures such as tibial plateau levelling osteotomy. Studies in the human field support their use in reducing surgical site infections, but the available evidence is limited to retrospective studies (Schmidt-Braekling et al, 2017). In the veterinary field, only in vitro studies are available (Azab et al, 2016; Ziąbka et al, 2020), so their use cannot be strongly recommended.
Future developments
Various other medical devices are employed to prevent surgical site infections, although their use is not widespread. Silver-coated central venous catheters have been investigated in human medicine, although studies of their effect on surgical site infections are conflicting (Heard et al, 1998; Choi et al, 2017). An in vitro canine study of silver-coated urinary catheters has shown promising results (Ogilvie et al, 2015). The use of antibiotic-impregnated calcium sulphate beads in a clinical setting has also been described (Peterson et al, 2021). Prospective clinical trials in this broader area are warranted.
The development of standardised and active surveillance programmes in veterinary medicine is likely to contribute to greatly improved outcomes for patients. As discussed previously, the variation in definitions and methodologies used between different institutions limits the author's ability to compare studies and draw conclusions. Most surgical site infections are also diagnosed through passive surveillance, such as when the patient returns for a postoperative check. This differs from active surveillance, whereby a systematic approach is used to establish the incidence of surgical site infections in a population. In a prospective veterinary study, 35% of surgical site infections would have been missed without active surveillance (Turk et al, 2014), highlighting the room for improvement of this within veterinary surgery.
As discussed previously, bacteria within biofilms are not always picked up on routine microbiological tests. Advanced microbiological techniques have been investigated in patients where clinical suspicion of an surgical site infection exists, but negative culture results return. Molecular diagnostics such as a bacterial ribosomal polymerase chain reaction (Wu et al, 2015) and fluorescent in-situ hybridisation (Malic et al, 2009) have higher sensitivity, and their use in veterinary patients should be investigated.
Non-invasive diagnostic tests have been used as a surrogate for infection status of the patient. In human patients with cystic fibrosis, Pseudomonas aeruginosa serology showed high sensitivity and specificity in differentiating intermittent colonisation from true infection (Pressler et al, 2009). These non-invasive techniques can be preferable in certain patient populations, for example in patients where the site of infection is inaccessible, such as in infected orthopaedic implants. Investigation and development of non-invasive tests for these situations would provide the clinician an adjunctive means of diagnosing surgical site infections.
Veterinary surgery currently extrapolates from and applies findings from the literature in human medicine. Prospective, randomised clinical trials answering specific clinical questions in this field of work would be beneficial.
Conclusions
Prevention of surgical site infections is preferable to treating them when they occur. Biofilms complicate the management of surgical site infections and their importance should not be overlooked. Active veterinary surveillance is warranted to allow us to further understand the true prevalence and costs of surgical site infections in veterinary surgery.
KEY POINTS
- Surgical site infections are common in small animal veterinary practice.
- Surgical site infections can be classified as superficial incisional, deep incisional, or organ-space.
- Biofilms within surgical site infections are more challenging to treat; and should be recognised by the practitioner.
- Further research is required to determine the best therapy for surgical site infections in general, and biofilm infections in particular.


