Reptiles are becoming more commonly owned and therefore presented in clinical practice, with owner expectations of medical and surgical care often being high. Reptile surgery is constantly evolving, with more novel surgical techniques being reported (Di Girolamo and Mans, 2016). It is essential to have a basic understanding of the unique reptile anatomy and physiology before undertaking any surgery (Bennett and Lock, 2000), and these will vary considerably between groups of reptiles and between different species within these groups (Bennett and Lock, 2000; Di Girolamo and Mans, 2016).
A full clinical examination and subsequent stabilisation before any surgery is essential, as many reptiles will often be dehydrated and nutritionally deficient (Bennett and Lock, 2000). Surgery should be delayed until the hydration status is improved, at the very least (Bennett and Lock, 2000). It is also important to bear in mind that reptiles are ectothermic, meaning their physiological mechanisms, including drug metabolism, are affected by their external temperatures, and are therefore optimised by ensuring that each individual is kept at its optimum temperature, which will vary between species (Bennett and Lock, 2000). As such, appropriate environmental temperatures should be provided from the preoperative period through to the postoperative period, to ensure optimum wound healing (Bennett and Lock, 2000; Alworth et al, 2011).
The position of both the patient and surgeon should be considered before and during surgery (Alworth et al, 2011). The patient should be positioned to allow appropriate visualisation of the surgical site, while gently extending the head and neck and simultaneously preventing coelomic contents from compressing the lower respiratory tract, to ensure optimum ventilation throughout the surgery. The surgeon's arms and wrists should rest on an appropriately elevated operation table, especially when performing microsurgery, to optimise motor control (Alworth et al, 2011). In the author's experience, using magnification (at least 2.5x) and an appropriately focused, ideally head-mounted light source will also improve the surgeon's motor control. The author recommends standard asepsis, which is just as important in reptiles as it is for other animals during surgery, including appropriate surgical attire. It should also be noted that excessive use of alcohol should be avoided, to minimise evaporative losses.
As a general rule, 0.4–0.8 ml per 100g of bodyweight is the maximum volume of blood that can be lost from a patient (Alworth et al, 2011). This means that minimising blood loss, especially in small patients, is essential. In the author's experience, sterile cotton tip applicators are excellent for small, confined spaces, as are the use of haemoclips. Radiosurgery uses radio waves to cause focal thermal tissue damage and is therefore useful to control haemorrhage during surgery, in comparison to electrosurgery, as there is minimal collateral damage (Alworth et al, 2011).
Owing to the vast anatomical differences between reptiles, it is wise to have easy access to a variety of different surgical equipment. For most reptiles, from 5kg to 50kg, normal surgical instruments are suitable. However, for patients under 1kg, fine tipped microsurgical equipment should be considered (Alworth et al, 2011). In the author's experience, oscillating saws and drills are often needed for orthopaedic surgeries, with narrow blades recommended for incising the plastron in chelonia coelomic surgery. This prevents the creation of a wide osteotomy which will delay wound healing (Bennett and Lock, 2000; Lock, 2000).
Soft tissue surgeries
Integument
The integument of reptiles will vary massively between different species, with the external structures of chelonia being significantly thicker and tougher than in other species. As a general rule, incisions should be made between the scales to avoid dysecdysis (Alworth et al, 2011; Di Girolamo and Mans, 2016). Integument surgery is often performed for wound management, the removal of neoplasia or of abscesses. When dealing with abscesses, it is important to remove the capsule of thick fibrous tissue that will be surrounding the pus, and send a sample away for bacterial and fungal culture and sensitivity testing (Alworth et al, 2011; Di Girolamo and Mans, 2016). The underlying structures should be suitably irrigated with an appropriately diluted antiseptic solution, such as povidone-iodine, and the skin closed using an appropriately sized monofilament, absorbent suture material (Stahl, 2003). Suture patterns that are suitable for closing reptile skin include both horizontal and vertical mattress sutures. These everting patterns are essential as reptile skin naturally inverts (Alworth et al, 2011; Bennett and Lock, 2000; Lock, 2000; Di Girolamo and Mans, 2016). Reptile healing takes seven times longer than a comparatively sized mammal, and as such sutures are recommended to be removed after 6–8 weeks (Alworth et al, 2011; Di Girolamo and Mans, 2016).
Aural abscesses are most commonly seen in terrestrial and aquatic chelonia and are often linked to hypovitaminosis A, poor environmental hygiene and environmental pollutants (Alworth et al, 2011; Tatli and Şen, 2016). In the author's opinion, the patient should be placed in sternal recumbency with its head and neck extended and rotated to allow the tympanic abscess to be uppermost. A semi-circular incision into the tympanum is made in a craniocaudal direction, the skin lifted, and a curette used to remove the pus, with samples taken for culture and sensitivity testing (Rowland, 2016). The incision can be extended to remove half, or sometimes the whole tympanum depending on the extent of the abscess. It is important that the eustachian tube is clear of purulent debris and the surgical site thoroughly flushed with an antiseptic solution, such as povidone-iodine, followed by sterile saline. The surgical site remains open to allow the owner to continue antiseptic flushing at home (Rowland, 2016).
Ophthalmic surgery
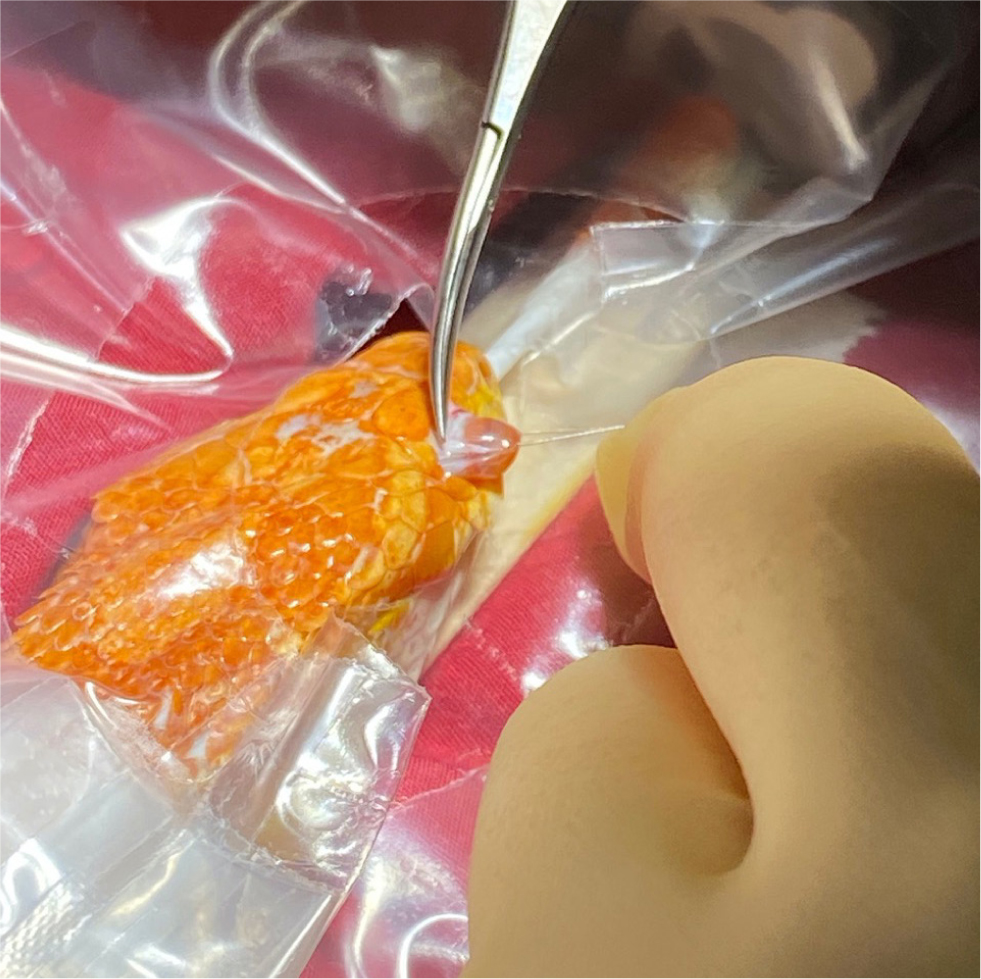
Subspectacular abscesses are common in snakes and will form when the lacrimal duct becomes blocked, resulting in secondary bacterial and fungal infections and subsequent abscess formation (Alworth et al, 2011) (Figure 1). The author suggests creating a 30–90° wedge to be removed from the ventral aspect of the spectacle, with samples taken for cytology, culture and sensitivity testing and the caseous material flushed away. It is important to ensure the lacrimal duct is patent, which can be assessed via the buccal opening found at the margin or the palatine teeth. The wedge is then left open to allow the application of topical ophthalmic medications.

It is the author's suggestion that in end stage ophthalmic disease, where there is irreversible ocular damage, globe prolapse or persistent pain, enucleations should be considered. A circular incision that encompasses the spectacle is made, the globe should then grasped with forceps, elevated and the surrounding attachments dissected. Haemoclips or suitably sized monofilament suture material, in combination with radiosurgery, should then be used to ligate the ocular vessels. The deficit is then left open (Figure 2).
Coelemic surgery
The complexity of coelomic surgery will vary depending on the species undergoing the procedure. It allows for the visualisation of most of the major internal organs, for various procedures, implantations and biopsies (Alworth et al, 2011). It is the author's suggestion that most lizards, except from laterally compressed individuals such as chameleons, should be placed in dorsal recumbency. There are two standard approaches that can be considered, the paramedian and median approach. The length of the surgical incision will vary depending on the procedure being performed.
The paramedian approach in lizards avoids the large midline blood vessel, the ventral abdominal vein, but can cause significant postoperative pain as the abdominal muscles will be incised (Wellehan and Gunkel, 2004; Alworth et al, 2011) (Figure 3). Sharp incision should be avoided to prevent significant haemorrhage (Martinez-Jimenez and Hernandez-Divers, 2007). In small lizards, the muscle and coelomic membrane can be parted using cotton applicators or radiosurgery.
With the midline approach, both sides of the coelomic cavity can be accessed, although there is an increased risk of iatrogenic trauma to the ventral abdominal vein. To avoid this vessel, the incision should be made caudal to the umbilicus and extended cranially, with the blood vessel being localised and moved laterally (Alworth et al, 2011). Wherever possible, coeliotomies should be closed with a double layer, but this is often not possible in small lizards with thin coelomic musculature. As mentioned previously, skin should be everted to optimise healing.
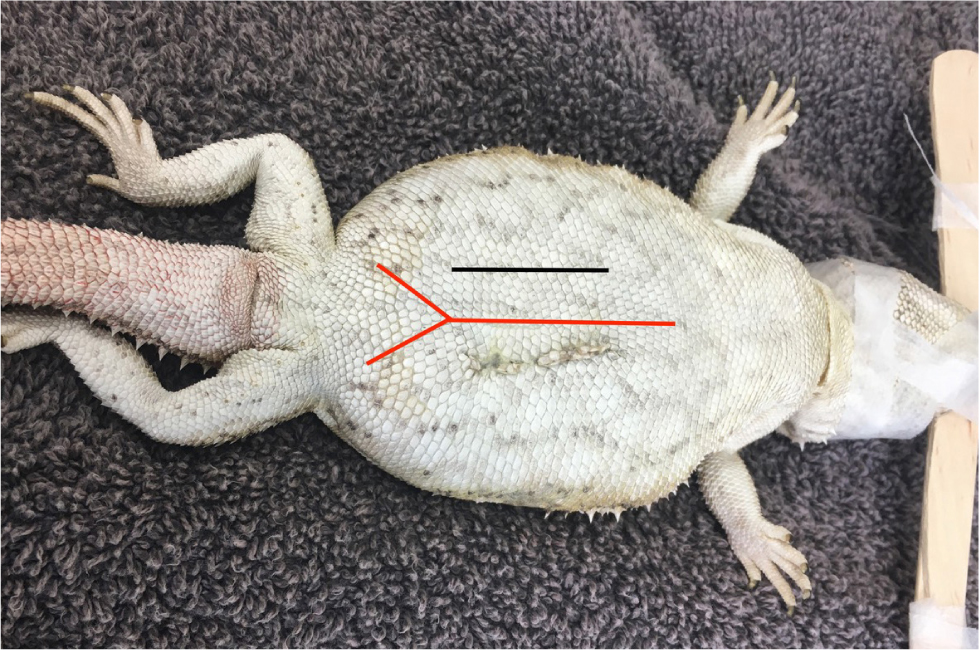
In the author's experience, in snakes, a single coeliotomy will not be enough to visualise all internal organs, which means that it is imperative that an accurate surgical site is established before surgery. The coeliotomy incision in snakes should be made between the first and second row of lateral scales (Figure 4). This will ensure the incision is positioned laterally and off the floor to allow the patient to remain ambulatory. The muscle layer should be blunt dissected, just ventral to the ribs, followed by the coelomic membrane to enter the coelomic cavity. A two-layer closure is recommended, with the coelomic membrane and muscle closed in a continuous or interrupted pattern. The skin should be closed in an everting pattern.
Chelonia coeliotomies are more challenging because of the shell (Alworth et al, 2011). There are two possible approaches to the coelom, the transplastron and prefemoral approach (Alworth et al, 2011) (Figure 5a and 5b). In the author's experience, the appropriateness of each will vary depending on the species and the target organ. The transplastron approach requires a temporary osteotomy through the plastron. The author suggests using a thin-bladed oscillating saw to create the temporary osteotomy, with the lateral incisions performed first, followed by the caudal and then cranial incisions to allow the pressure to be released and the coelomic membrane to fall away from the underside of the shell, ensuring the underlying soft tissues structures are protected. The edges of the osteotomy should be bevelled at a 45° and the depth of the bone incisions probed with a 25G needle to ensure full thickness is achieved (Alworth et al, 2011).
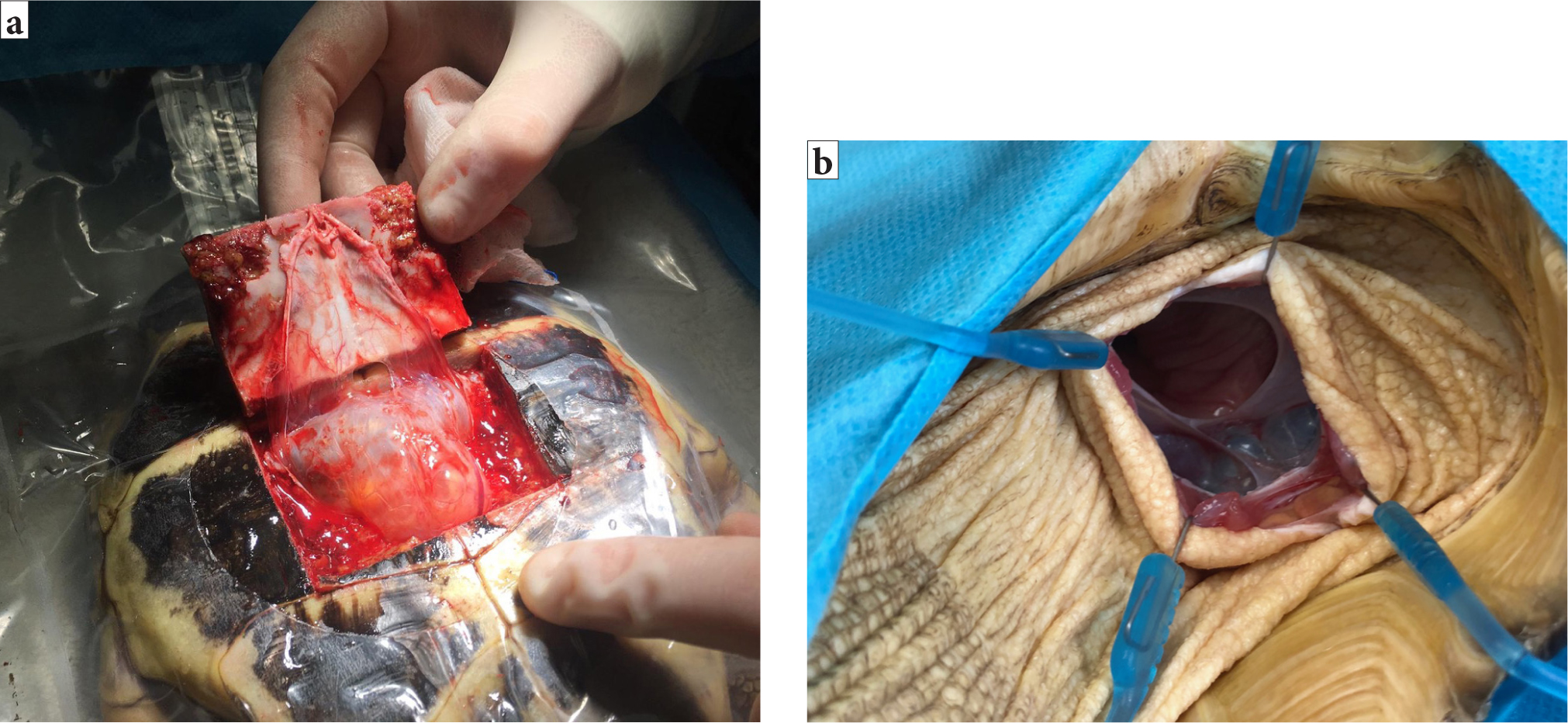
The author suggests that the new bone fragment should be reflected cranially and the soft tissues blunt dissected from the underside. The caudal attachments should be preserved in an attempt to preserve blood supply to the fragment (Alworth et al, 2011) and swab soaked in sterile saline used to cover the tissues to prevent them from desiccating. Care should be taken to avoid the paired abdominal veins present on the surface of the underlying coelomic membrane (Figure 6a). Incision into the coelomic cavity is made in between these paired abdominal veins (Figure 6b).
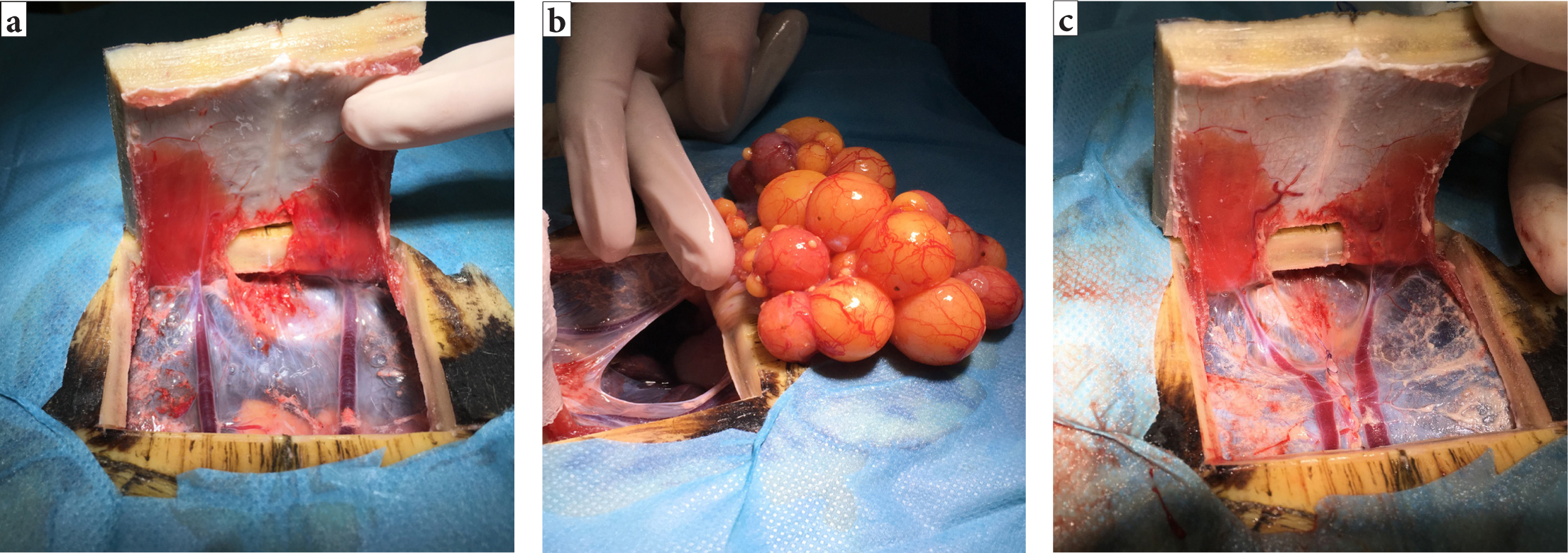
Closure of a chelonia coeliotomy should involve simple continuous closure of the coelomic membrane with an appropriately sized absorbable monofilament (Figure 6c), the bony section should then be replaced and fixed in place using a non-heat generating polymer such as Kooliner (KoolinerTM; GC America Inc.) (Figure 6d). In the author's experience, the healing process for plastronotomies will depend on the size of the bony gap and the quality of the repair. The plastron takes approximately 12–18 weeks to heal.
The pre-femoral approach allows entrance to the coelomic cavity through the soft tissues in front of the hind limb (Alworth et al, 2011). The patient should be placed in lateral recumbency with the surgical site uppermost, with the hind limbs retracted backwards (Figure 7). A craniocaudal incision is made in the middle of the fossa. The underlying soft tissues are blunt dissected cranial to the sartorius and ventral to the iliacus muscles, revealing the coelomic aponeurosis of the transverse and oblique abdominal muscles (Alworth et al, 2011). This coelomic aponeurosis is fibrous, and some force may be required to breach the membrane. A lone star retractor should be used to hold the skin, subcutaneous tissues and coelomic membrane to improve surgical exposure (Figure 5b). Closure routinely is accomplished in two layers (Alworth et al, 2011).
Reproductive surgery
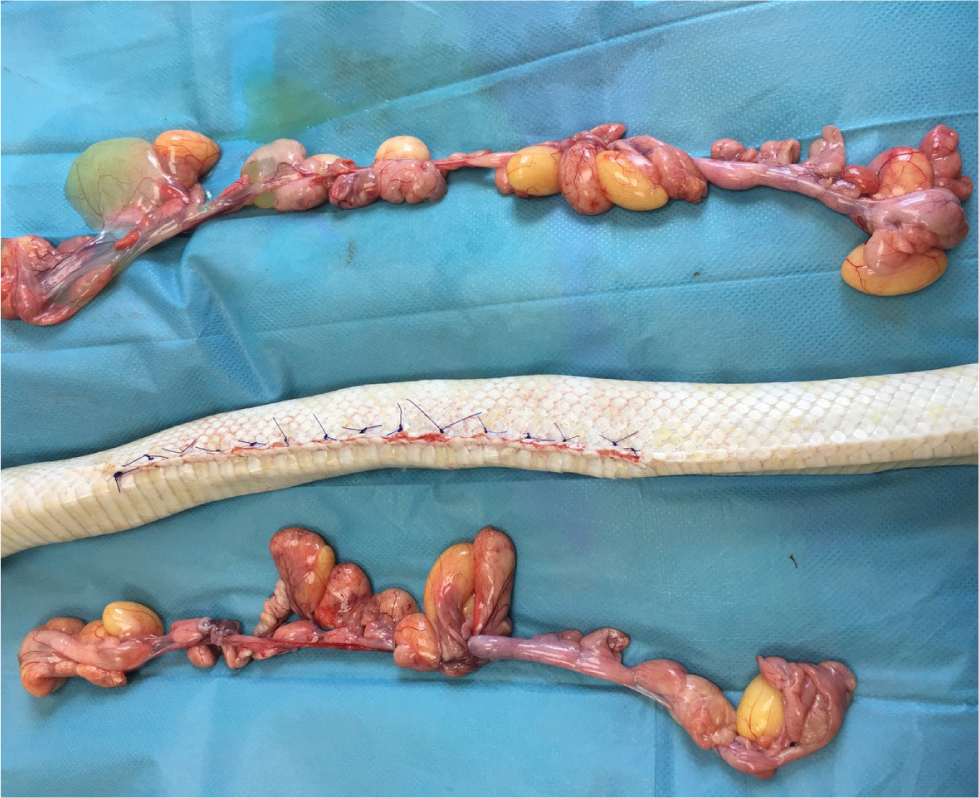
The reproductive physiology of reptiles varies depending on the order to which the reptile belongs (Lock, 2000). Oviparous reptiles, such as chelonia, produce eggs, whereas squamates can be oviparous or viviparous (bare live young). Reproductive diseases requiring surgical intervention are very common in reptiles, with multiple potential causes (Funk, 2002; Hedley, 2016; Lock, 2000). Ovariectomies can be performed as a preventative measure, as well as a treatment, for reproductive disease (Alworth et al, 2011). In non-reproductively active female lizards, the ovaries are very small and located adjacent to the dorsal renal veins and the adrenal glands in the mid to caudal coelomic cavity (Lock, 2000; Alworth et al, 2011). When there is ovarian disease, the ovaries will be substantially larger and will resemble yellow/orange bunches of grapes (Figure 8) (Alworth et al, 2011). The author suggests that to perform an ovariectomy, vascular clips or a suitably sized monofilament suture material should be placed along the ovarian ligament and associated blood vessels, before dissecting the gonad. If the oviducts are normal, there is no need to remove them. Ovaries in stasis should be elevated out of the coelomic cavity to allow full exposure of the suspensory ligament, which will contain many blood vessels that branch directly off the aorta and renal veins (Figure 8).
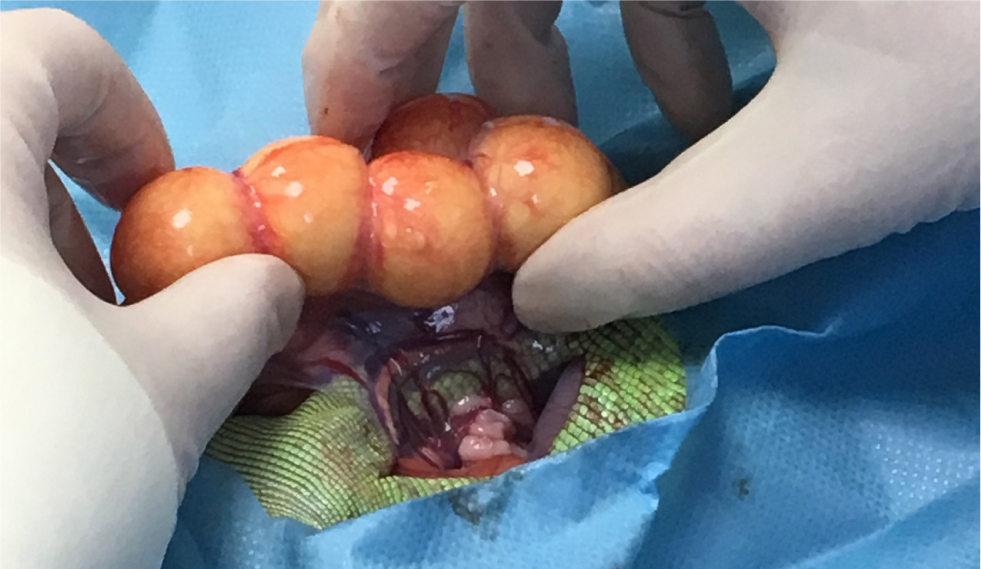
In comparison to lizards, reproductive surgeries in snakes and chelonia are more invasive (Alworth et al, 2011). In the author's experience, snakes will either require multiple, or singularly large coeliotomies, and surgeries in chelonia may involve an osteotomy if the pre-femoral approach is not a possibility. Chelonian ovaries originate close to the ventrolateral aspect of the kidneys which, when reproductively active, will extend into the central coelomic cavity. The ovarian suspensory ligament in chelonia and snakes is extensive, therefore it is very important to ensure all the ovarian tissue is localised and exteriorised before ligating the blood vessels within. As with lizards, if the oviducts are normal there is no need to remove them.
Salpingotomies and salpingectomies are most commonly performed in cases of dystocia, with a salpingotomy being performed when breeding capacity needs to be preserved (Alworth et al, 2011). The reptile oviduct contains a large number of blood vessels which will need to be ligated. Owing to the extent of the oviduct in snakes, there is often a need for multiple coeliotomies (Stahl, 2002). Reptile oviductal tissue is often incredibly fragile, although the structure of chelonia oviduct is thicker than those found in snakes and lizards.
Orchidectomies are reported in lizards (Alworth et al, 2011). The testes are located adjacent to the dorsal renal vein and adrenal glands in the mid to caudal coelomic cavity. They are suspended by a short broad ligament from the dorsal body wall (Alworth et al, 2011). The left adrenal gland is located between the left testis and the left dorsal renal vein, therefore care needs to be taken, even more so when operating on the left, to minimise the risk of iatrogenic trauma (Alworth et al, 2011). Vascular clips should be used to ligate the vessels associated with the testes, which can be better visualised if the testis is retracted slightly (Alworth et al, 2011).
Gastrointestinal surgery
In the author's experience, the most common and straightforward gastrointestinal surgery performed in reptiles is placement of a oesophagostomy tube. This procedure should be considered when repeat feedings are performed, or if oral medications are required, but the owner is unable to medicate accurately (Lock, 2000; Alworth et al, 2011). In the author's experience, this procedure is most commonly performed in chelonia, but is possible in other species. The author suggests that the patient should be heavily sedated or under a light plane of anaesthesia, then a tube of an appropriate diameter for the individual should be premeasured from the cranial rim of the plastron to the junction of the pectoral and abdominal scutes and marked with a non-toxic marker (Alworth et al, 2011). The patient should then be placed in lateral recumbency and a small pair of curved haemostats inserted into the mouth and down the oesophagus and pushed gently against the caudolateral aspect of the neck, as far down the neck as possible. A small incision over the point of the haemostats is made through aseptically prepared skin, taking care not to inadvertently lacerate the dorsolateral jugular vein and the ventrolateral carotid artery (Alworth et al, 2011). The closed haemostats are pushed through the hole, then gently opened and the end of the premeasured tube grasped and retraced into the oesophageal lumen. The tube should then be gently pulled out of the mouth and the redirected back down the oesophagus, into the stomach. Radiographs should be performed to confirm correct placement and the tube sutured in place either with a butterfly tape sutured to the skin, or using a Chinese finger trap suture. The tube is then taped in place and the leg and cranial rim of carapace taped to prevent the tube being accidentally pulled out (Figure 9a–g).

The author suggests that gastrotomies, enterotomies and enterectomies are performed in a similar fashion to mammals, but it should be noted that reptile tissues are comparatively thinner and more friable. The author recommends that the area of the gastrointestinal tract is exteriorised, which will vary in ease depending on the species. Gastrointestinal tracts in chelonia will be harder to exteriorise than snakes and lizards. Once exteriorised, sterile saline-soaked swabs should be used to pack and protect the coelomic cavity while the procedure is performed if there is space (Figure 10). Standard intestinal closure is recommended and the cavity flushed copiously before coelomic cavity closure, ideally two layers.
Urinary tract surgery
The main surgery of the urinary tract is a cystotomy. This surgery will be required for the removal of bladder stones, but also the removal of eggs in chelonia, which can be retropulsed into the bladder in cases of dystocia. Not all reptiles have bladders, this organ is only present in chelonia and some lizard species (Alworth et al, 2011). The bladder in chelonia can be accessed via the transplastron approach, but also via the pre-femoral approach. Via either route, stay sutures should be placed to prevent coelomic contamination (Figure 11). Once completed, the bladder should be closed using an appropriately sized absorbable monofilament suture material, via a single layer closure, then leak tested. The coelomic cavity should then be copiously flushed before closure.
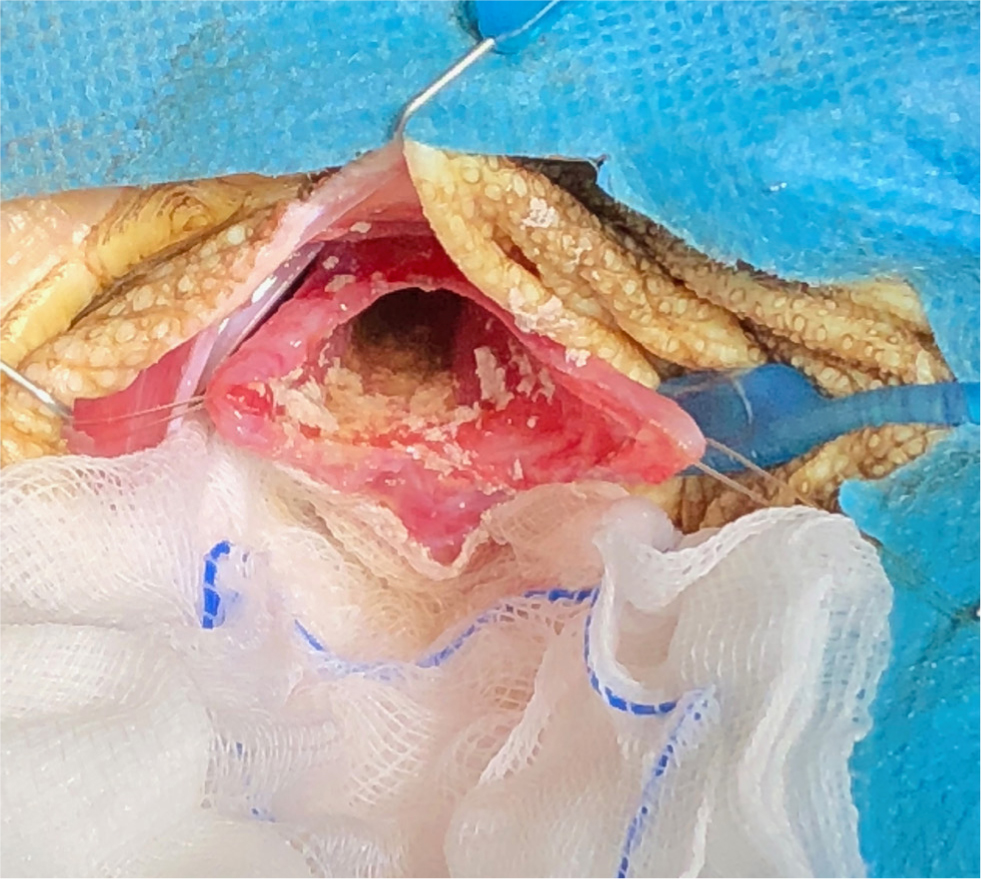
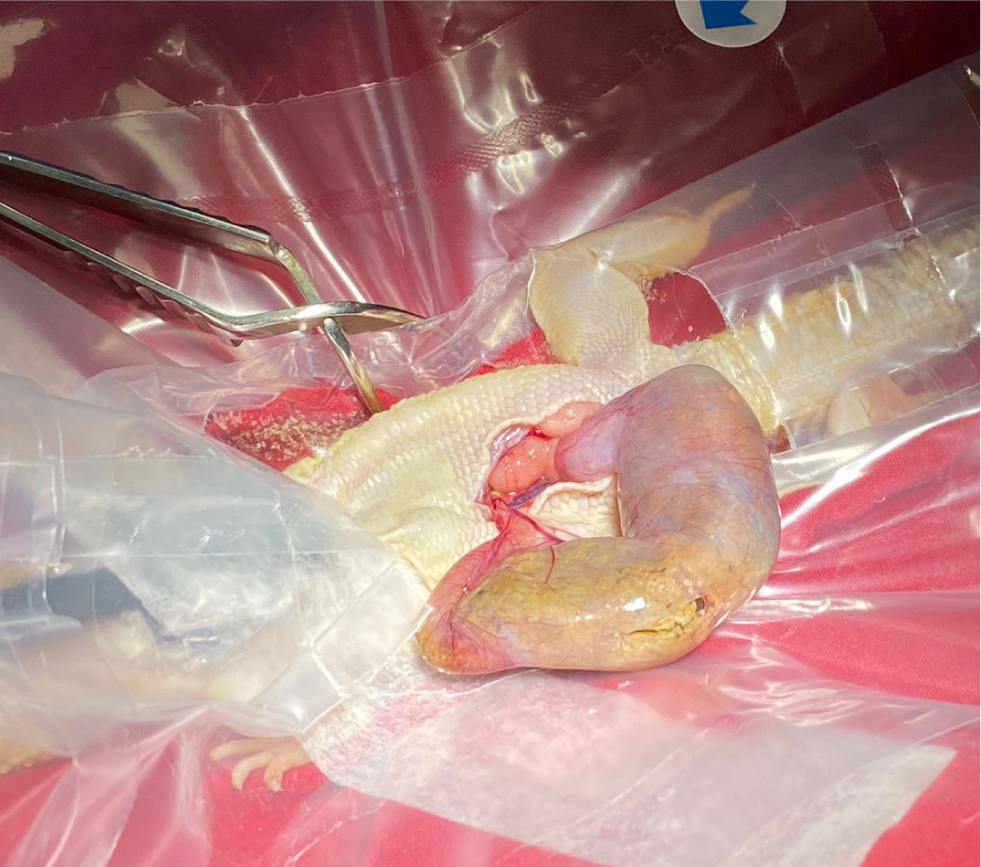
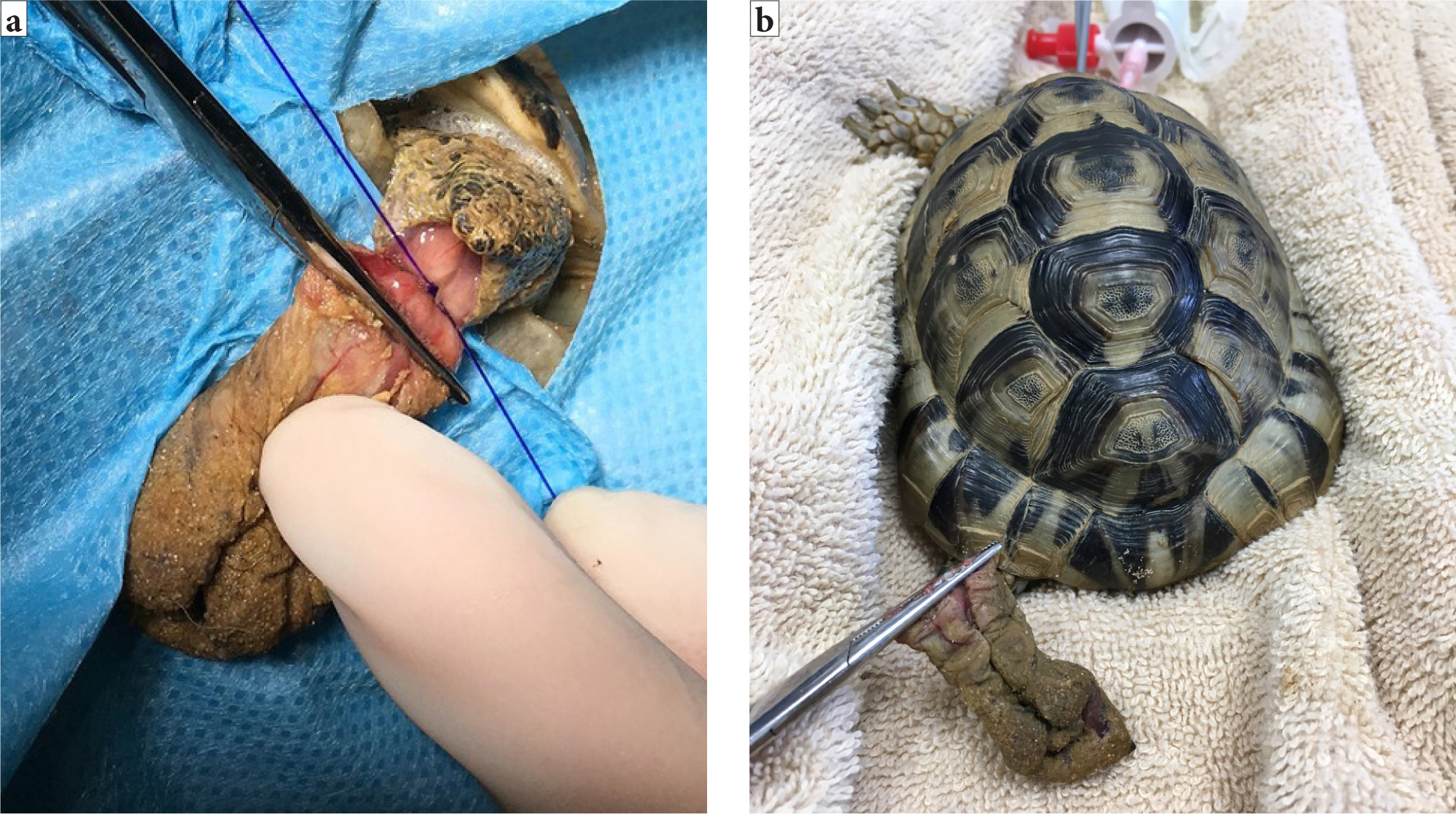
The bladder is one of the organs that can prolapse in reptiles. Other structures include the cloaca, intestines, oviduct, hemipenes and phallus in chelonia (Wellehan and Gunkel, 2004). It is important that, before any surgery, the tissue is identified and the underlying cause of the prolapse identified. With the phallus in chelonia and hemipenes in other male reptiles, if there is irreversible tissue trauma, an amputation can be performed (Knotek et al, 2017). This organ is used for copulation only, not urination. If the tissue is viable, it can be cleaned and replaced with the cloaca closed temporarily using simple interrupted sutures either side, not a purse string, which is associated with traumatic damage to the nerves and musculature of the cloaca (Alworth et al, 2011). If an amputation is needed, a circumferential or transfixing ligature should be placed at the base of the phallus and the tissue amputated distally, and this will of course interfere with future breeding (Martinez-Jimenez and Hernandez-Divers, 2007) (Figure 12a–b).
Orthopaedic surgery
Fracture repair in reptiles follows the same principles as in other species, with full radiographic assessment essential before stabilisation and fixation. Depending on the fracture type and position, various techniques may be possible, such as external coaptation, external and internal fixation and in severe instances, amputation (Mitchell, 2002). In the author's opinion, digit amputations should be performed at the level of the metacarpus or metatarsus; if the problem requiring amputation is proximal to the carpus or tarsus, then a full limb amputation through the most proximal joint, especially in large lizards that would likely traumatise a stump, should be considered. The author suggests that a tourniquet should be placed proximally, alongside the use of radiosurgery and appropriately sized monofilament suture material or haemoclips to control haemorrhage. Soft tissues should be preserved to permit closure. Tail amputations are commonplace in practice, mostly in lizards, but also in snakes. Some species are able to undergo autoamputation, which can be used during surgery. The author suggests that while under anaesthesia and once appropriately anaesthetised, the tail can be grasped proximal to the injury and the tissue twisted and snapped through a fracture plane of the coccygeal vertebrae. In these species, the tail will eventually grow back. In species that do not autoamputate, an incision is made in between the vertebrae and the soft tissues preserved to allow closure.
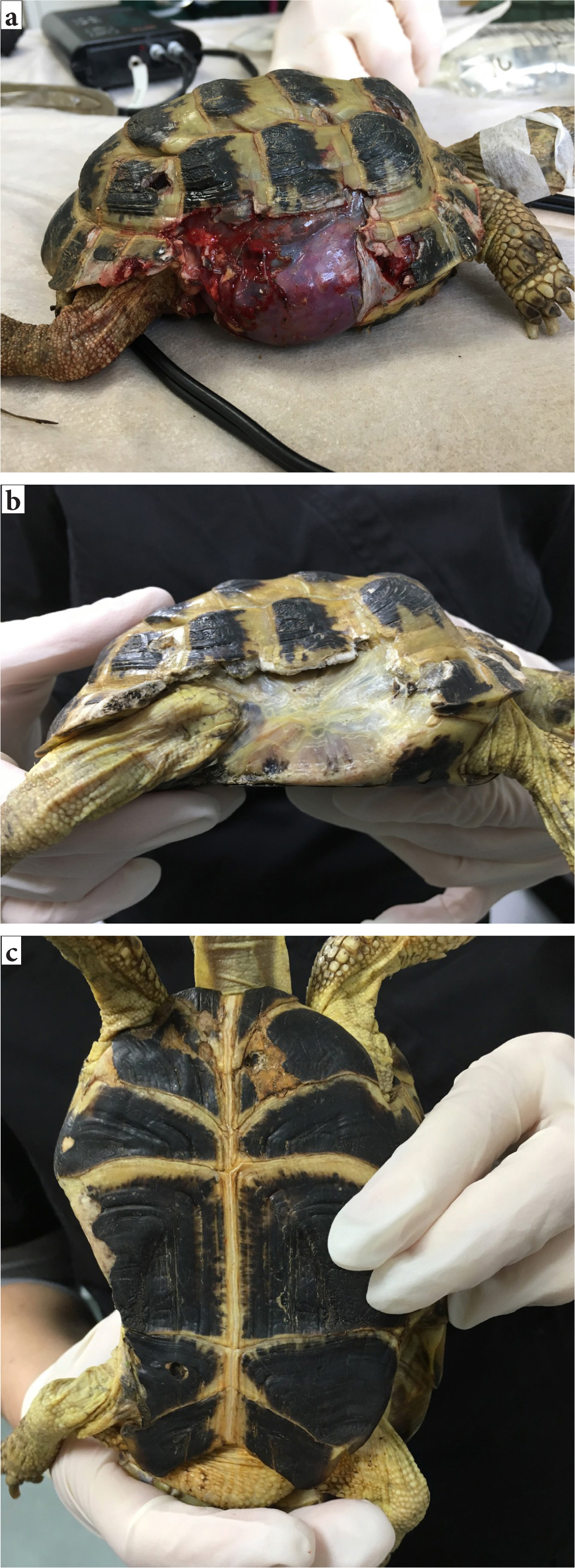
Traumatic shell fractures in chelonia will have a variable prognosis depending on the extent of the trauma and whether the coelomic membrane and contents are affected (Figures 13a–c). Standard wound management techniques are important to initiate, alongside shell fracture stabilisation. Fragments can be replaced using screws and wire, or with more inventive techniques such as use of bra clasps or cable (Music and Strunk, 2016). Techniques that allow observation and access for cleaning the fracture sites are preferable to the historical techniques that involved complete coverage and therefore entrapment of possible foreign material, such as epoxy or acrylic, as these practices increase the possibility of abscess formation and subsequent sepsis (Kishimori et al, 2001).
KEY POINTS
- It is essential to have a basic understanding of the unique reptile anatomy and physiology before undertaking any surgery.
- A full clinical examination and subsequent stabilisation before any surgery is essential, as many reptiles will often be dehydrated and nutritionally deficient.
- Appropriate environmental temperatures should be provided from the preoperative period through to the postoperative period, to ensure optimum wound healing.
- A surgeon's position, equipment, magnification and lighting will all aid in improving surgical care and technique.
- Swift and correct diagnosis and surgical intervention in many cases of reptile disease allow for the best possible outcome in many patients.


