The female reproductive tract of a bird consists of an ovary, oviduct and cloaca. Most female birds only have a functional left ovary and oviduct. While rudimentary remnants of the right ovary and oviduct may occasionally be observed, functionality is extremely rare. The kiwi (Apteryx spp.) is an exception, in which two functional ovaries are observed. The single left oviduct is positioned within the coelomic cavity to be able to receive oocytes from both ovaries (King and McLelland, 1984b). Paired ovaries and oviducts are reported commonly but irregularly in birds of prey (Kinsky, 1971). The oviduct consists of five parts: the infundibulum, magnum, isthmus, shell gland and vagina. The oviduct opens into the urodeum of the cloaca.
The male reproductive tract consists of paired testes and ductus deferens. In some species, males have a phallus, which may or may not be intromittent. The testes are symmetrical, paired and located internally, near the cranial end of the kidney. The ductus deferens carries spermatozoa from the epididymis to the urodeum of the cloaca. In passerine birds and budgerigars, the caudal end of the ductus deferens forms a convoluted ‘mass’ called the seminal glomus, observed as a cloacal promontory – a bulge, palpable adjacent to the vent. The seminal glomera are the main storage sites for sperm in these species (King and McLelland, 1984b). There are no accessory sex glands as there are in mammals. Most birds lack a phallus and mating occurs via ‘cloacal kissing’ (Videvall et al, 2018). Anseriformes, tinamous and ratites possess an intromittent phallus. Some birds, including domestic chickens and turkeys, possess a small non-intromittent phallus on the ventral lip of the vent (King and McLelland, 1984c).
Both sexes have a cloaca – a space into which the terminal portions of the gastrointestinal tract, reproductive tract and urinary tract open, which exteriorises at the vent. The cloaca is formed of three chambers, the cranial coprodeum, into which the rectum terminates, the urodeum, into which the reproductive and urinary tracts terminate and finally, the caudal proctodeum, a short compartment between the urodeum and the vent. The opening to the bursa of Fabricius, an important immune tissue, is in the dorsal midline of the proctodeum (King and McLelland, 1984a).
Relevant physiology
The reproductive system in birds is under control of the hypothalamic–pituitary–gonadal axis. Gonadotropin-releasing hormone (GnRH) is the main factor responsible for gonadotropin secretion. The central nervous system receives information regarding daylength, temperature, availability of food, availability of a mate and availability of nesting sites/materials, and these reproductive stimulators lead to increased secretion of GnRH from the hypothalamus. Three different forms of GnRH have been identified in birds, avian GnRH-1, -2 and -3. GnRH-1 is thought to be the main stimulator of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion in birds (Mans and Pilny, 2014).
The main gonadotropins in birds are FSH and LH, which affect the function of the ovaries and testes (Ritchie, 2014). Prolactin has potent anti-gonadotropic and anti-gonadal effects. The onset of incubation behaviour and the production of crop milk by species that produce it (such as columbiformes) are associated with prolactin secretion (Figure 1). In the female, FSH is the key hormone for folliculogensis and steroidogenesis and is responsible for follicular maturation and differentiation. In the male, the major role of FSH is the stimulation of spermatogenesis. In the female, LH has the major function of stimulating ovulation. In the male, as in mammals, LH stimulates the Leydig cells to produce and secrete testosterone (Ritchie, 2014).

During oviposition, prostaglandin F2α (PGF2α) and vasotocin stimulate contractions of the uterus, in the presence of calcium. Prostaglandin E2α (PGE2α) causes contraction of the shell gland but also stimulates relaxation of the lower uterine sphincter (Hudelson and Hudelson, 1996).
Female avian reproductive disorders
Chronic egg laying and hyperoestrogenism
Birds will innately reproduce when environmental conditions are favourable. Chronic, unrelenting reproductive stimulation or activity and recurrent egg laying are seen commonly in avian practice. This can lead to a range of problems, including hypocalcaemia, metabolic bone disease, egg binding, body wall rupture, cloacal prolapse and undesired behaviours, among others (Bowles, 2002; Pollock and Orosz, 2002; Amer et al, 2018). In captivity, there are several aspects of the domestic setting that may act as stimuli for reproductive activity in pet birds, such as the inadvertent provision of nesting sites and materials, provision of an abnormally long photoperiod and feeding a high energy diet.
Aside from chronic laying, the display of reproductive behaviours, evidence of hyperostotic polyostosis on radiographs and the persistent finding of biochemistry changes consistent with reproductive activity such as hypercalcaemia and hypercholesterolaemia, are other signs of chronic hyperoestrogenism (Baumgartner et al, 1995; Campbell-Ward, 2013). For birds that are chronic egg layers, removal of eggs is likely to stimulate the bird to lay more. Over time, this may lead to depletion of calcium stores, egg binding or metabolic bone disease (Welle, 1999). Treating chronic egg laying and hyperoestrogenism first and foremost requires correction of the underlying cause(s) mentioned below. The use of hormone therapy and treatment with GnRH agonists may also be beneficial, but not in the absence of correcting the underlying causes or treatment is unlikely to be successful.
Inadvertent provision of nesting sites and materials
For pet psittacine birds especially, any dark hiding place or crevice may be perceived to be a nest. Often, caged birds may be provided with huts, or soft hides in their cage or alternatively, during times out of the cage, they may be able to access cupboards, drawers and areas behind furniture and appliances. Similarly, they may also be able to move among bed linen and pillows. These may all be perceived as potential nesting areas (Van, 2006). Any material which can be shredded up, for example newspaper from the floor of a bird cage, or fibres from shredded toys may also be perceived as nesting material (Spitler-Nabors and Myron, 1983), stimulating egg production (Figure 2).

Provision of an abnormally long photoperiod
Photoperiod has been demonstrated to be a major stimulator of reproductive activity in many species of birds (Nakane and Yoshimura, 2019). Few wild birds receive more than 12 hours of daylength and many of the species that are commonly kept as pets are relatively equatorial birds, that receive around 12 hours of light and 12 hours of darkness. In captivity, birds may often be kept in human living areas, with televisions, computers and human activity until late in the evening. Many captive birds may regularly receive 12–16 hours of daylength or, in extreme cases, longer. Restricting the photoperiod to 12 hours is recommended and a separate, smaller ‘sleep cage’, which can be placed in a darkened room or covered in an area without light, noise or stimulation, may be a good idea. The sleep cage is more conducive to the normal routine of wild psittacine birds, which roost in a different area to their main foraging sites during the day (Wilson, 2001).
Feeding a high energy diet
Diets which contain a high energy content are known to be a stimulus for reproduction. Psittacine birds that are fed predominantly on seed mixes are a classic example of this. Converting to a more complete, nutritionally formulated food is almost always recommended for non-breeding, pet birds (Rosen, 2012).
Dystocia and egg binding
Egg binding is defined as the delayed passage of an egg through the reproductive tract, whereas dystocia is the mechanical obstruction of an egg in the caudal reproductive tract. Therefore, all birds suffering from dystocia are suffering from egg binding, but not all birds with egg binding are suffering from dystocia. There are a variety of causes of egg binding, including mechanical obstruction or partial obstruction, the formation of abnormally large or misshapen eggs, smooth muscle contraction failure, salpingitis, oviductal hypoplasia, excessive production of eggs, hypocalcaemia, coelomic masses and more. Stress, dietary deficiencies and suboptimal husbandry may all predispose a bird to egg binding (Abou-Zahr et al, 2019). Torsion of the oviduct has also been described as a cause of egg binding in psittacine birds (Harcourt-Brown, 1996). Clinical signs include straining, coelomic distension, ataxia, dyspnoea and non-specific signs of illness (Abou-Zahr et al, 2019).
The egg can usually be palpated between the pubic bones, although this may not always be the case, particularly where the egg has an abnormally soft or poorly calcified shell. The author has seen a few cases of egg binding where two fully developed eggs are present within the coelomic cavity at once. Diagnostic imaging is useful to identify the presence of an egg(s) and to assess for likely causes, especially abnormalities with the shape or size of the egg, and for causes of obstruction. Radiography, ultrasonography and computed tomography may all be useful in these cases. A complete blood count and plasma biochemistry is recommended for assessment of potential underlying causes, such as hypocalcaemia (Calvo Carrasco and Sabater, 2017) (Figure 3).
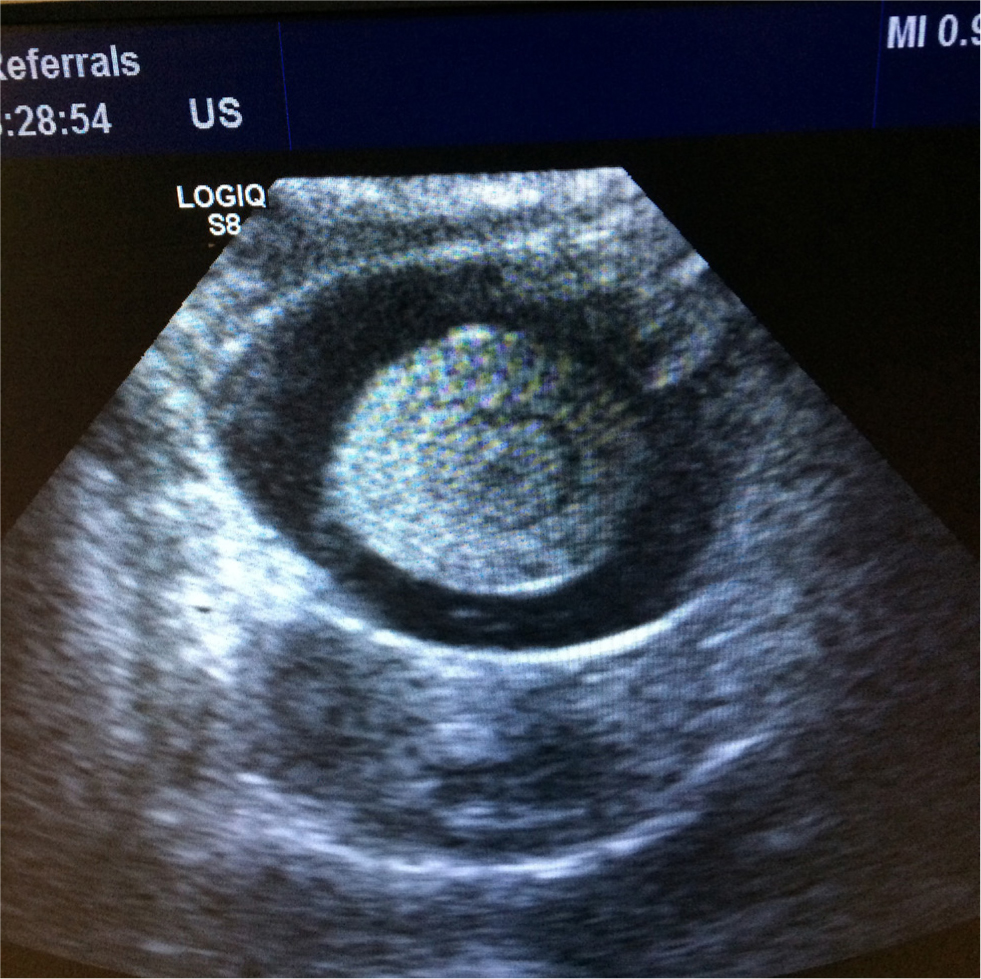
Initial treatment to stabilise the patient is critical, with heat, fluid therapy, oxygen and calcium (if indicated) included in the basic treatment protocol (Joyner, 1994). Oxytocin can be used, which targets the uterus and seems to have few systemic side effects, although its use in birds is controversial because it is not an avian hormone, and its efficacy is yet to be scientifically validated. Arginine vasotocin, the equivalent of oxytocin in birds, is not commercially available (Scanes, 2015). Oxytocin does not assist with relaxation of the uterovaginal sphincter. However, PGE2 does and also stimulates uterine contractions. It has been used with good success as a topical gel applied into the cloaca (Hudelson and Hudelson, 1994). For cases involving a partial obstruction, or those that are refractory to medical management, ovocentesis can be considered. This involves collapsing the egg, following removal of its contents, to help its passage. Ovocentesis may be performed via the cloaca in ‘percloacal ovocentesis’, or via the skin and body wall in ‘percutaneous ovocentesis’, although with this method there is the risk of causing salpingitis by introducing bacteria from the skin, so an aseptic technique is recommended before placing the needle. In some cases, the collapsed egg may be removable via the vent, in other cases, the bird will usually pass the collapsed egg within a few days of the procedure. In cases where ovocentesis is not an option, or does not resolve the problem, a surgical caesarean section may need to be considered to remove the egg (Abou-Zahr et al, 2019).
Ovarian and oviductal disease
Ovarian cysts are seen occasionally. Follicular cysts may be acquired or congenital. Congenital cysts are most common in budgerigars and canaries (Schmidt et al, 2016). Oophoritis may be associated with local spread of infection from neighbouring air sacs or other areas within the coelmic cavity, or from haematogenous spread. Ovaries are enlarged and appear grossly discoloured. Infection may be caused by viruses (for example herpes), bacteria, fungi and mycobacteria (Schmidt et al, 2016). Ovarian atrophy or atresia may be seen secondary to aflatoxicosis. Several ovarian neoplasms are reported in birds. Granulosa cell tumours, ovarian adenomas, ovarian carcinomas and teratomas are examples of some commonly encountered in practice. These birds may present in a similar way to birds with renal tumours, with a unilateral lameness often being observed (Simova-Curd et al, 2006). Surgical removal of the avian ovary is extremely challenging because of its close association with the cranial division of the kidney and its profound vascularisation. Attempts to remove the ovary in adult birds are usually unsuccessful and are either incomplete or result in fatal haemorrhage (Lierz et al, 2016).
Cystic oviducts may be congenital or acquired, and may affect the left oviduct or a retained or incompletely regressed right oviduct. Inflammation of the oviduct may have infectious and non-infectious causes. Oviductal torsion or volvulus is seen occasionally and if not rapidly diagnosed, will usually lead to death. Oviductal impactions occur often, usually as a result of salpingitis or egg binding. Several neoplasms of the avian oviduct are reported, including adenomas, adenocarcinomas and leiomyomas (Schmidt et al, 2016). Surgical removal of the avian oviduct is possible with a salpingohysterectomy. In some cases, cessation of ovarian function and ovulation seems to occur following this procedure, but in other cases it does not (Hernandez-Divers et al, 2007; Pye et al, 2001). Continued ovulation from the ovary in a bird without an oviduct is likely to result in egg yolk coelomitis.
Cloacal disease
Not all cloacal disease is related to reproduction or the reproductive tract. Cloacal papillomatosis is very common in Amazon parrots, and is caused by psittacid herpes virus infection (Styles et al, 2004). Other cloacal disorders include cloacaliths, cloacal impactions and cloacitis. In psittacine birds, cockatoos are predisposed to cloacal disease, especially female birds. Prolapse of the cloaca itself has been suggested to be the predominant cloacal disease reported (Styles et al, 2004). There are a variety of causes of cloacal prolapse, including gastrointestinal problems such as endoparasites, bacterial or fungal enteritis, and reproductive abnormalities such as egg binding and dystocia. Frequent masturbation by male and female birds may also be a predisposing factor (Rosen, 2012). Treatment involves replacement of the prolapse and often, passing of a temporary cloacal suture(s). It is essential to address the underlying cause. In many cases, a surgical cloacapexy, and sometimes a cloacaplasty, are needed to prevent recurrence in the future (Van Zeeland et al, 2014) (Figure 4).
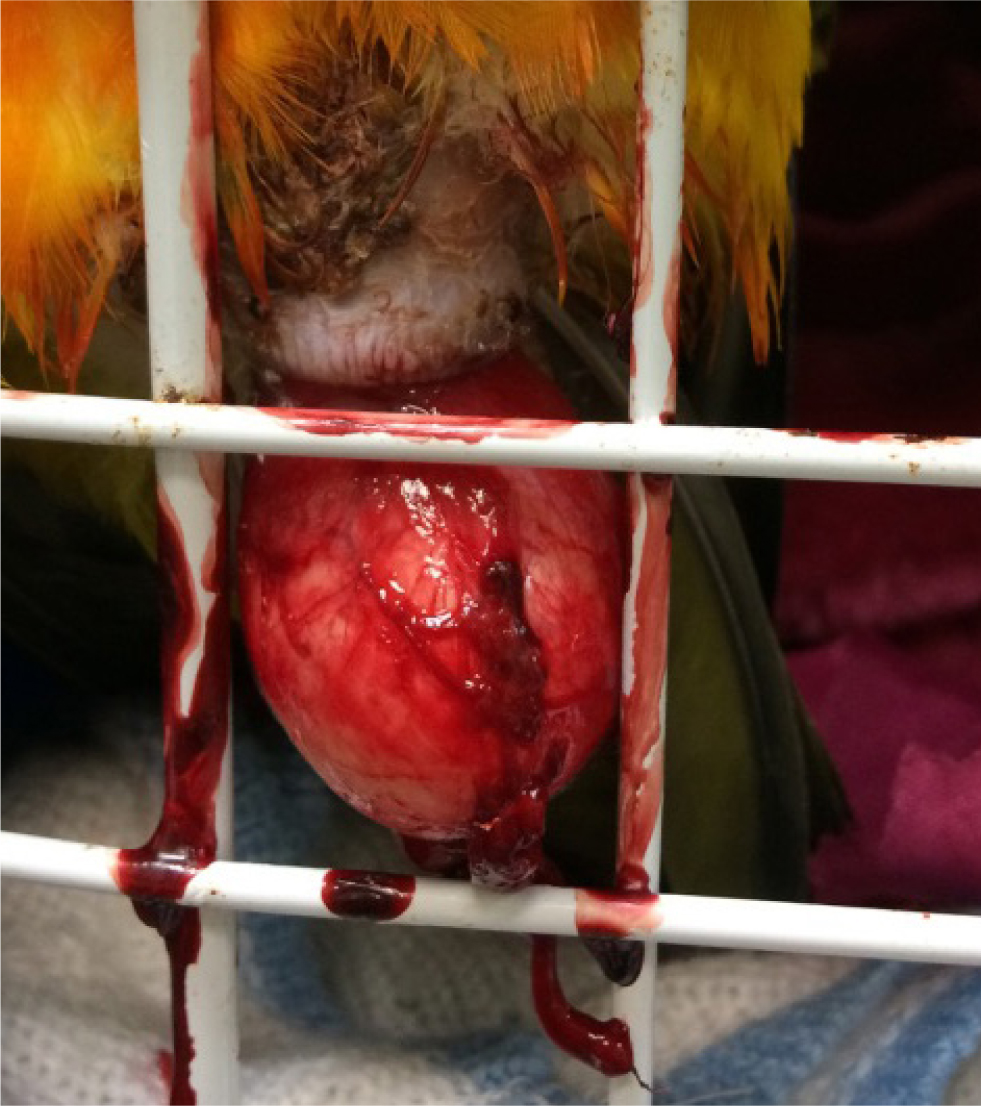
Egg coelomitis
This occurs when the infundibulum fails to engulf the ovum, when there is reverse peristalsis of ova back into the coelomic cavity from the oviduct, or if there is an oviductal rupture. In rare cases, it may result from trauma to the ovary from rough handling (Gingerich and Shaw, 2015). Cystic ovarian disease, oophoritis and ovarian neoplasia, as well as oviductal disease, may result in egg coelomitis. Clinical signs include depression, abdominal distension and dyspnoea, usually with a cessation of egg laying. Ultrasonography is very useful and coelomocentesis can be performed to gain samples of coelomic effusions for cytology and culture. Haematology may show a profound leucocytosis from the inflammation caused by yolk material irritating the serosal surfaces (Raftery and Jones, 2019). Treatment of the underlying cause, use of non-steroidal anti-inflammatory drugs and antibiotics if an infectious element is suspected, and removal of ascitic fluid are all important aspects of management. One of the most important considerations is the prevention of further ovulations and GnRH agonist implants can be used for this. In some cases, surgical coelomic lavage and removal of yolk material may be indicated and, in some cases, salpingohysterectomy may also be required (Morishita, 1996; Gingerich and Shaw, 2015; Greenacre, 2015; Raftery and Jones, 2019).
Male reproductive disorders
Testicular disorders
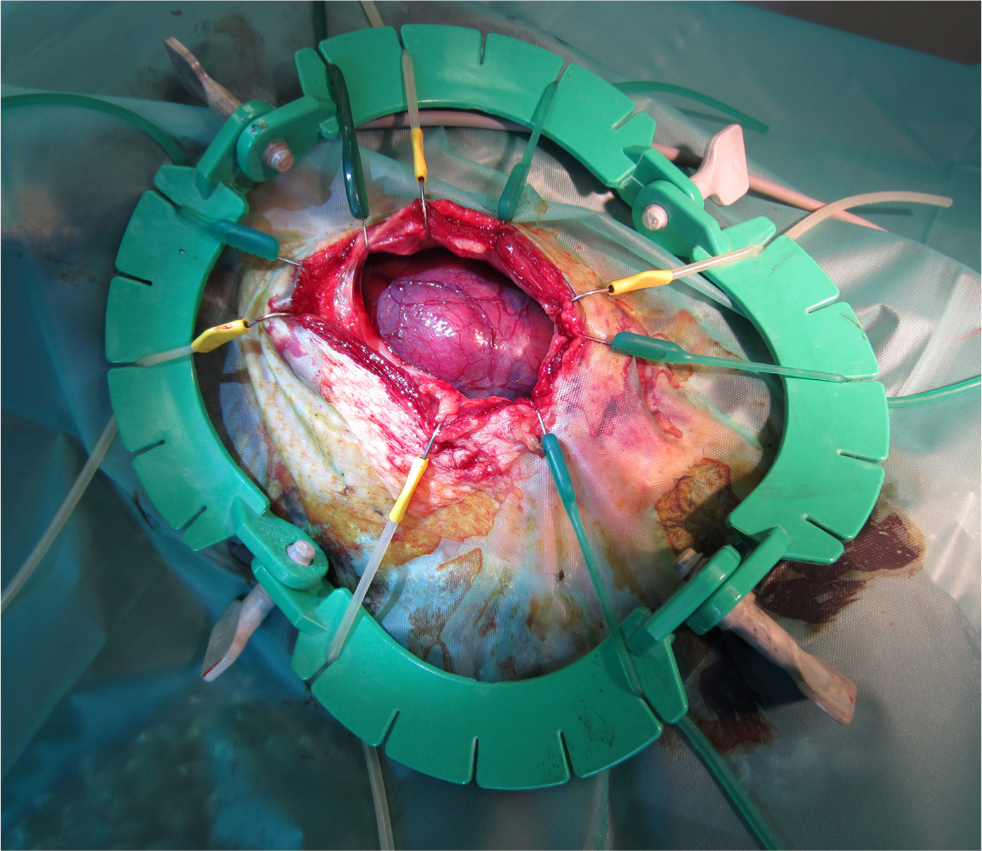
Degenerative changes affect male fertility. A variety of toxins may affect avian testicles, for example furazolidone, mercury and copper fungicides. Cystic dilatations, called spermatoceles, of the rete testis or tubules of the epididymis may be observed. These may be congenital or may develop secondary to trauma or inflammation. Orchitis may result from trauma, bacterial or fungal infections extending from elsewhere in the coelom or the air sacs, or haematogenous infections (Schmidt et al, 2016). Tumours of the testes may result in coelomic distension. Several types of testicular tumours are reported, including seminomas, Sertoli cell tumours, interstitial cell tumours, lymphoma and teratomas (Reavill et al, 2004) (Figure 5).
Prolapse of the phallus
Prolapse of the phallus is infrequently reported. It occurs mainly in Anseriformes and ratites. It can be caused by trauma, infection, inflammation or even frostbite (Schmidt et al, 2016). Prolapse seems particularly common in the breeding season and may follow over-exertion or bullying (Bexton and Couper, 2014). Cleansing and reduction of the prolapsed organ may be performed, alongside analgesia and antimicrobials if indicated. Temporary cloacal sutures may be needed initially. However, in many cases, recurrence occurs despite treatment efforts and surgical amputation of the penis is indicated (Raftery and Jones, 2019).
The use of deslorelin in avian patients
The GnRH agonist deslorelin (Suprelorin 4.7mg implant/Suprelorin 9.4mg implant, Virbac Limited, UK) has become widely used in avian medicine for the treatment of reproductive disorders and is worth particular mention. Some studies describe the efficacy in avian species. Studies looking at deslorelin use in birds have indicated the following:
- In pigeons (Columba livia) 4.7 mg deslorelin implants stopped egg laying in females for at least 49 days (Cowan et al, 2014)
- In cockatiels, 4.7 mg deslorelin implants stopped egg laying in females for at least 180 days (Summa et al, 2017)
- In Japanese quail, 4.7 mg deslorelin implants stopped egg laying in females for an average of 70 days (Petritz et al, 2013)
- In a non-peer-reviewed study in domestic chickens, it was reported that a 4.7 mg deslorelin implant stopped egg laying in females for an average of 180 days, while a 9.4 mg deslorelin implant stopped egg laying for an average of 319 days (Petritz et al, 2013)
- Another non-peer-reviewed study in pscittacines suggested that a 4.7 mg implant stopped egg laying in females for approximately 3 months in 100% of the birds (Van Sant and Sundaram, 2013)
- A study in two male turkeys showed that both two 4.7 mg deslorelin implants and two 9.4 mg deslorelin implants reduced conspecific aggression and suppressed testosterone levels for several months (Molter et al, 2015).
The implants are placed subcutaneously, often in the interscapular region, or intramuscularly, often in the pectoral musculature, usually under general anaesthesia. A small bleb of tissue adhesive is useful to prevent loss of the implant after placement (Mans and Pilny, 2014).
Conclusions
Reproductive disease is common in birds. Medical management, such as the use of GnRH agonists can be useful in the treatment of some disorders. However, a considerable amount of further research is needed to demonstrate its efficacy in many species and in both sexes, particularly after repeated or sustained use.
In other disorders, surgical management is indicated. Above all, environmental triggers must be dealt with before long-term success can be achieved when treating many reproductive disorders in avian medicine..
KEY POINTS
- Reproductive diseases are common in birds, especially in those birds that have been selectively bred to maximise their potential to ovulate on an ongoing basis.
- Reproductive disorders are frequently seen in birds housed in single-bird households and where owners have no intention of breeding them.
- There are many anatomical and physiological differences between birds and other species and a brief understanding is useful in the approach to reproductive disorders in birds.
- Chronic egg laying, egg binding, dystocia, ovarian disease, oviductal disease and egg coelomitis are all examples of common reproductive disorders in females.
- Testicular disease and prolapse of the phallus are examples of common reproductive disorders in males.
- Treatment of reproductive disease may involve medical and/or surgical management but it is often more pertinent to manipulate the bird's husbandry and environment to minimise reproductive stimuli.


