Some pain in animals can be straightforward to recognise and classic behaviours are displayed, such as aversion to touch and vocalisation (Reid et al, 2007). Other types of pain are significantly more challenging to recognise and may only produce behavioural signs such as lethargy or restlessness. Owing to a lack of verbal communication with animals, successful management of their pain depends on our ability to recognise it. This article provides an overview of the pathophysiology of the different types of pain, with a focus on the development and management of chronic pain.
States of pain
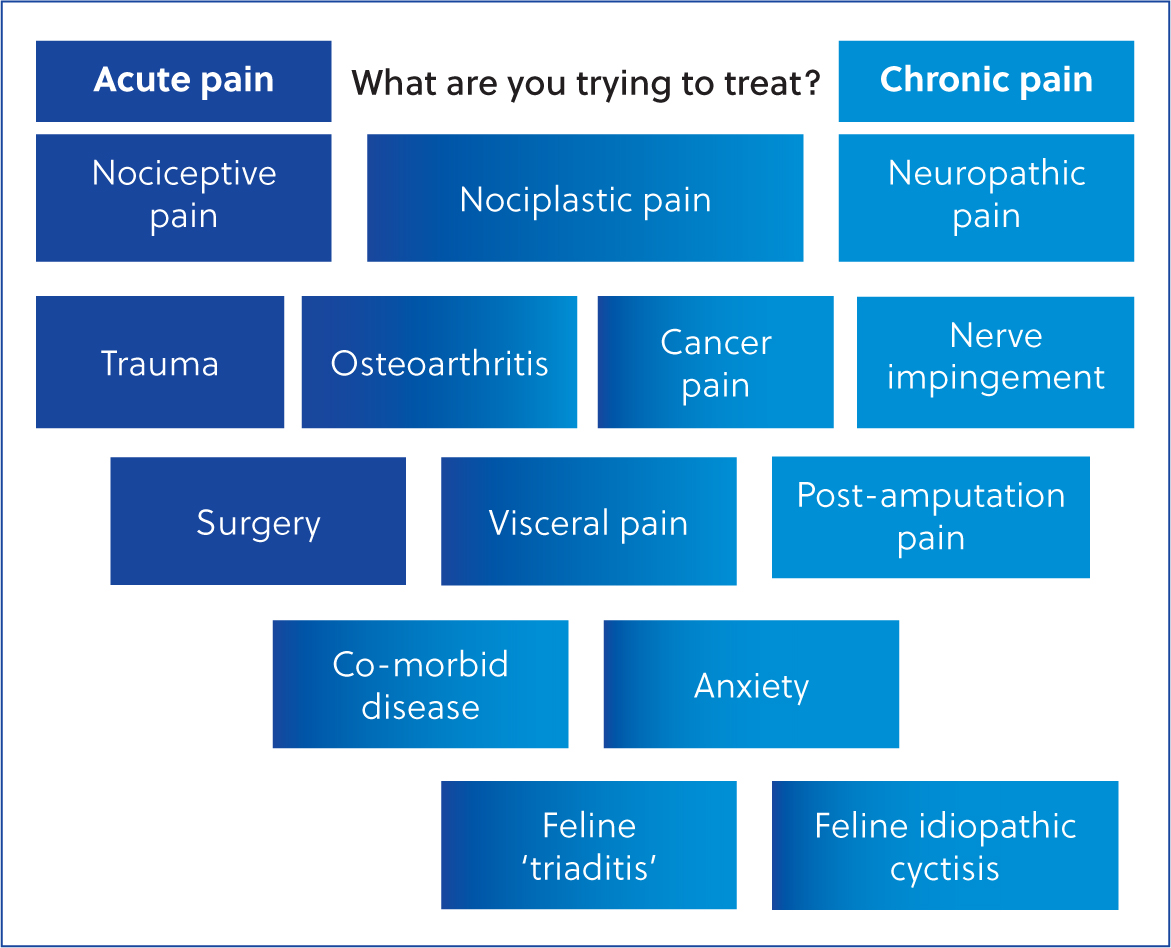
Pain is an emotive and sensory experience secondary to nociceptive or potentially nociceptive stimuli. It can be categorised as acute pain, lasting less than 12 weeks, or chronic pain which exceeds 12 weeks duration, as defined by The British Pain Society (2023) and the International Association for the Study of Pain Classification of Chronic Pain for the International Classification of Diseases 11 Coding (Treede et al, 2019). The transition from acute to chronic pain involves many complex changes (Voscopoulos and Lema, 2010) and there is little evidence in animals that strict timescales are observed. Furthermore, since animals are unable to ‘self-report’ it becomes more difficult to detect a point of transition to chronic pain, especially where pain is ‘waxing and waning’ in nature. Therefore, a more helpful definition may be that chronic pain persists beyond the expected time of healing, and may or may not be associated with an identifiable cause or actual tissue damage (Reid et al, 2015). This ‘persisting pain’ features in osteoarthritis and neoplasia, where there are ongoing features of both acute and chronic pain (Gwilym et al, 2008; Reddi, 2015).
Nociception describes a sensory signalling pathway which facilitates aversive responses to nociceptive stimuli that may damage an organism. Receptors for these stimuli are known as nociceptors, and can be activated by by thermal, mechanical, or chemical stimuli (Tracey, 2017). Alongside the efferent aversive pathway, nociceptive neurons are also able to trigger inflammatory pathways within the proximity of the stimulus and generate areas of neuroinflammation distant from the initial noxious stimulus (Tracey, 2017). Prolonged proinflammatory states can result in nociceptor sensitisation, whereby they are activated by non-noxious stimuli such as light touch (known as allodynia) and/or activated to a greater extent by noxious stimuli (hyperalgesia) (Tracey, 2017). Sensitisation can also occur within the spinal cord and brain (central sensitisation), whereby descending facilitatory pathways promote afferent nociceptive signal transmission and descending inhibitory control may become deficient. This is an example of neuronal plasticity and is fundamental for the transition of acute pain to chronic pain. Neurokinin-1 and serotonergic signalling (McCleane et al, 2003; Takeda et al, 2012) may play important roles in central sensitisation, along with an array of other chemical mediators. It is the more persistent changes within the nociceptive pathway that may be responsible for the development of chronic pain after acute pain is experienced (Voscopoulos and Lema, 2010).
Neuropathic pain is distinct from nociceptive pain as it is caused by an insult or pathology specifically within the nervous system (The British Pain Society, 2023). Chronic neuropathic pain occurs via changes in expression of voltage-gated sodium channels and both peripheral and central inflammatory signalling (Vosco-poulous and Lema, 2010; Galvin and McCrory, 2021). It can be challenging to manage because the pathophysiology is distinct from that in other pain states.
Nociplastic pain is a relatively new term used to describe chronic pain when nociception is altered; there is no risk or actual tissue damage and there is no pathological change within the nervous system (Kosek et al. 2016). Nociplastic pain is a product of dysfunctional nociceptive signalling pathways and involves central and peripheral sensitisation, thus allodynia and hyperalgesia are commonly present (Fitzcharles et al, 2021).
During the development and continuation of chronic pain, it is likely that nociceptive, nociplastic and neuropathic pain states occur in combination (Fitzcharles et al, 2021). This is illustrated in Figure 1. This promotes the concept of chronic pain as a ‘syndrome’ with poorly established mechanisms and a variety of clinical signs.
Goals for pain management
Currently, the single most effective step in managing chronic pain is to prevent its occurrence (Dahl and Kehlet, 2011). This is best achieved by aggressive management of acute pain, a strategy known as ‘preventative analgesia’, but this is not always possible or effective (Dahl and Kehlet, 2011). As with chronic pain, effective recognition of acute pain is also important. Pain scoring systems developed for acute pain can assist with this and are useful to assess the effectiveness of an analgesic intervention. Examples of validated pain scores for dogs and cats include the Glasgow Composite Pain Scoring Systems (Reid et al, 2007, 2017), the Colorado Canine Acute Pain Scale (Holten et al, 2001) and the UNESP-Botucatu Multidimensional Composite Pain Scale for cats (Brondani et al, 2013).
Given the subjective nature and individual experience of chronic pain, self-reporting in humans is regarded as essential for accurate assessment (Achterberg et al, 2021). It is generally accepted that animals are likely to experience chronic pain in a similar manner to people, but explicit self-reporting is not possible (Burma et al, 2017). This complicates diagnosis and assessment of chronic pain states in species encountered in veterinary practice, particularly the emotional elements, which strongly feature (Achterberg et al, 2021). Observed behavioural assessment forms the mainstay of evaluation for companion animals and veterinary surgeons rely heavily on the owner to report any behavioural changes (Wisemann-Orr et al, 2004). Changes in behaviours such as activity levels, attitude and sociability have been included in health-related quality of life questionnaires, designed particularly for chronic pain assessment (Wisemann-Orr et al, 2004). Assessments are predominantly directed towards specific disease states associated with chronic pain, such as osteoarthritis (Brown et al, 2007, Heim-bjorkman et al, 2009; Zamprogno et al, 2010, Benito et al, 2013) and spinal cord injury (Budke et al, 2008). More recently, assessments designed for all states of chronic pain in both dogs and cats have been developed (Reid et al, 2018a, 2018b; Noble et al, 2019). The concept of structural behavioural observation and associated scoring systems are reviewed extensively by Reid et al (2018b). An important goal for the patient is to improve the score on the chosen assessment tool, therefore accurate record keeping and repeated scheduled reviews are critical. Reducing progression of the disease is also an important aim of therapies that may not be included in the scoring systems, depending on the underlying pathology.
It is important to discuss the long-term goals of chronic pain management with the owner to develop a consensus as to whether the intervention is appropriate, to consider additional therapies and lifestyle adjustments, and to evaluate response to treatment. Clients are effective at evaluating the success of analgesic intervention for chronic pain management in cats (Bennett and Morton, 2009) and quality of life in dogs with spinal injury (Levine et al, 2008). This means that history-taking forms a key component of clinical decision-making regarding the management of chronic pain.
It is also important to strive to achieve other client-led goals concurrently, including working within the remit of any financial constraints, supporting clients with appropriate frequency of contact, and suggetsing interventions which are reasonable to accommodate based on the client's schedule (Davis et al, 2019).
Therapies for acute and chronic pain
Acute pain can often be effectively prevented and managed with the array of efficacious analgesic agents licenced for use in dogs, ranging from full mu-agonist opioids to non-steroidal anti-inflammatory agents. A variety of routes of administration are also available, such as systemic administration and locoregional approaches. Pre-emptive analgesia aims to prevent noxious signalling to the spinal cord (Dahl and Kehlet, 2011). While considered optimal, a pre-emptive strategy is not always possible or effective, for example, intra-operative nociception can occur during an exploratory laparotomy if there is a failure of full anaesthesia after epidural administration of lidocaine.
Effective management of patients experiencing chronic pain is challenging as a result of various factors. These include difficulty recognising chronic pain and its underlying cause(s), difficulty managing the complex psychosocial implications of the condition, veterinary-prescribing legislation, and a lack of understanding of off-licence medication options. Treatment plans for patients with chronic pain must be designed to provide maximum benefit and minimum harm and respect the client's wishes for their pet. The concept of rational prescribing is discussed extensively by Max-well (2016) and an approach is outlined, beginning with a discussion regarding the expectations and goals surrounding therapy. Patient factors including signalment and concurrent medication are considered along with drug factors such as pharmacological characteristics, evidence of effectiveness and cost.
As nociceptive, nociplastic and neuropathic pains are likely to feature in combination, drug therapies that are effective against all of these pain states may need to be employed, so it is important to consider the pathways involved in the generation of these pain states to select effective therapies.
Figure 2 shows the pain pathway. The first step in the pathway is transduction of the pain stimulus into an electrical signal via activation of the nociceptors. This signal is transmitted from the peripheral to the central nervous system and passes through the dorsal horn of the spinal cord. Neurons project this signal into the brainstem, thalamus and finally the cerebral cortex, where pain is perceived. Modulation of signals occurs in the brainstem and dorsal horn of the spinal cord. There is a large selection of drugs that act at different levels of the pain pathway that may be effective at managing patients with chronic pain (Table 1).
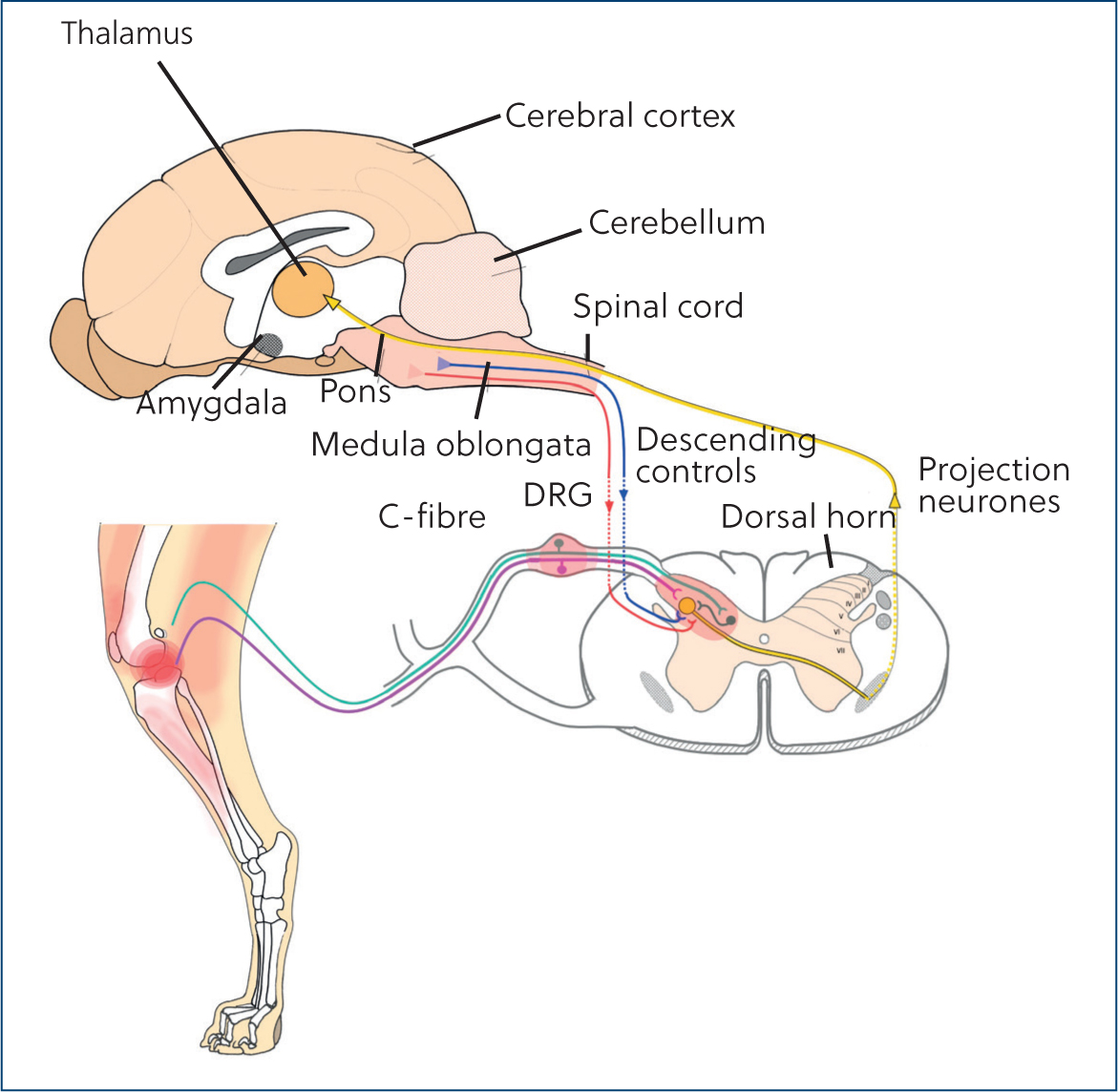
Table 1. Drugs prescribed to manage chronic pain and the level of pain pathway they target
| Drug | Pathway | Reference |
|---|---|---|
| Local anaesthetics | Transduction, transmission | Taylor and McLeod (2020) |
| Non-steroidal anti-inflammatory drugs | Transduction, modulation | Gunaydin and Bilge (2018) |
| Anti-nerve growth factor monoclonal antibodies | Transduction, modulation | Bimonte et al (2021); Enomoto et al (2018) |
| Alpha-2 agonists | Transmission, perception | Pertovaara et al (1991) |
| Gabapentinoids | Transmission, modulation | Rose and Kam (2002) |
| Opioids | Transmission, perception | Ballantyne and Mao (2003) |
| NK1 receptor antagonist | Transmission | Corrêa et al (2019); Suzuki et al (2002) |
| N-methyl-D-aspartate receptor antagonist | Modulation, perception | Li et al (2019) |
| Paracetamol | Modulation | Anderson (2008) |
| Tricyclic antidepressants | Modulation, perception | Giorgi and Owen (2012) |
| SSRIs, SNRIs | Modulation, perception | Patetsos and Horjales-Araujo (2016); Ito et al (2018) |
When using pharmacological intervention for the management of chronic pain, it is very important to consider legislation surrounding prescribing. In the UK, the first-line treatment for outpatients should be licenced for use for the target species for pain management. These are often non-steroidal anti-inflammatory drugs, drugs containing paracetamol, or tramadol. Drugs that are not licenced for use may be prescribed according to the cascade, using clinical judgement to avoid compromising patient welfare (Veterinary Medicines Directorate, 2015). These drugs are often used in addition to licenced medication, so it is also important to consider whether a licenced drug is being prescribed according to the cascade. For example, paracetamol and codeine tablets (Pardale-V; Dechra) are only licenced for use as an analgesic for five consecutive days, although they are frequently used for a longer duration.
Finally, there are a variety of non-pharmacological techniques that may be effective in addition to prescription of medication for chronic pain. These have been reviewed by MacFarlane et al (2014) and includes lifestyle changes such as weight loss, physiotherapy, controlled exercise and acupuncture. At present, there is insufficient evidence to consider any non-pharmacological therapy as a sole treatment for chronic pain.
Case example: forelimb amputation in a dog following trauma
Table 2 shows the case presentation, diagnosis and management of a dog presenting following a road traffic collision. The client declined referral and opted for amputation owing to financial constraints. On the morning of surgery, the dog looked depressed in its kennel and still resented having its left forelimb touched. Despite this, surgical management was successful, and the dog appeared increasingly bright over the subsequent 24 hours and began eating and drinking.
Table 2. Case presentation, diagnosis and management of a Labrador presenting after a road traffic collision
| Signalment and History |
|
| Initial management and diagnosis |
|
| Overnight plan |
|
| Surgical intervention |
|
| Postoperative management |
|
Postoperative checks at 5 and 10 days showed that the incision was healing well, and the client was happy with the outcome. The dog was reported as quiet and a little less interactive than normal, but the client was not concerned. A few months later, the client called and was worried her dog was not back to his ‘old self’. She was concerned about his behaviour and reported that the dog was paying a lot of attention to the surgical site and rubbing it, randomly vocalising and appeared restless in the evenings. She had given the dog some left-over meloxicam for the previous 5 days, but did not see an improvement. On re-examination at the clinic, the surgical site appeared healed, with no evidence of infection or inflammation. The vet suspected the dog was experiencing chronic, neuropathic pain, and prescribed 10 mg/kg gabapentin every 8 hours per os, alongside continuation of the meloxicam, and the client was asked to phone back in 2 weeks with an update.
Was the pre-surgical management of acute pain adequate in this case?
It is very difficult to tell. If an animal is not explicitly evaluated for pain at a preliminary examination, it may be missed. Not every animal will vocalise, and not every vocalising animal will be in pain. Looking depressed can be an indicator of acute pain, as can resentment of the painful site being palpated (Reid et al, 2007). Formulating a structured acute pain assessment plan using validated scoring systems allows better interpretation as to how a patient has responded to analgesia overnight. Even better would be the inclusion of a rescue analgesia plan (for example, additional 0.1 mg/kg methadone intravenously), with set thresholds for intervention as outlined by these pain scoring systems. It is important to control pre-amputation pain promptly and effectively, as it has been linked to more postoperative pain episodes (Menchetti et al, 2017).
Was intraoperative management of acute pain adequate in this case?
Probably not. Increasing respiratory rate, heart rate and blood pressure can all be signs of nociceptive signalling during general anaesthesia. Rather than increasing the anaesthetic depth, consideration should have been made to splash or infiltrate local anaes-thetic around the exposed nerves proximally, before nerve transection. Administration of an additional dose of methadone, and/or the perioperative use of a ketamine infusion may have also reduced nociceptive signalling (Wagner et al, 2002).
Was the postoperative management of acute pain adequate in this case?
Without using a validated acute pain scoring system, it is not possible to tell. Perioperative use of ketamine has been shown to be effective at reducing pain in dogs undergoing limb amputation, therefore adding this to the protocol may have been beneficial (Wagner et al, 2002). Scoring systems can also be useful to assist with deciding when to taper or switch opioid use postoperatively. Pain before and after amputations is reported as similar (Menchetti et al, 2017), so even in the absence of an unstable fracture, effective postoperative pain management is still critical.
Why was it important to recheck the dog at 2 months post-surgery in this case?
It is important to assess whether there are postoperative complications such as incisional healing that need addressing. Phantom limb pain describes pain experienced from a limb that is no longer present (Flor, 2002), but it is important to differentiate this from post-surgical pain, or pain caused by inflammation and infection, which generally improves with healing (Menchetti et al, 2017). The behaviours described by the client are consistent with post-amputation chronic pain (Boesch et al, 2021). There is scant evidence in the literature to suggest which specific analgesic drugs are effective at managing chronic pain behaviours post-limb amputation because the pathophysiology is not fully understood. Long-term pathophysiological changes in the peripheral and central nervous system are thought to occur (Kuffler, 2018).
Was the continuation of meloxicam and addition of gabapentin a reasonable choice, given the suspicion of chronic post-amputation pain?
It is important to consider licenced drugs as a first line therapy, under UK prescribing regulations (Veterinary Medicines Directorate, 2015). However, if treatment with authorised products alone is ineffective, it is reasonable to use the prescribing cascade for medications such as gabapentin. In humans, gabapentin may relieve phantom limb pain (Bone et al, 2002; Abbass, 2012), but in dogs it is unclear whether it is effective. There is evidence to suggest it is not effective when used as an adjunctive analgesic for acute pain in the perioperative period for dogs undergoing forelimb amputation (Wagner et al, 2010). However, Wagner et al (2010) administered gabapentin every 12 hours, but pharmacological evidence suggests at least 8 hourly dosing is required to maintain therapeutic plasma concentrations (Kukanich and Cohen, 2011). A study using quantitative sensory testing to evaluate response to therapy with gabapentin in dogs with neuropathic pain gave some encouraging results, but they should be interpreted with caution (Ruel et al, 2020).
Given the lack of clarity in the literature regarding specific recommendations for prescribing for animals in chronic pain, regular patient rechecks and the use of specific pain scoring systemics for chronic pain are recommended. It is important to counsel clients on the risk of adverse effects associated with long-term use of non-steroidal anti-inflammatory drugs (Luna et al, 2007) and be continually mindful of the contraindications in the summary of drug characteristics. Non-pharmacological interventions can be considered alongside medication, if feasible for the client and patient.
Conclusions
The complex pathophysiology of the different types of pain is a significant part of why they can be challenging to manage. There is a range of licenced and effective pharmacological therapies for acute pain, but the unique features of chronic pain make effective intervention more challenging. In particular, the complex psychosocial elements of chronic pain and client expectations can be difficult to manage. This is compounded by a relative lack of understanding of off-licence prescribing options available for chronic pain. This sought to provide an overview of some of these medications, along with discussion on how to assess the effectiveness of intervention strategies used for pain management. It also highlights the potential importance of effectively managing acute pain with the aim of reducing the incidence of chronic pain in animals.
KEY POINTS
- There are different types of pain that exhibit different clinical signs.
- The complex pathophysiology of the development and continuation of pain means it can be challenging to effectively manage.
- There are a variety of pharmacological management options, but the relevant prescribing legislation should be adhered to.
- It is important to consider the needs of both the client and the animal when devising pain management plans and review progress on a regular basis.


