Pheochromocytomas are tumours of the adrenal medulla which develop from the chromaffin cells within the inner medulla, with a high rate of metastasis and local invasion (Gilson et al, 1994b; Barthez et al, 1997). This is an uncommon tumour type, most often diagnosed in older dogs, but without any sex or breed predisposition.
Pathophysiology and clinical signs
Clinical signs associated with pheochromocytomas are secondary to the release of catecholamines, local infiltration and metastatic disease (Gilson et al, 1994b; Barthez et al, 1997). Catecholamine release results in hyperstimulation of alpha- and beta-adrenergic receptors, leading to hypertension, tachyarrhythmias and, in some cases, sudden death. Catecholamine release is often paroxysmal in nature, thus clinical signs can be intermittent. Hypertension is reported in approximately 50% of cases (Barthez et al, 1997) and can cause associated clinical signs including signs of renal dysfunction such as polyuria and polydipsia, epistaxis and acute onset blindness after retinal detachment. Tachyarrhythmias can cause weakness and episodic collapse. Local tumour invasion into the vena cava, predominantly via the phrenicoabdominal vein, can give rise to tumour thrombi and obstructed venous return, leading to pelvic limb oedema or ascites. Distant metastasis occurs in up to 40% of cases in sites such as the regional lymph nodes, liver, spleen, lung and vertebral column (Gilson et al, 1994b; Barthez et al, 1997; Spall et al, 2011). Therefore, clinical signs attributable to metastasis may be many and varied. On occasion, a pheochromocytoma is found incidentally on abdominal imagining during investigation for other disease processes and has no associated clinical signs.
Diagnostic approach
Whether an adrenal mass is identified incidentally or suspected based on clinical presentation, a logical step-wise diagnostic approach should be undertaken, seeking answers to three core questions that will be important for the approach to ongoing treatment. These are:
- Is there evidence of metastatic disease?
- What degree of local invasion is present?
- Is the tumour functional?
Diagnostic imaging is critical to evaluating dogs with pheochromocytomas and can determine both metastatic spread for staging purposes and the degree of local invasion. The degree of metastasis and local invasion are important in helping establish the likely prognosis and whether surgical resection is suitable. The degree of local invasion is particularly relevant to pheochromocytomas for surgical planning, as these tumours have a greater propensity for vascular invasion compared with adrenal cortical tumours (Barrera et al, 2013; Gregori et al, 2015). Imaging modalities that may be considered include abdominal ultrasound, radiographs, computed tomography (CT) and magnetic resonance imaging (MRI). Radiographs have limited use in investigating the primary tumour because of the relatively low sensitivity of this modality, resulting from the low incidence of mineralisation with pheochromocytomas. However, radiography can be used to investigate the presence of thoracic metastasis. Abdominal ultrasound, abdominal CT and MRI are more useful modalities for determining the degree of local invasion (Figure 1). One study demonstrated a 92% sensitivity and a 100% specificity when the degree of vascular invasion of canine adrenal masses at surgery or necropsy was compared with preoperative CT images (Schultz et al, 2009). The added advantage of CT imaging is that thoracic imaging can happen concurrently.

Unfortunately, no imaging modality has been shown to effectively distinguish between pheochromocytoma and other adrenal tumours. It is important to establish the tumour type because the functionality of the tumour (secretion of cortisol, catecholamines, aldosterone, progesterone or steroid hormone precursors), or whether the mass is non-functional, will determine the perioperative management plan.
Ultrasound-guided cytology samples can be an effective method of distinguishing the origin of adrenal tumours and recent retrospective publications indicate the procedure is not unsafe (Bertazzolo et al, 2014; Sumner et al, 2018; Pey et al, 2020). However, case numbers in these studies are small, making assessing the safety challenging, so larger scale prospective studies to validate the findings are required. Thus, because of legitimate concerns that sampling could result in sudden catecholamine release, which would lead to a life-threatening hypertensive crisis, preoperative cytological sampling of adrenal masses is often avoided.
Routine blood and urine tests should be undertaken to establish comorbidities, but no pathognomonic changes in baseline blood tests are associated with pheochromocytomas (Gilson et al, 1994b; Herrera et al, 2008). Changes in blood work attributable to hyperadrenocorticism can point towards the diagnosis of a functional tumour of the adrenal cortex rather than the medulla, and tests such as a low-dose dexamethasone suppression test should be undertaken.
Hypokalaemia, particularly when combined with hypertension, should prompt investigation of hyperaldosteronism. Tests establishing the urine normetanephrine:creatinine ratio and plasma normetanephrine are now available. Initial studies, although small, show that these tests are easy to perform and are accurate in the diagnosis of pheochromocytoma in dogs (Salesov et al, 2015). Urinary normetanephrine:creatinine ratio is particularly useful because the sample can be collected at home by the owner, thus avoiding an iatrogenic increase in metanephrine and catecholamine excretion caused by a stressful clinic visit for the dog (Kook et al, 2007).
Perioperative management
The treatment of choice for functional pheochromocytoma is adrenalectomy, and long-term survival is achievable (Schwartz et al, 2008). However, surgery is associated with relatively high mortality, as well as surgical and anaesthetic complication rates, so it should not be undertaken lightly. Non-functional ‘incidentalomas’, which are often slow growing and not associated with clinical signs, may only need to be monitored. For all functional adrenal tumours, including pheochromocytomas, perioperative management, anaesthesia and surgery should be carefully planned to achieve the best outcome.
When adrenalectomy for pheochromocytoma is performed electively, rather than as an emergency procedure, and when appropriate preoperative medical management (namely the non-selective alpha-adrenergic receptor antagonist phenoxybenzamine) is instituted to counteract side effects associated with catecholamines, mortality rates are reported to be 13% (Herrera et al, 2008). While it is not directly comparable, this is a large reduction compared to historically reported data (Gilson et al, 1994a; Barthez et al, 1997). Despite this improved mortality rate, treatment with phenoxybenzamine does not reduce the occurrence of intraoperative arrhythmias (Herrera et al, 2008) and blood pressure derangements are not eliminated, which should be taken into account when planning appropriate anaesthetic and postoperative care plans for these animals. Pre-treatment with phenoxybenzamine commonly causes postoperative hypotension in humans, secondary to the prolonged duration of action of this drug. Thus, there is currently some discussion in human medical literature regarding the best treatment protocols in the preoperative period (Fang et al, 2020).
Following diagnosis and leading up to surgery, exposure to stress and extreme exercise should be minimised as much as is practically possible to reduce the likelihood of catecholamine release. Additional tests, such as fundic examination to identify retinal bleeding or detachment, and electrocardiography (ECG) to identify any arrhythmias, are also advised. Blood typing and cross-matching should be considered before surgery in case a transfusion is required, particularly where vascular invasion or extensive local invasion is present. Hypertension and arrhythmias should be treated before surgery to reduce risks related to anaesthesia, such as acute intraoperative episodes of hypertension and tachyarrhythmias.
Phenoxybenzamine, a non-competitive alpha-adrenergic receptor blocker should be initiated 2–3 weeks before surgery. The recommended starting dose is 0.5mg/kg orally every 12 hours and can be increased to a maximum dose of 2.5mg/kg until clinical signs of hypotension occur. If phenoxybenzamine is ineffective alone in normalising blood pressure, or if tachycardia persists, second-line drugs such as propranolol or atenolol can be added, but only after instituting alpha-adrenergic receptor antagonists, otherwise hypertension can be exacerbated with loss of beta-receptor-mediated vasodilation (Maher and McNiel, 1997).
Careful monitoring of blood pressure, ideally with an arterial catheter, ECG and effective communication between the surgeon and the anaesthetist, is important so that catecholamine surges caused by the manipulation of the tumour during surgery can be anticipated and rapidly addressed. If this occurs, a short-acting alpha agonist such as phentolamine to treat intraoperative arterial hypertension can be used, with or without a vasodilator such as nitroprusside. Anaesthetic drug regimens should avoid drugs that are either arrhythmogenic, or will potentiate catecholamines. Beta-adrenergic antagonists such as propranolol, to treat severe sinus tachycardia, and lidocaine to treat ventricular arrhythmias should also be available to the anaesthetist.
Should metastatic disease be identified preoperatively, or if surgery is not an option, the medical preoperative management steps discussed above can be continued longer-term. The effectiveness of chemotherapy and radiotherapy have yet to be evaluated in large-scale studies in dogs (Lunn and Boston, 2020).
Surgical management
Surgical anatomy
A good knowledge of surgical anatomy is vital because of the close association between the adrenal glands and major blood vessels. The two adrenal glands are located in the retroperitoneal space, craniomedial to either kidney. The phrenicoabdominal vein crosses the ventral surface of each gland, and can act as a useful anatomical marker, although retroperitoneal fat accumulation can sometimes obscure it from sight. The more cranially located right adrenal gland is adhered to the wall of the caudal vena cava. The left adrenal gland sits in a space between the adjacent aorta medially and the left renal artery caudally. The surrounding vascular and adrenal capsular adventitia can be continuous, which has relevance to surgical dissection.
Surgical approach
Open adrenalectomy can be performed either via midline coeliotomy or via an ipsilateral flank laparotomy. The latter approach may make retroperitoneal exposure easier, but the disadvantage is that full abdominal exploration is precluded. Therefore, the midline approach is most commonly performed. Laparoscopic adrenalectomy for dogs with an adrenal tumour in either sternal or lateral recumbency is also described with favourable outcomes. Case selection is important and minimally invasive approaches are best avoided for cases with vascular invasion because of the higher risk of bleeding (Jiménez Peláez et al, 2008; Naan et al, 2013; Mayhew et al, 2014).
Surgical technique
Surgical dissection if often challenging, especially when vascular invasion has occurred and because of the depth of the surgical field. Therefore, adequate abdominal retraction is vital. Vascular invasion is common with canine pheochromocytoma and is reported in 35–55% of cases (Bouayad et al, 1987; Kyles et al, 2003; Barrera et al, 2013), with the risk of perioperative mortality increasing with more extensive vascular invasion (Barrera et al, 2013). However, the presence of tumour thrombi, and thus the requirement for vascular dissection via venotomy, does not necessarily preclude surgical removal of the tumour. When vascular invasion occurs, the tumour thrombus typically extends down the phrenicoabdominal vein and into the caudal vena cava, but does not tend to adhere to the walls of the vessels. Although a proportion of cases will not be amenable to surgical resection, recent publications have described adrenalectomy, employing various venotomy techniques, with largely successful outcomes (Knight et al, 2019; Lipscomb, 2019; Mayhew et al, 2019; Chiti et al, 2021).
Vessel-sealing devices and right-angled forceps for dissection are particularly helpful for adrenalectomy. If venotomy is required, Rumel tourniquets, Potts scissors and partial occlusion clamps such as Satinsky clamps should be available. Careful blunt dissection of the neoplastic adrenal gland is commonly initiated at the lateral aspect, furthest from major blood vessels located medially. A stay suture in the adrenal capsule can assist manipulation of the gland, which can then be medially retracted to expose underlying vessels to allow further ligation. The phrenicoabdominal vein is isolated and attenuated at the entry point to the vena cava or, if the tumour extends into the vena cava, depending on the extent of the thrombus, venotomy of the caval (Kyles et al, 2003), phrenicoabdominal (Mayhew et al, 2018) or renal vein (Chiti et al, 2021) is performed to extract the thrombus. Venotomy is assisted by placing Rumel tourniquets at either end of the tumour thrombus (Figure 2). These are tightened before performing the venotomy.
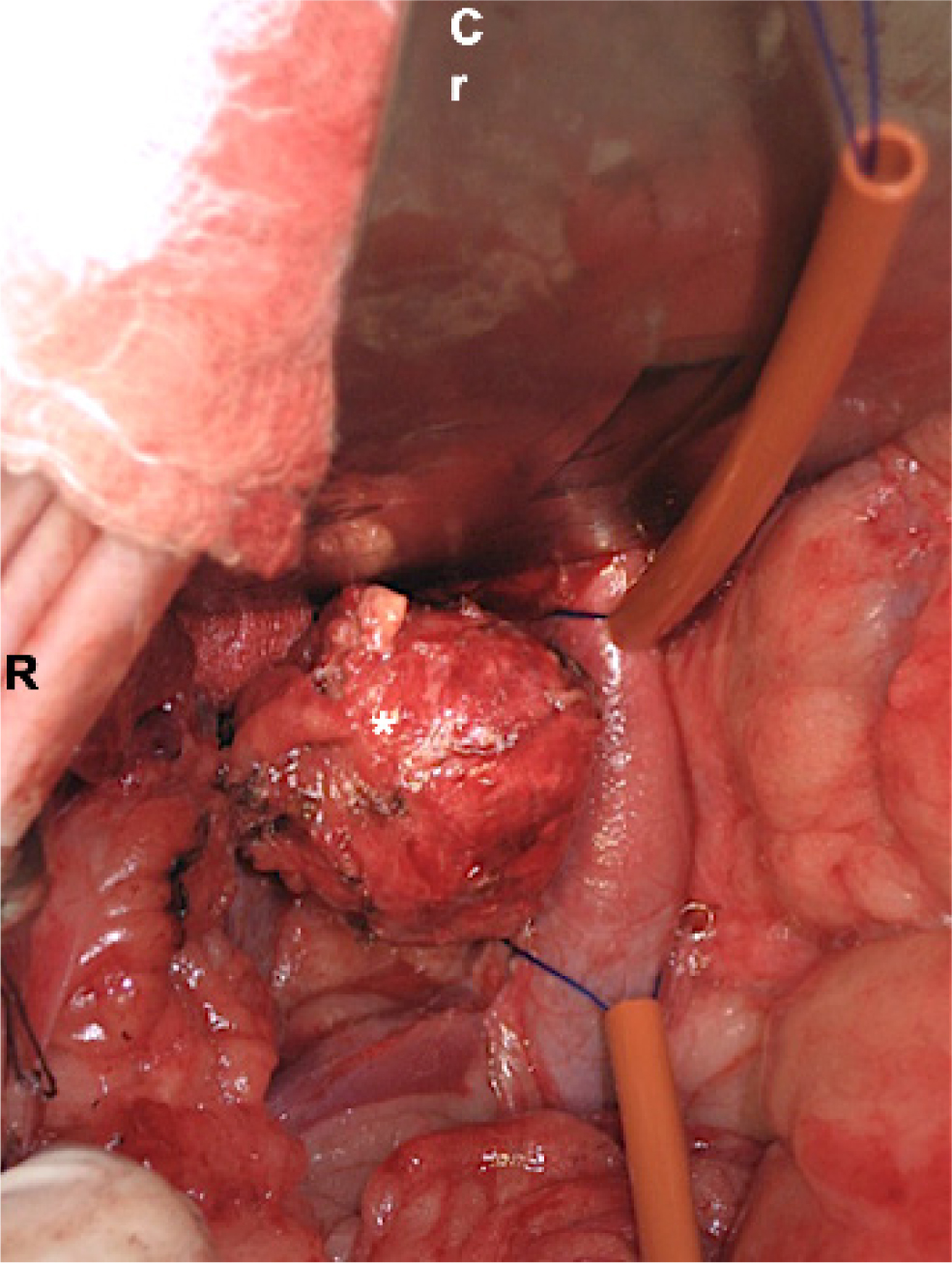
Alternatively, when the caval thrombus is small, a phrenicoabdominal vein venotomy can be performed and the tumour thrombus is milked backwards into the phrenicoabdominal vein before being removed through the phrenicoabdominal venotomy site. Once the tumour thrombus is extracted, the partial occlusion clamp is placed tangentially across the venotomy to allow partial blood flow past the clamp. The tourniquets are released, the venotomy is sutured with a single or double simple continuous pattern and the clamp is removed. Venotomy closure is occasionally performed under complete vascular occlusion when the venotomy is too long for the partial occlusion clamp to be placed around it, or when the location of the venotomy precludes space for a clamp. Occlusion of the caudal vena cava has been shown to be safe experimentally in healthy dogs for up to 8 minutes (Hunt et al, 1992), although a greater degree of tolerance might be expected in dogs with pre-existing occlusion caused by a tumour thrombus, a finding that is reflected in more recent clinical literature (Knight et al, 2019). A concurrent nephrectomy is sometimes required if renal parenchyma or renal vessels are invaded.
Prognosis
The prognosis for dogs without metastatic disease, that have had surgical resection and survived the perioperative period, appears to be good, with mean survival times of 374 days reported (Schwartz et al, 2008).
Conclusions
Pheochromocytoma is a tumour that can be difficult to diagnose. Historically, surgical management has been associated with high mortality but with careful case selection, appropriate preoperative imaging, pre- and postoperative medical management and advanced anaesthesia, good surgical outcomes and survival times can be achieved.
KEY POINTS
- Pheochromocytoma is a highly invasive tumour derived from the chromaffin cells within the adrenal medulla.
- Functional pheochromocytomas secrete excessive quantities of catecholamines and can have life-threatening consequences.
- Adrenalectomy is the treatment of choice.
- Careful perioperative planning and the ability to react quickly to rapid and life-threatening blood pressure changes are key to successfully managing pheochromocytomas.
- Venous invasion is not always a barrier to good surgical outcome and when patients survive the perioperative period, with complete resection, longer median survival times can be achieved.


