There are several considerations UK veterinary professionals and owners need to know regarding cannabidiol (CBD) in veterinary medicine. Legislation, product quality, evidence base and prescription on the cascade all need to be taken into account before deciding whether to prescribe CBD for an animal.
There is no authorised product
In the UK, there are currently no CBD-based products that have been granted a UK veterinary marketing authorisation. The Veterinary Medicines Directorate (VMD) state that any CBD products for use in animals is a veterinary medicinal product, therefore require a marketing authorisation before they can be sold or supplied in the UK. The VMD consider that veterinary products containing CBD are veterinary medicines and should be regulated as such on the basis that products containing CBD fulfil the definition of a veterinary medicine in the Veterinary Medicines Regulations (VMR, 2013) by virtue of the effects they have.
As there are currently no CBD products authorised in the UK for veterinary use, a veterinary surgeon may prescribe a legally obtained human CBD product under the provisions of the prescribing cascade. It is important to note that administration of an unauthorised product containing CBD without a veterinary prescription is an offence under Regulation 8 of the VMR (VMD, 2018), which states that no person may administer a veterinary medicinal product (which CBD is classed as under the VMD) to an animal unless the product has a marketing authorisation for its administration, or it is administered in accordance with Schedule 4 (administration of a veterinary medicinal product outside the terms of a marketing authorisation) or Schedule 6 (exemptions for small pet animals) (VMD, 2015).
Prescribing appropriate products
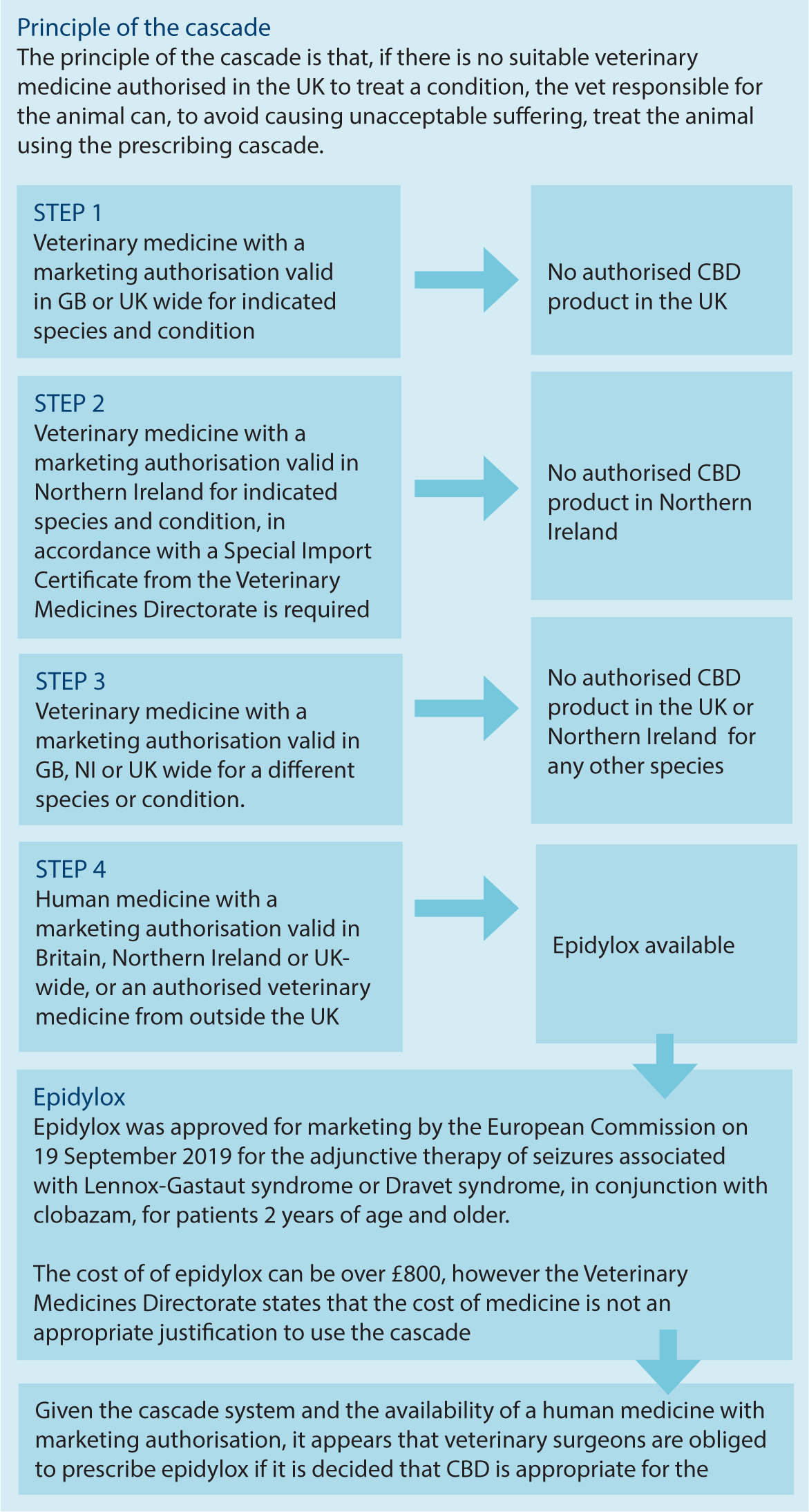
Prescription of a reliable and appropriate product may not be as easy as initially anticipated (Figure 1). Human product equivalents containing cannabinoids do exist, for example, the NHS (2021) discusses Epidyolex (a pure CBD product with a market authorisation for a specific form of childhood epilepsy), Sativex (a THC and CBD product with marketing authorisations for muscle spasticity in patients with multiple sclerosis), and Nabilone (a synthetic THC product with marketing authorisation for managing chemotherapy-induced nausea). Epidyolex as a pure CBD product is the most appropriate and comparable product for this discussion; it is a prescription-only medicine, and a controlled drug under Schedule 5. These drugs are generally cost-prohibitive to prescribe. However, the VMD stated: ‘Where there is an authorised veterinary medicine available, the prescribing vet should always consider it as the first line treatment. The vet should only prescribe an alternative medicine under the cascade if there is a valid clinical reason. The cost of the medicine is not an appropriate justification to use the cascade.’
Cannabis vs cannabidiol
Many people get confused between the difference between CBD and cannabis. CBD is a compound that is derived directly from the cannabis plant, a type of flowering plant in the Cannabaceae family. Cannabis is a complex plant, with 400 chemical entities of which more than 60 are cannabinoid compounds; the major compounds being delta-9-tetrahydrocannabinol (THC) and CBD. THC is well known for its negative effects; the recreational use of cannabis can have persistent adverse effects on mental health. THC is the main psychoactive constituent of cannabis, and most, if not all, of the psychoactive effects associated with the use of cannabis are caused by THC (Niesink, 2013).
In the UK, there are two main pieces of legislation pertaining to cannabis. These are the Misuse of Drugs Act 1971, which controls drugs that are ‘dangerous or otherwise harmful’, in which cannabis is categorised as a class B drug, and the Misuse of Drugs Regulations, which regulates the availability of controlled drugs by placing them in one of five schedules to the regulations.
However, the compound CBD does not elicit the negative psychological symptoms associated with THC, nor has it been found to be addictive. It has actually been suggested that CBD may have antipsychotic properties, and therefore does not fall under dangerous drug legislation.
In the same way that UK animal supplements cannot make medicinal claims, CBD-containing products that are marketed for medicinal purposes in humans are considered by human medicine regulators (the Medicines and Healthcare products Regulatory Agency) to be medicines, thus they require a marketing authorisation.
Herbal medicines for humans are monitored and regulated separately, whereas herbal medicines for animals are regulated under the same criteria as normal veterinary medicines. If there are CBD products for animals claiming medicinal benefits without scientific proof in the UK, they are currently illegal and can potentially leave pets at risk.
Administration of an unauthorised product containing CBD without a veterinary prescription is an offence under Regulation 8 of the VMR. Companies supplying CBD products for human use in line with the requirements of the Medicines and Healthcare products Regulatory Agency must not indicate or recommend their products for use on animals (VMD, 2018).
Over the counter products
Ensuring that patients are receiving a quantifiable and precise dose is important with any medication. Vets must be confident in what the product contains, that what it states on the label is accurate to its contents, and that there are no contraindications for use owing to unknown substances, such as contaminants.
It is important to ensure the quality of products so patients are not receiving illegal substances, such as THC, that may induce undesirable, unpredictable and negative psychological effects. The Home Office (2019) stated that there needs to be a defined commercial end use. Therefore, with regards to human CBD supplements, the Home Office only issues licences for cultivation of plants from approved seed types, with a THC content not exceeding 0.2%; the ‘0.2%’ reference is used solely to identify varieties that may potentially be cultivated (Home Office, 2019).
An ethical dilemma faced by vets is the potential for fake products being used by owners, who are not aware of the legal constraints that govern the use of CBD in animals. Over the counter products have been proven to have unreliable contents, with laboratory tests from the Centre for Medicinal Cannabis (2019) finding that 62% of the UK high street products studied did not contain the CBD content promised on the label and 37% contained high levels of THC (Advisory Council on the Misuse of Drugs, 2021). This was supported by a study that found 45% of the selected products had measurable levels of THC (mean content 0.04%) or cannabinol, a mildly psychoactive cannabinoid (mean content 0.01%), and are thus technically illegal within the UK (Centre for Medical Cannabis, 2019). Another study found only 31% of products accurately reported the amount and concentration of CBD that they contained (Bonn-Miller, 2017).
In response to such studies highlighting the issues with product quality in CBD products, the Food Standards Agency have said that CBD products are a ‘novel food’, meaning products such as CBD food supplements, oils and tinctures are classed as ‘novel foods’ under food safety regulations. The Food Standards Agency requires that any products being sold as novel products must have been sub-mitted for, or received, market authorisation (General Pharmaceutical Council, 2022; Food Standards Agency, 2022).
Knowing when to prescribe CBD products
Looking at the current legislative constraints, it is virtually impossible for a veterinary surgeon to prescribe CBD products to their patients in most real-world situations.
The evidence behind CBD is beyond the remit of this article, but it is not unreasonable to say that our knowledge of its use in veterinary medicine is still very much in its infancy. If veterinary surgeons are to comply with the cascade, a CBD product would be quite low on the list of tools to use in the management of most relevant cases. That is not to say that with the current evidence there may be some complex cases where the vet team may ultimately end up considering CBD after trying all other licenced products, but use as a first- or even second-line medication does not seem to be in accordance with the VMR.
This is where we hit a dilemma, because there is a human prescription product available for use in veterinary medicine, in the form of Epidyolex, which is definitively above the ‘food supplement’ type products on the cascade, and therefore must be used first. However, in most cases, it will be cost-prohibitive to prescribe, as the cost for Epidiolex oral liquid (100 mg/mL) is around £850 per 100ml (MIMS, 2022). However, the VMD state that cost is never an appropriate justification to proceed down the cascade… which leaves vets in a bit of a tricky situation.
Future direction
At present, vets seem to end up in a no-win situation. They can turn a blind eye to the illegality by their clients, which often stems from a place of misunderstanding and ignorance rather than malice, they could breach the law themselves, or they can look for an alternative option. However, none of this is helpful when vets are faced with a frustrated client who believes CBD will save their pet, and that vets are being unhelpful by not enabling them to obtain it. It is also a long and complicated topic to muster the energy to discuss during consultations, where vets are already stretched for time with their case loads and work force issues.
Until more companies undertake the necessary studies and apply for marketing authorisation for CBD products, vets look to be stuck on the horns of a triple dilemma.
KEY POINTS
- Regulation of cannabidiol products in animals is complex.
- Prescription of an appropriate product may be difficult.
- Owners often breach medicine regulations by administering cannabidiol to their pets without a prescription.

