Rabbits (Oryctolagus cuniculus) are very popular pets, with approximately 1.5 million of them being kept in the UK (UK Pet Food, 2023). Improvements in how medical and surgical care is provided to rabbits is resulting in them often living longer (Fisher, 2010), with many owners having high expectations of veterinary surgeons (Graham, 2006). This series of articles gives a broad overview of a number of surgical procedures performed in this species, as well as covering general pre-, peri- and postoperative specifics to help veterinarians and veterinary nurses meet these expectations. Anaesthesia and analgesia protocols are beyond the scope of these articles.
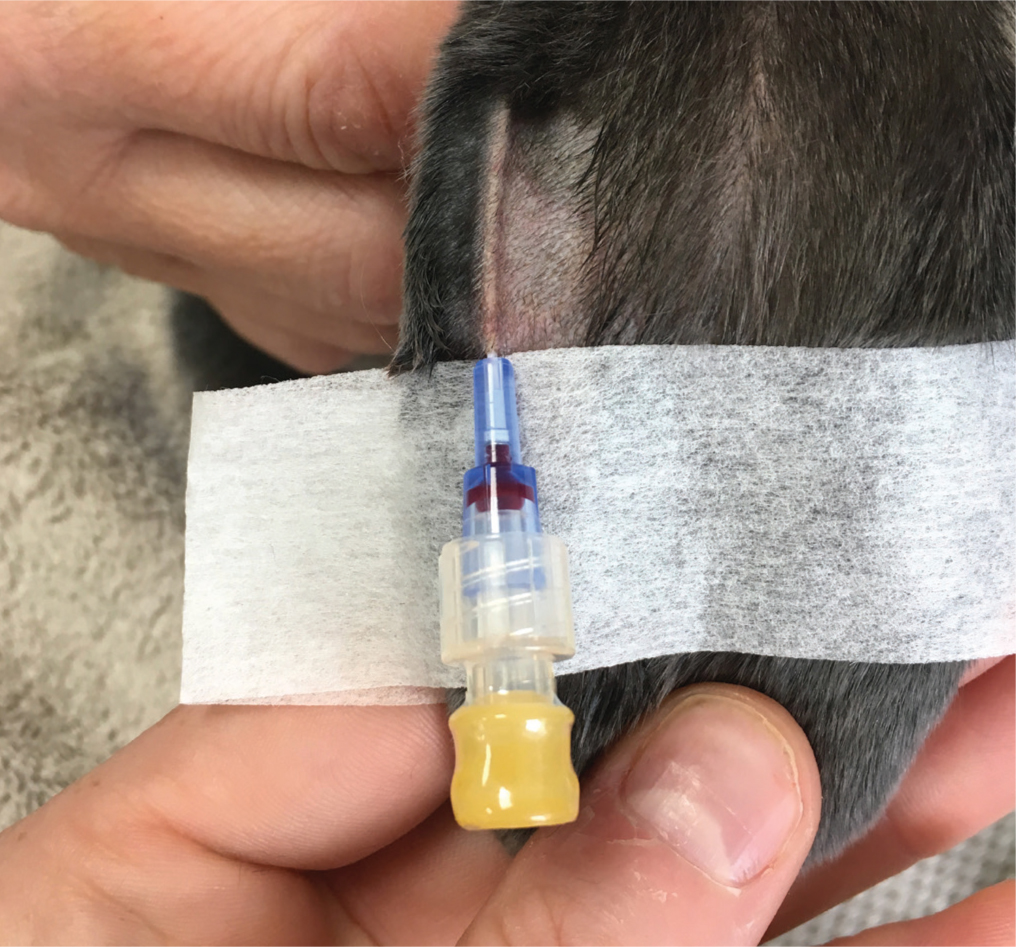
Rabbits are a prey species, and have evolved to conceal signs of illness to escape predation (Paul-Murphy, 2007; Huynh et al, 2016; Miller and Leach, 2023). For this reason, as well as dramatic differences between a rabbit's anatomy and physiology, they cannot be thought of as small dogs or cats (Mullen, 2000). Rabbits, unlike cats and dogs, do not need to be fasted before surgery, as they are unable to vomit (Szabo et al, 2016). As with all species, a thorough, systematic examination is a vital part of patient assessment before any surgery (Antinoff, 1999; Graham, 2006; Szabo et al, 2016). It is wise to remember that if inappropriately handled, rabbits can be prone to back and hind limb fractures (Graham, 2006). Any rabbit deemed unwell on a clinical examination should be stabilised before surgery (Redrobe, 2002; Szabo et al, 2016). Any dehydration should be suitably corrected, with intravenous fluid therapy (Szabo et al, 2016) (Figure 1). If intravenous catheter placement is not possible, fluids can be administered subcutaneously or intraosseously (Fisher, 2010).
All fur should be carefully removed from the surgical site, with an approximately 2–3 cm margin around the incision site (Bennett, 2000; Kroner et al, 2016). Care must be taken to avoid inadvertently damaging the fragile underlying skin (Kroner et al, 2016; Szabo et al, 2016). Only the minimum amount of fur should be removed to prevent a drastic decrease in insulation and subsequent hypothermia while still maintaining a sterile surgical field (Kroner et al, 2016). Anaesthetised small mammals are prone to hypothermia because of their large surface area to volume ratio, the cooling effect of anaesthetic gases, suppression of thermoregulation by pre-anaesthetic drugs and analgesics, excessive wetness when cleaning and the use of surgical spirit (Fisher, 2005). As such, clinicians must remain vigilant to avoid exacerbating this risk. It is recommended to use a warm, dilute 0.05% chlorhexidine solution (Hibiscrub, Regent Medical Ltd) for surgical preparation in most cases (Redrobe, 2002; Kroner et al, 2016; Szabo et al, 2016).
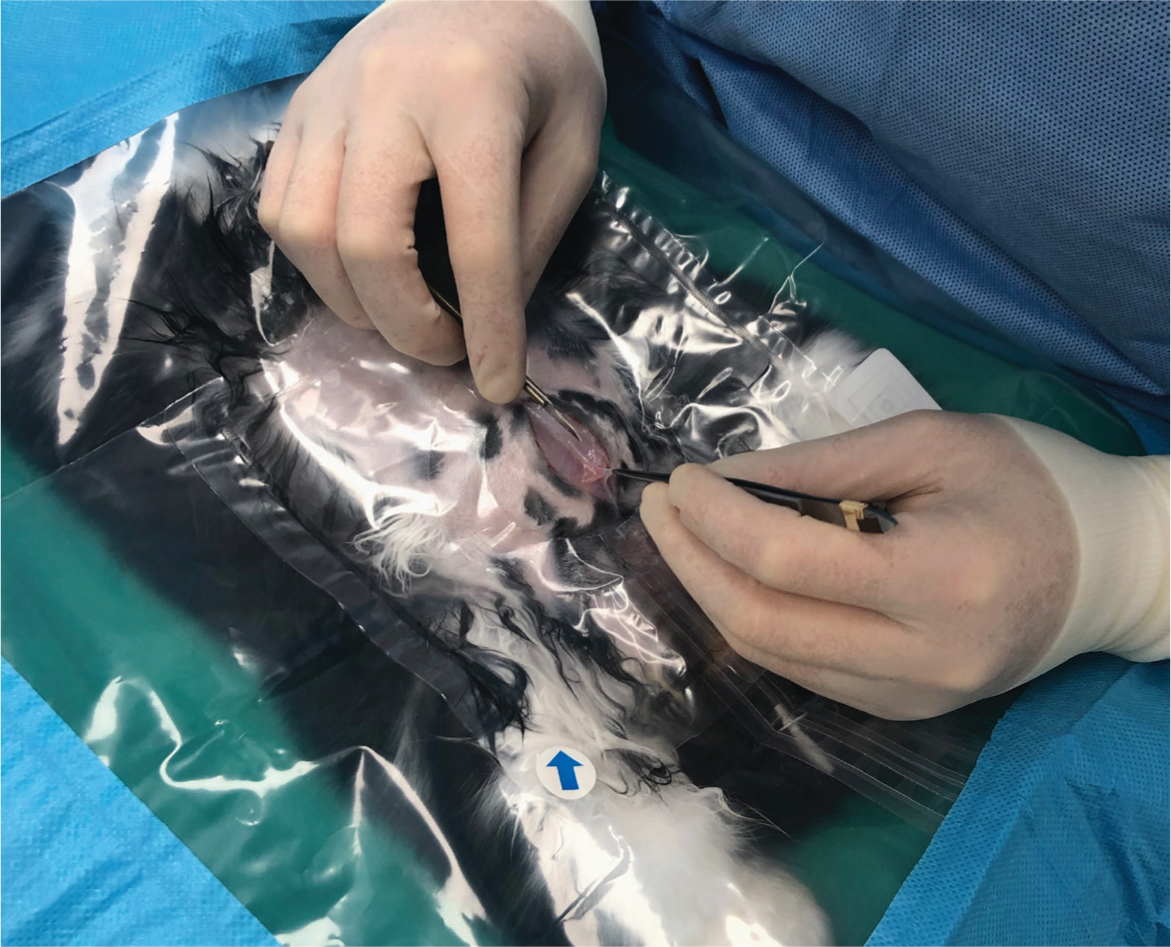
Ideal draping material should be impermeable to fluid, resistant to mechanical damage and remain in place during manipulation (Kroner et al, 2016). This author recommends using transparent, slightly adhesive drapes with a fenestration that matches the size of the proposed incision site, surrounded by an absorbent edge that covers the rest of the operating table. The transparent centre, laid over the patient, allows full visualisation for accurate anaesthetic monitoring (Figure 2). These drapes are also good at trapping heat, thus aiding in intraoperative thermoregulation. Other intraoperative methods of keeping the patient warm include thermostatically controlled conductive heat (heated surgical tables or water circulating heating pads) or the Bair Hugger forced air system for longer procedures and very small patients (Fisher, 2005).
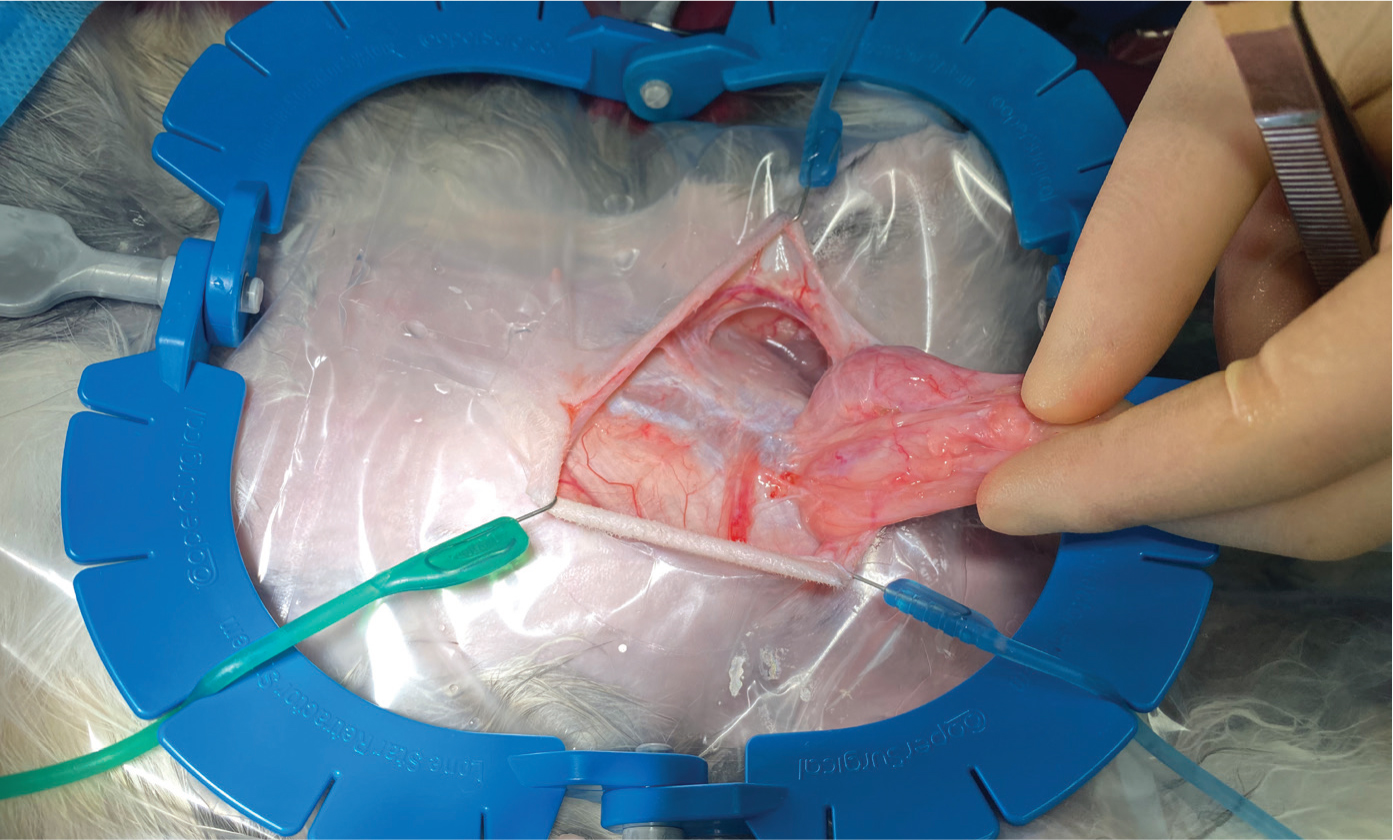
For most procedures, this author uses a standard feline spay kit. It is advisable to have easy access to microsurgical instruments, containing at least microsurgical needle holders, scissors and thumb forceps. Microsurgical instruments can have rounded handles and are counterbalanced for better hand control, resulting in less muscle fatigue and more precise movement. These items will often a lack a locking mechanism, which tends to allow more precise control needed during more intricate surgeries (Fisher, 2005; Kroner et al, 2016). This author finds a Lone Star Retractor an extremely useful tool for improving surgical site exposure in rabbit surgeries (Figure 3). The plastic frame is size-adjustable, can be autoclaved and the elastic stays with a hooked end can be placed at the incision margin (Redrobe, 2002; Kroner et al, 2016). For many surgical procedures in rabbits, magnification is strongly recommended for proper visualisation of the surgical field (Kroner et al, 2016). Loupes provide the added benefit of allowing the surgeon to maintain an ergonomically correct position (Kroner et al, 2016).
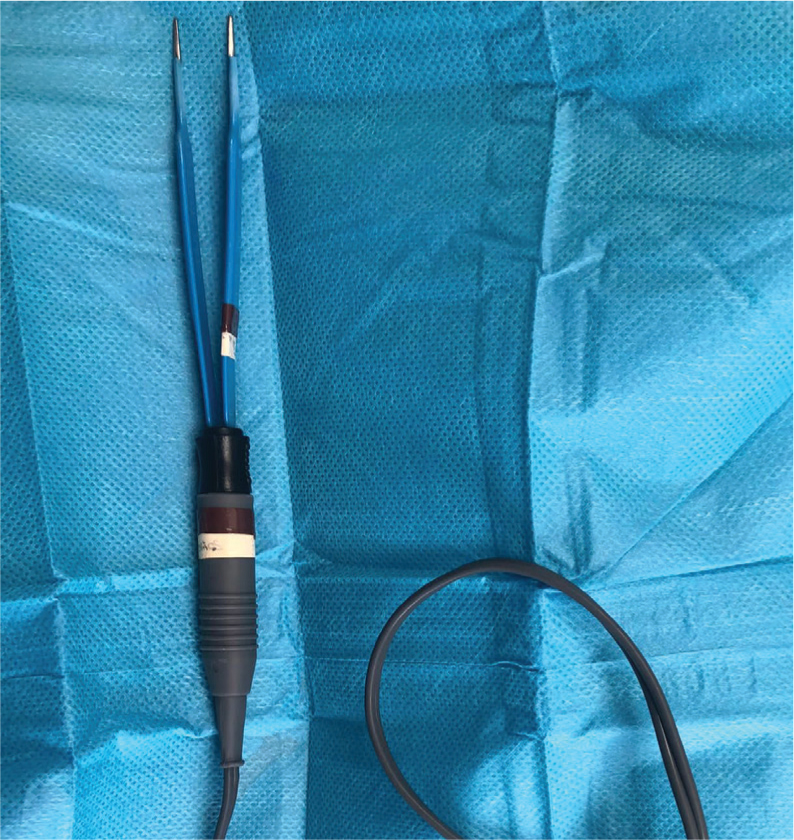
On average, the rabbit has a blood volume of 57 ml/kg body weight (5.7% bodyweight) (Redrobe, 2002). Loss of 15–20% of the total blood volume results in tachycardia and arterial constriction, compromising the blood supply to the skin and gastrointestinal tract, with an acute 25% loss potentially proving fatal (Redrobe, 2002). As such, haemostasis is an incredibly important factor that should not be overlooked. This author recommends using a combination of gauze pads, cotton tip applicators, haemostatic sponges, as well as bipolar cautery or a Ligasure (Figure 4). The Ligasure has been shown to be more efficient and cause less thermal injury than conventional bipolar electrocautery when used for coagulating rabbit gastric veins and arteries in an experimental study (Chassang and Bismuth, 2019).
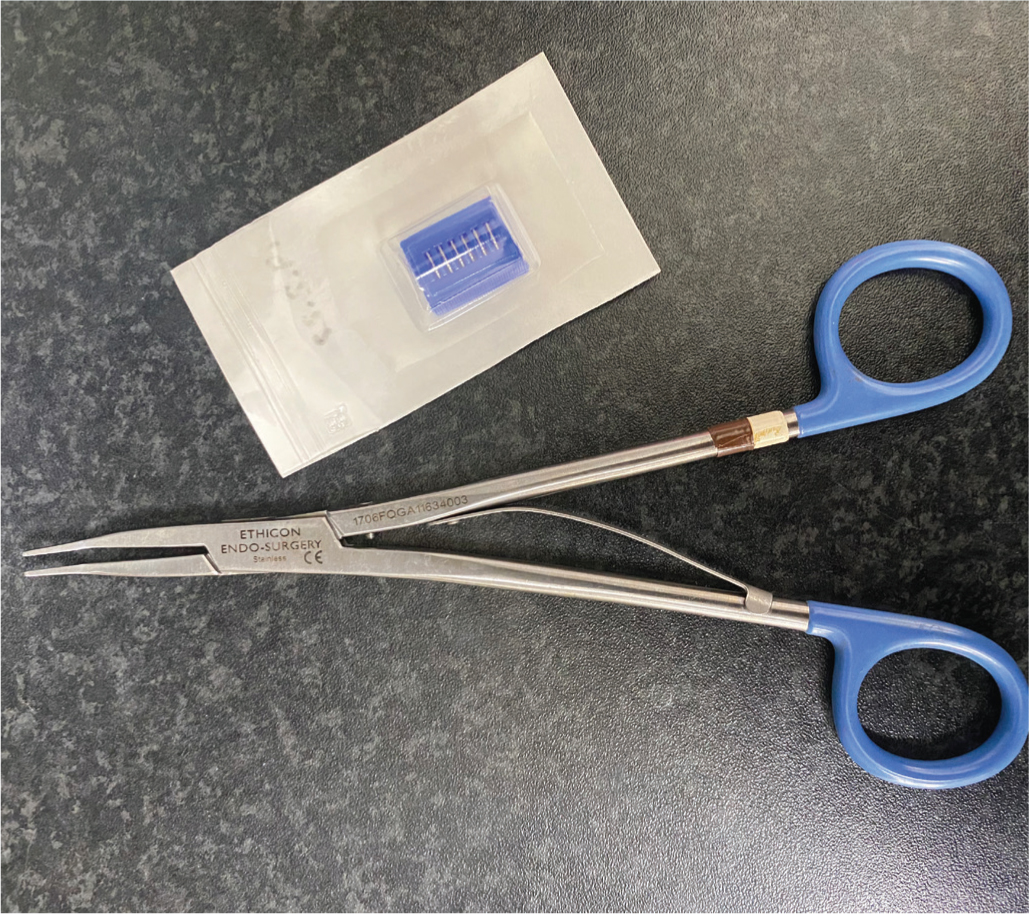
A surgeon's goal when choosing a suture material is to diminish loss of tissue function and limit the adverse effects of tissue repair (Kroner et al, 2016). As rabbits are particularly prone to adhesion formation, absorbable, monofilament suture material, such polydioxanone (PDS, Ethicon) and poliglecaprone (Monocryl, Ethicon), between 3-0 and 6-0 are most commonly used (Setzen and Williams, 1997; Graham, 2004; Kroner et al, 2016). Polypropylene (Prolene, Ethicon), although monofilamented but nonabsorbable, produced a relatively thick capsule response, perhaps because of its stiffness and rigidity (Setzen and Williams, 1997; Graham, 2004). This author also routinely uses small and medium haemostatic clips (Ligaclip, Ethicon) and the appropriate size applicators to quickly and efficiently ligate blood vessels (Figure 5). Although it is less common than in other exotic mammal species such as rodents in this authors experience, rabbits may still attempt to remove external sutures, and as such this author advises all skin sutures to be placed in a subcuticular or intradermal suture pattern, as well as a gentle, atraumatic tissue handling during any surgery.
Skin surgery
When considering rabbit skin tumours, basal cell tumours (trichoblastomas) were the most frequently diagnosed at 20%, with spindle cell sarcomas at 9.5%, collagen nevi/hamartomas at 8.4%, squamous papillomas at 6.4%, and mammary carcinomas at 6% out of 672 cases (Kanfer and Reavill, 2013). The same study showed that 46% of all cutaneous neoplasms in rabbits were malignant (Kanfer and Reavill, 2013). All skin masses should be removed as soon as they are noticed, using the same techniques used in cats and dogs, closing the skin as mentioned previously.
Because rabbits have well-encapsulated and caseated abscesses, management can be quite challenging (Graham, 2004). Complete surgical excision of skin abscesses is recommended as the best treatment option, often in conjunction with adjunct antibiotic therapy (Graham, 2004).
Nasal surgery
Surgeries performed on the nasal cavity of rabbits include rhinotomies and rhinostomies. This author suggests that the position and number of osteotomies must be planned before surgery, by using advanced imaging such as computed tomography scanning. The size of the osteotomy should be tailored to the reason for the rhinotomy (eg rhinoscopy, biopsy sample, microbial culture, exploratory rhinotomy, debridement or catheter placement) (Lennox, 2012; Jekl, 2021). When performing either a dorsal or lateral rhinotomy, the patient should be intubated, placed in an appropriate orientation and the surgical site shaved and aseptically prepared (Lennox, 2012; Jekl, 2021). This author advises the use of a local block along the line of the incision into the periosteum. The soft tissues are then blunt dissected to expose the underlying bones. The periosteum is gently lifted with a periosteal elevator – a Lone Star Retractor can be used to hold the soft tissues away from the underlying bone (Figure 6). A unilateral or bilateral osteotomy of the nasal bone is performed using a dental bur, remaining as central as possible. If debriding, often using a Volkmann curette (Figure 7), the presence of haemorrhage should be closely monitored.
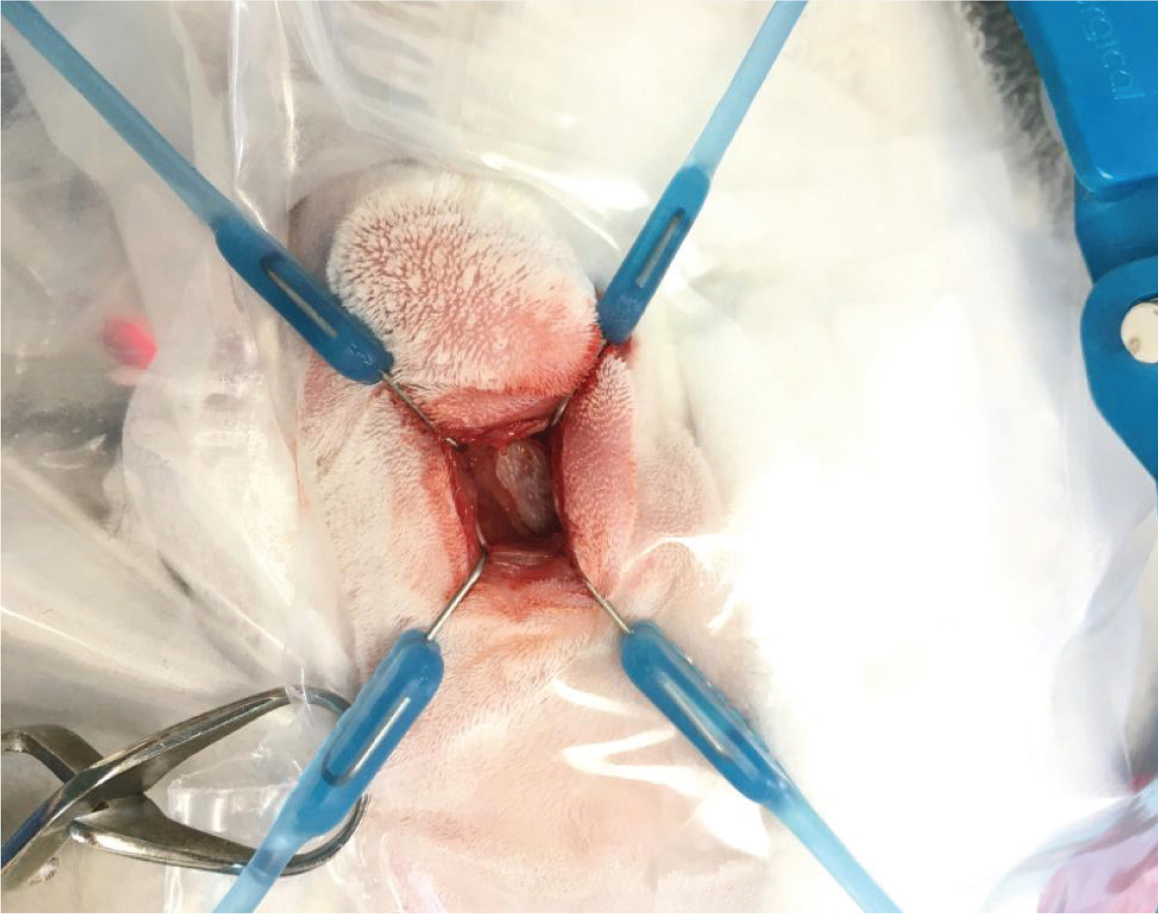

The area should be thoroughly flushed with warmed saline after suitable sampling, with the patient's nares tilted down slightly to prevent a build-up of the fluid being used to flush the area, as well as aspiration. Once complete, a suture can be used to close the periosteum and skin over the surgical site; however, the periosteum is fragile, so this can be difficult (Lennox, 2012).
When continued post-operative flushing is needed, a temporary rhinostomy can be considered (Lennox, 2012; Jekl, 2021). This is performed in the same way as the rhinotomy, but instead of a full closure, the skin is sutured around the stoma or an implanted, fenestrated urinary catheter. This can be used by an owner at home for days or weeks post-surgery. If an indwelling catheter is left in place over time, the periosteum will often heal over the fenestrations of the catheter, followed by skin granulation (Lennox, 2012). Continued flushing and administration of antibiotic therapy can continue indefinitely through this site (Lennox, 2012).
Nasogastric tube placement
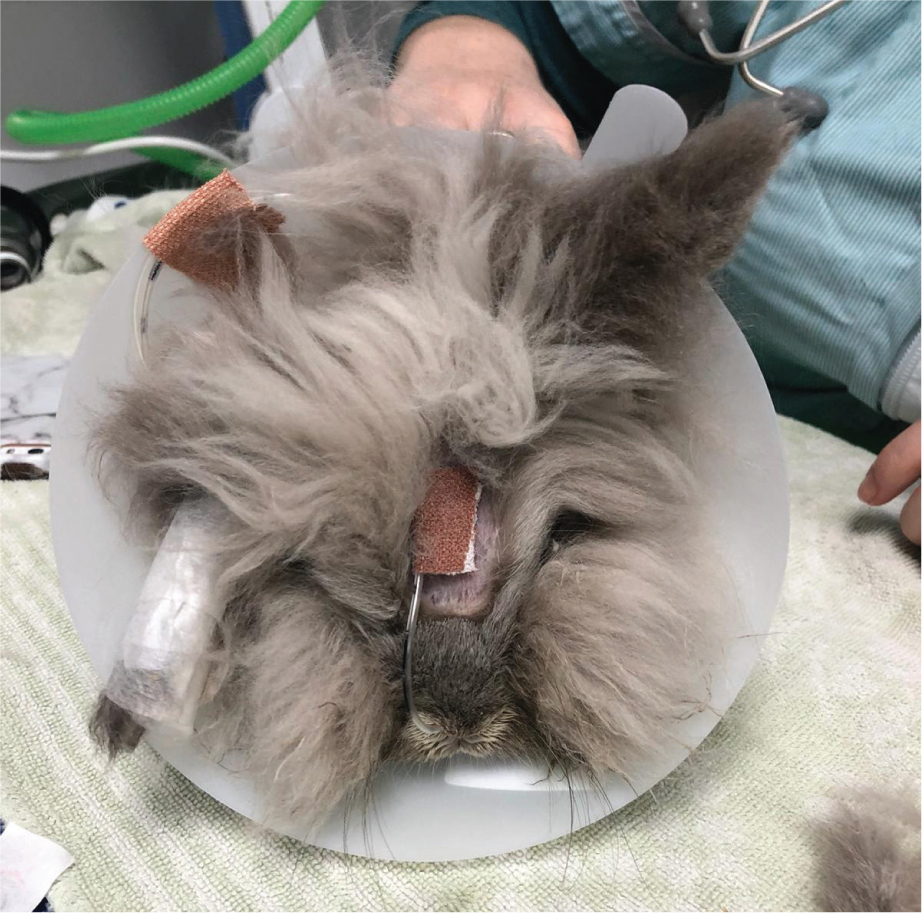
This author uses nasogastric tubes in patients that are unable to take routine support feeding, usually after oral surgery. A 3-5 French feeding tube, placed under a light plane of sedation or local anaesthesia is usually used. The largest tube possible should be used while not causing iatrogenic trauma (Rosen, 2011). The tube is carefully inserted through the ventromedial nasal meatus and passed ventrally and medially, while the patient's head is gently flexed, to a pre-measured point. Placement should be confirmed using radiography (Ritzman, 2014). The tube can be secured in place by suturing a tape tab to the top of the patient's head. The patient should then be fitted with an Elizabethan collar to prevent the patient from scratching or removing the tube (Figure 8). If the patient has upper respiratory tract disease, the placement of a nasogastric tube is not appropriate (Rosen, 2011).
Oral surgery
Understanding rabbit dental anatomy and physiology is essential for accurate diagnosis, treatmentand assessment of prognosis of conditions involving the oral cavity (Martínez-Jiménez et al, 2007). Having access to proper dental equipment such as appropriate low speed dental burs, lighting, magnification, a mouth gag, cheek retractors, a dental spatula, Crossley luxators for incisors and cheek teeth, as well as extraction forceps is also essential (Capello, 2016a) (Figure 9). Rabbits have continuously growing (elodont) teeth with long crowns (hypsodont) and no anatomic roots (aradicular) (Capello, 2005; DeCubellis, 2016). Therefore, if a rabbit is either born with, or develops, a malocclusion, its teeth will overgrow, leading to maladjustment of the occlusal angle with impaired mastication and potential lingual and buccal mucosal trauma that will require interventions (DeCubellis, 2016) (Figure 10). All dental interventions should be performed under an appropriate level of sedation or anaesthesia to allow accurate corrections and minimise the patient's stress (Crossley, 2000; Graham, 2006).

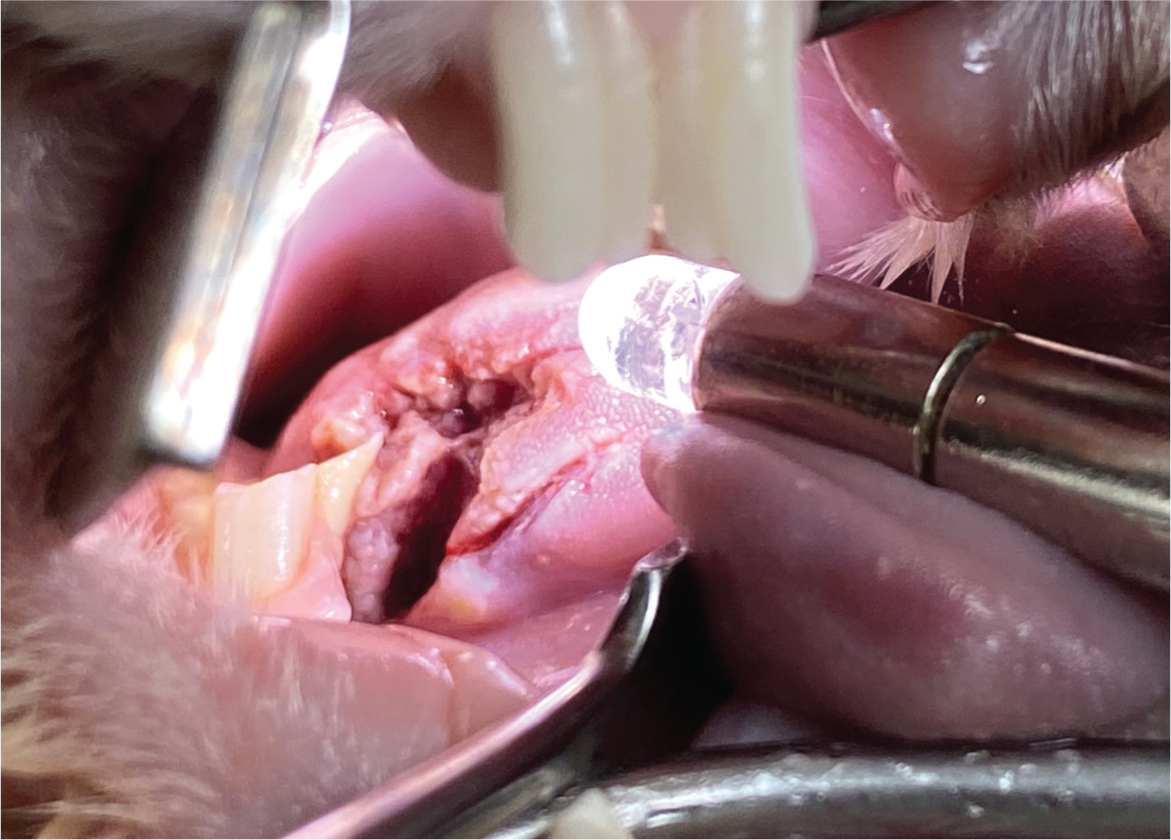
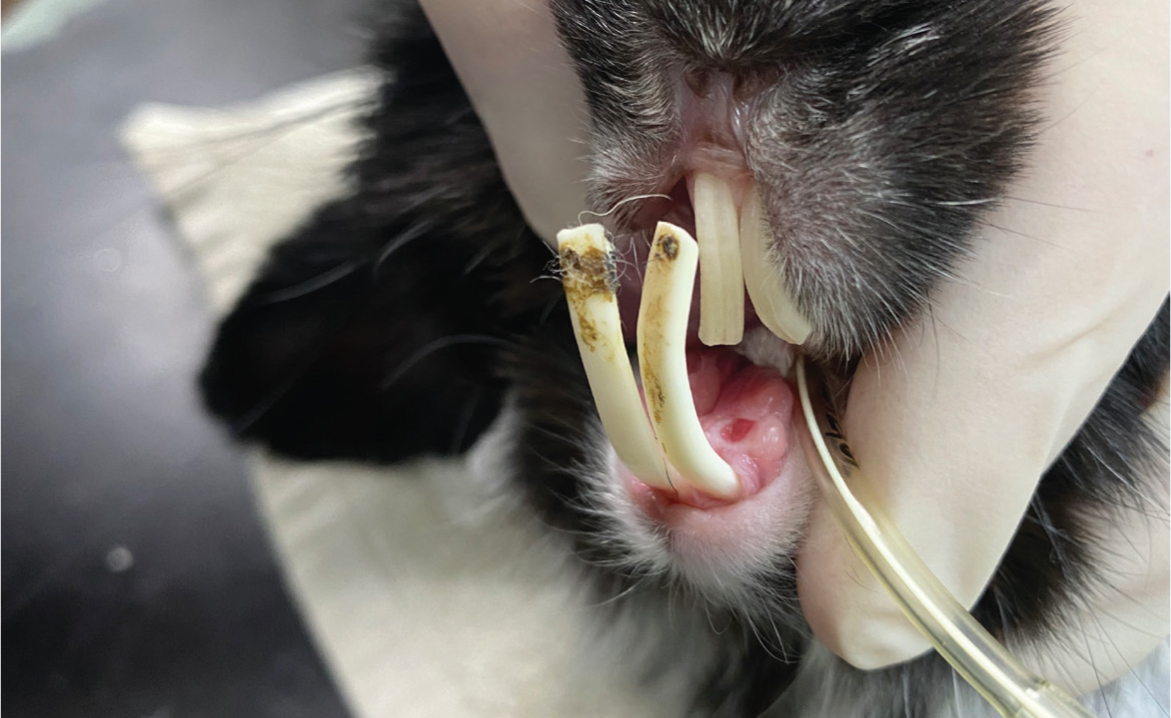
There are few circumstances in which this author would consider removing the teeth of a rabbit. These include persistent malocclusion of incisors (Figure 11), where regular trimming is not an option or when teeth are fractured, wobbly or associated with an apical root infection. This author finds that incisors can be extracted whilst still allowing the rabbit a good functional prognosis; however, when dealing with cheek teeth, the goal of therapy should be retention of as many cheek teeth as practically possible because their primary role is to crush food (Fisher, 2005). When removing incisors, this author uses an 18-guage needle to incise through the gingival attachments around each of the incisor teeth. Crossley luxators are then inserted into the periodontal space to break down the periodontal ligaments with gentle, repeated rotation of the instrument (Figure 9b). Once the tooth is loose, extraction forceps can then be used by grasping the tooth as close to the gingival margin as possible and slowly increasing force, following the curvature of the root (Figure 12), and with slight torsional movements applied to the tooth. After extraction, damage of the geminal tissue is performed by inserting a needle into the alveolus to curette the alveolar walls, which helps to prevent possible tooth regrowth (Fisher, 2005). The gingiva can then be closed using 4-0 absorbable suture material such as poliglecaprone (Monocryl, Ethicon). However, this is not recommended if there is evidence of infection. The same principles can be applied when removing cheek teeth, with the Crossley luxator for cheek teeth being used to break down the periodontal ligaments of all four aspects of the cheek teeth (Figure 9c). Ease of removal will vary depending on the degree of disease present, but this can be challenging as a result of the short clinical crown and long reserve crown.

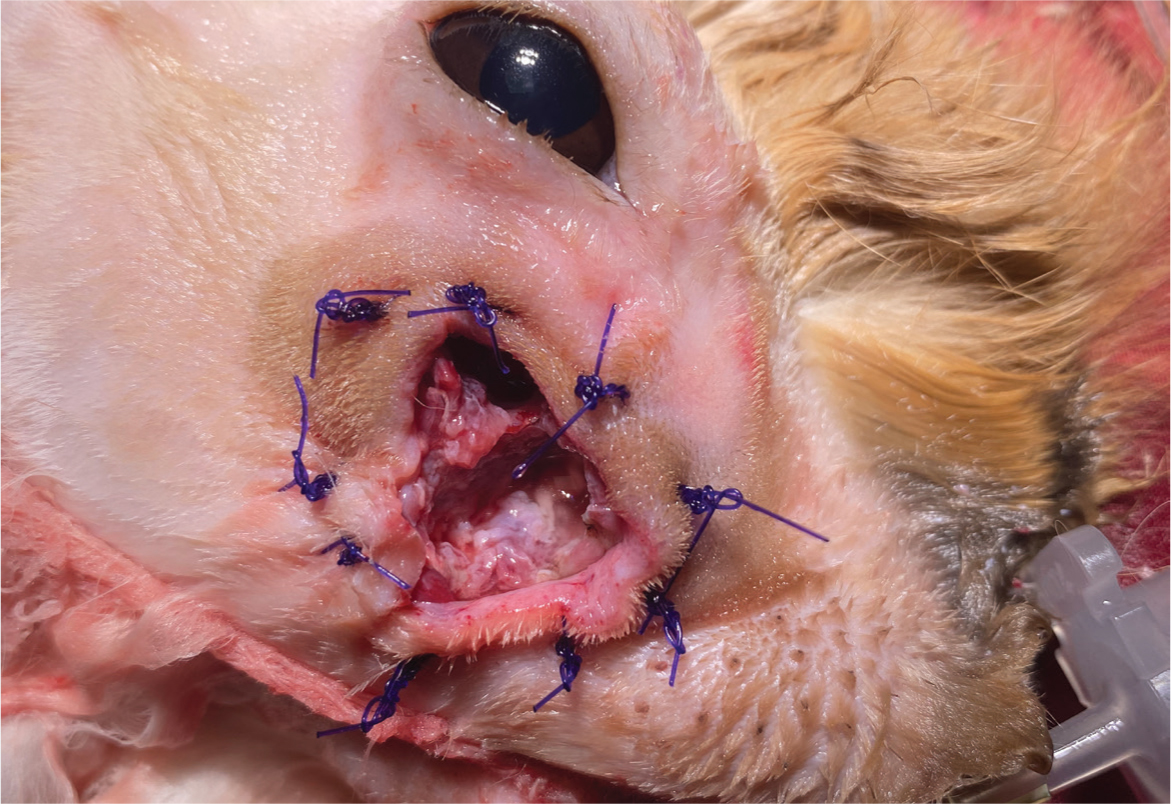
When approaching rabbit abscesses, complete surgical excision should be attempted where possible; however, this is not usually possible in periapical abscesses (Reusch, 2005). Medical therapy alone is unrewarding, although it is an important adjunct to the dental and surgical treatment (Capello, 2016b). The aim of surgical treatment is to remove as much of the abscess and its capsule as possible, associated tooth roots and any osteomyelitic bone, which will frequently involve a combined intra and extraoral approach (Reusch, 2005; Capello, 2008; 2016a). Marsupialisation of the soft tissues around the area of the affected bone is this author's treatment of choice, as it allows for continued post operative flushing, application of antiseptics and constant direct monitoring of the healing of the wound (Figure 13). During debridement of deep tissue, this author recommends obtaining samples for culture and sensitivity testing to allow a targeted antibiotic therapy.
Ophthalmological surgery
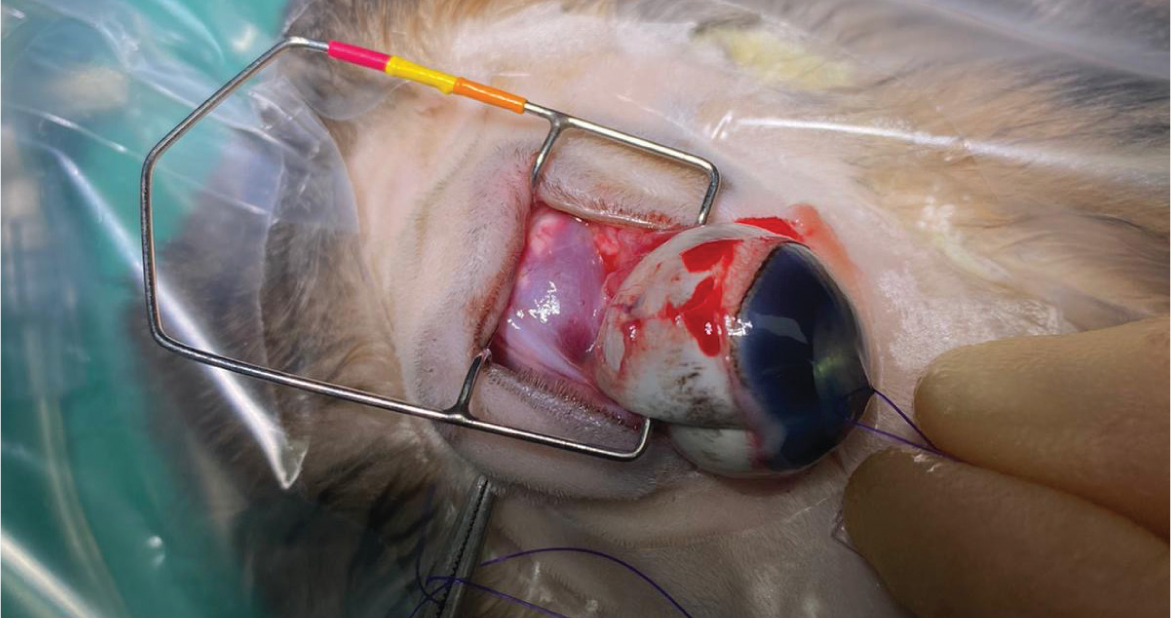
The three surgical techniques described for enucleation are the transconjunctival, transpalpebral and lateral approach (Holmberg, 2007). The transpalpebral and lateral approaches are associated with a reduced chance of leaving neoplastic or infectious material from the globe or adnexa within the orbit (Holmberg, 2007). However, because of the inherent risks of haemorrhage in rabbits because of the presence of a large venous sinus (Redrobe, 2002), the transconjunctival enucleation is preferred (Mullen, 2000; Redrobe, 2002; Holmberg, 2007; Diehl and McKinnon, 2016). The fur around the eye should be removed and the surgical site and surfaces of the eye cleaned with 2% povidone-iodine solution (Videne, Ecolab). An eyelid speculum should be placed to allow clear visualisation of the extraocular muscles, which are sharply dissected with tenotomy scissors at the point of insertion to the globe, thereby decreasing intraoperative haemorrhage (Holmberg, 2007; Diehl and McKinnon, 2016). Once all muscle attachments are released, the globe should rotate freely in the orbit (Holmberg, 2007) (Figure 14), and a haemostatic clip can be placed on the optic nerve and associated blood vessels to allow removal of the globe. Moore et al (2020) found the use of the LigaSure electrosurgical vessel-sealing device to be superior to previously described methods of haemostasis during enucleation in rabbits. Care must be taken not to cut the posterior sclera, especially in animals with septic endophthalmitis or an intraocular tumour, to avoid contamination of the orbit (Holmberg, 2007). After the globe has been removed, the conjunctiva, third eyelid and associated glandular structures as well as the eyelids should be removed. It is at this stage that haemorrhage is most likely to occur in this author's experience. Failure to completely remove these structures and their associated glands can result in cyst formation postoperatively (Redrobe, 2002; Holmberg, 2007; Diehl and McKinnon, 2016). If there is haemorrhage, direct pressure and use of haemostatic sponges can help encourage clot formation. The skin should be apposed and closed in an intradermal pattern using an absorbable monofilament suture material (Figure 15).
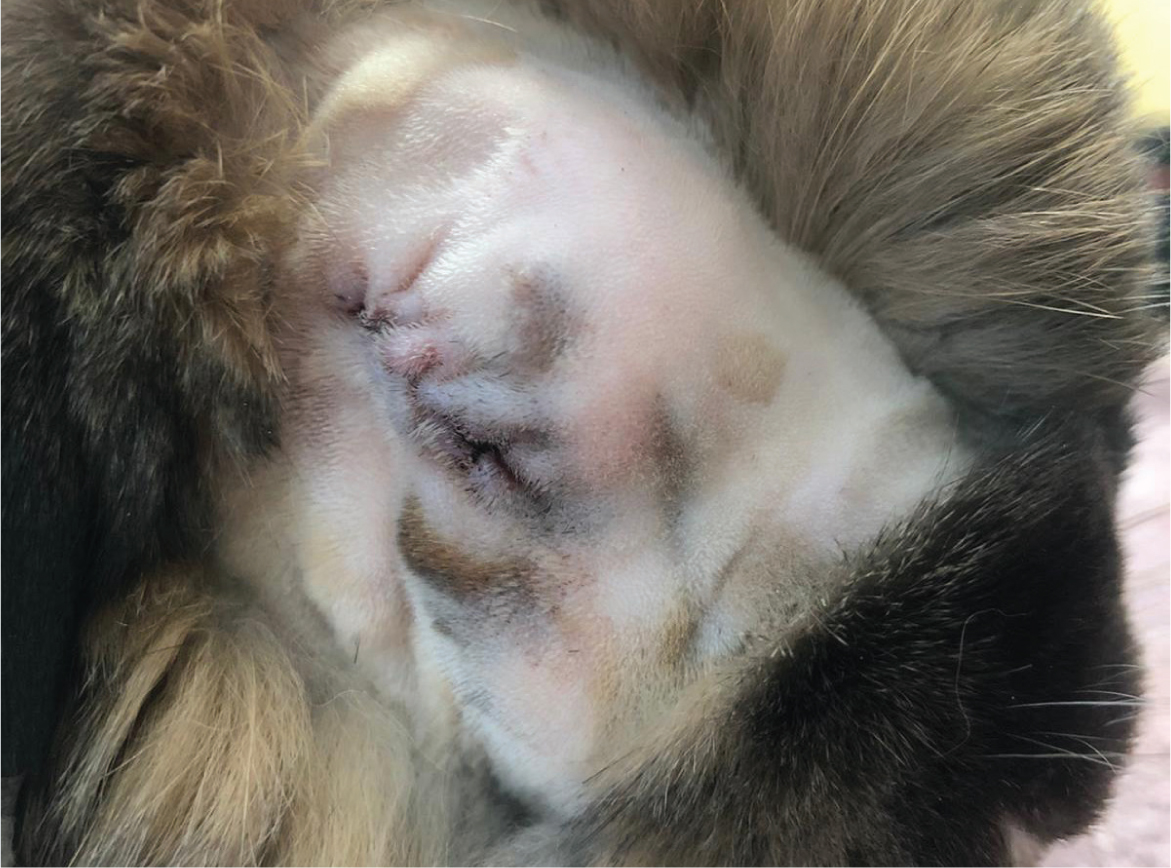
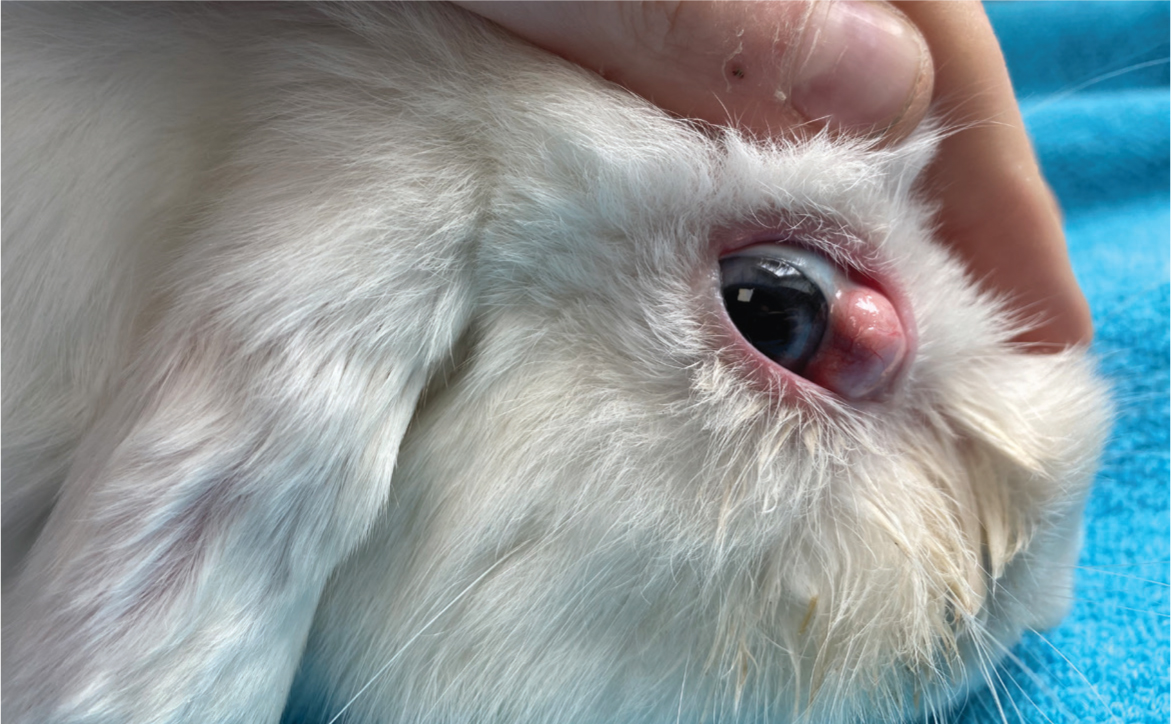
Prolapse of the deep gland of the third eyelid results in a large tissue mass protruding from under the third eyelid in the medial canthus of the eye (Wagner and Fehr, 2007) (Figure 16). Surgical replacement of the gland involves using the pocket technique described by Morgan and co-workers (Morgan et al, 1993; Janssens et al, 1999; Capello, 2016c), and is this author's preferred technique for replacement. Patient preparation is the same as for an enucleation. Using two fine-tipped haemostats, the free edge of the nictitating membrane is grasped and gently retracted, further exposing the prolapsed gland. Using a fine pair of forceps, the mucosal surface is grasped and pulled over the deep gland to confirm there is enough tissue to create a pocket prior to incisions. Two approximately 5 mm incisions are made posterior to the nictitans conjunctiva, parallel to either side of the prolapsed gland, about 2–3 mm from each side of the gland (Capello, 2016c). The mucosal incisions are then sutured over the prolapsed gland with monofilament, absorbable 5-0 poliglecaprone suture (Monocryl, Ethicon). A needle is passed through the lateral margin of the lateral incision, then twice through the medial margin of the medial incision, in a horizontal mattress fashion (Capello, 2016c). The prolapsed gland can then be gently reduced using a wet cotton-tipped applicator, while the horizontal mattress suture is simultaneously tightened over the gland on the posterior aspect of the nictitans (Capello, 2016c). The nictitans can then be reduced with a single transfixing suture through the ‘T-shaped’ cartilage using the same absorbable 5-0 poliglecaprone suture, anchoring the nictitating membrane to the periosteum of the orbital bone to help prevent re-prolapse (Capello, 2016c). Before tying off this anchoring suture, the opening of the nasolacrimal duct must be identified to ensure patency (Capello, 2016c).
Aural surgery
Unfortunately, medical management alone for rabbit otitis externa and media often yields poor results (Eatwell et al, 2013), not only because of inadequate drug penetration into the external and middle ear but also because of inappropriate antimicrobial selection (Monge et al, 2023). Sampling the tympanic cavity (before surgery), to identify bacteria and determine their antibiotic susceptibility is rarely performed because it requires anaesthesia and a myringotomy (Monge et al, 2023). There are a number of surgical approaches to rabbit ear disease reported in the literature, with lateral ear canal resections being recommended for persistent otitis externa (Chow, 2011) (Figure 17). Multiple approaches have been reported for the management of otitis media (Mancinelli and Lennox, 2017), including ventral and lateral bullae osteotomies (Chow et al, 2009; Chow, 2011; Csomos et al, 2016) and total ear canal ablations, with bullae osteotomies (Chow, 2011; Chow et al, 2011). Partial ear canal ablations with lateral bullae osteotomies have historically been favoured when both external and middle ear disease have been discovered in rabbits (Mancinelli and Lennox, 2017) (Figure 18).
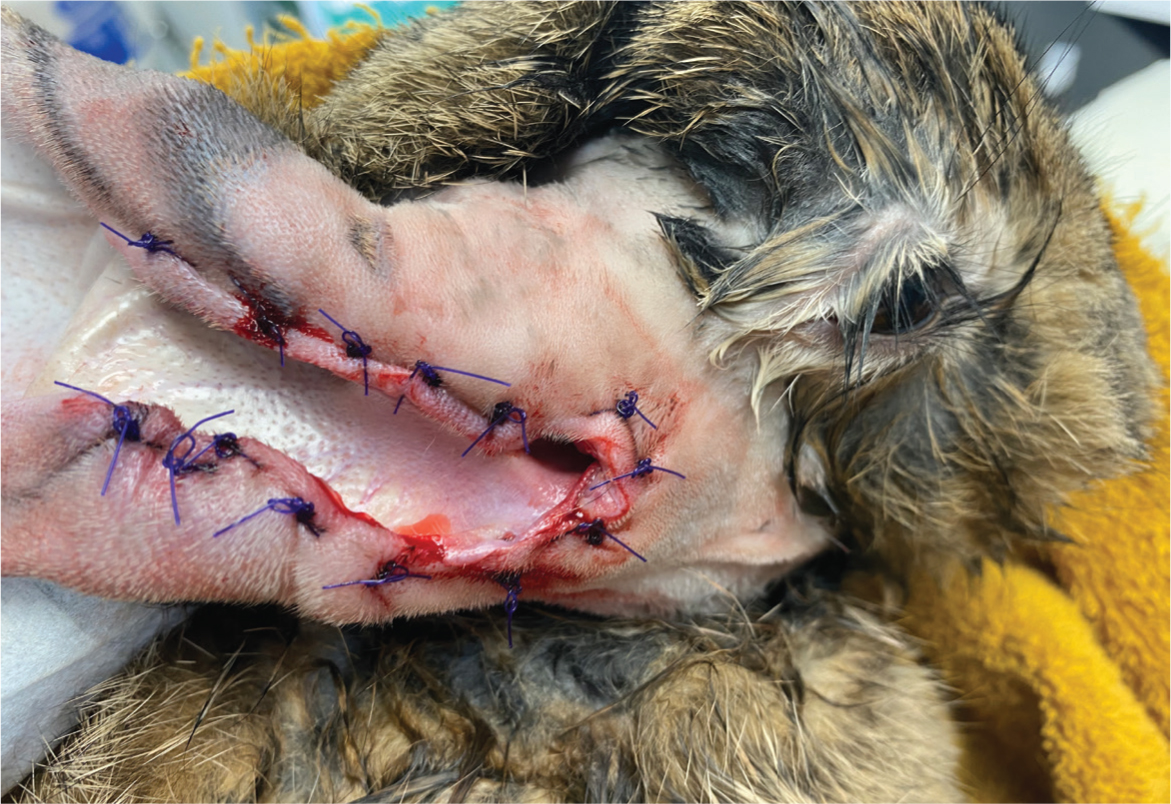
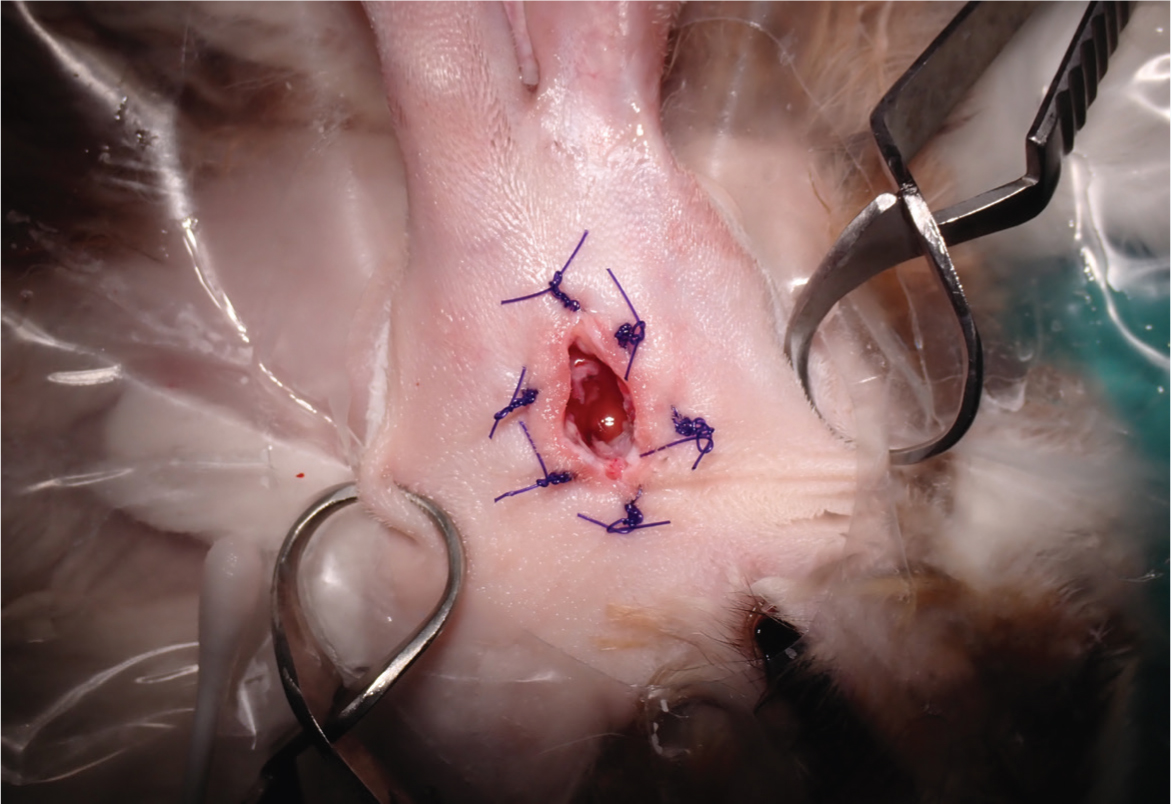
This author recommends lateral ear canal resections are performed when there is evidence of external ear disease only, with the surgery (predominantly performed in lop ear rabbits) aiming to increase drainage and ventilation of the ear canal. Monge et al (2023) discussed combining a lateral ear canal resection with marsupialisation of the tympanic bullae. This procedure has been adopted by this author over partial ear canal ablations with lateral bullae osteotomies because of improved results. When performing a lateral ear canal resection with marsupialisation of the tympanic bullae, the rabbit should be placed in lateral recumbency, after the ear has been clipped and aseptically prepared (both inside and outside of the ear). Two parallel skin incisions are made lateral to the ear canal from the base of the ear to the pretragic notch and intertragic notch while avoiding the blood vessels that supply the pinna. A long-stemmed cotton tip applicator is placed into the ear canal to the junction of the ear canal cartilage and annular cartilage, allowing approximate guidance to the position of the tympanic bulla opening. An incision is made at this site to join the latera parallel skin incisions. The skin flap is removed, and the sub-cutaneous tissues dissected from the auricular cartilage, allowing exposure of the tragal and ear canal cartilages. Two parallel cuts in the lateral wall of the tragal and ear canal cartilages are made. This cartilage flap is transected ventrally at the opening of the annular cartilage and excised. Care must be taken to separate the ear canal epithelial lining from the tragal and ear canal cartilages along the cut edge of the ear canal, so that it can be reattached to the skin without tension on the wound. The annular cartilage is then bluntly freed from the surrounding tissue and excised, exposing the osseous part of the ear canal. Rongeurs are used to remove the lateral aspect of the osseous ear canal and enlarge the tympanic bulla opening. Care must be taken during this step to avoid damaging the facial nerve. The tympanic cavity is curetted with a Volkmann curette (Figure 7). Care must be taken to avoid the dorsomedial aspect as this is where the vestibular or oval window of the inner ear is located. The tympanic cavity should then be gently flushed, after appropriate sampling, with warm sterile saline. The site can then be exposed during endoscopy to ensure the area has been fully debrided. The epithelial layer of the exposed ear canal is sutured to the skin overlying the pinna. Subcutaneous tissue around the bulla osteotomy opening is marsupialised to the surrounding skin using polydioxanone 3-0 suture (PDS, Ethicon) in a simple interrupted pattern.
Conclusions
It is essential to understand rabbits unique anatomical and physiological differences from the more commonly seen domestic species. It is advisable to pay close attention to haemostasis and tissue handling regardless of the level of intricacy of the surgery being performed. When performing rabbit surgery, monofilament, absorbable suture material is recommended by this author. The recommendations stated in this article should be at the forefront of the surgeon's mind when performing any of the surgeries mentioned in this and the next in the series of rabbit surgery articles.
KEY POINTS
- Make sure suitable equipment is ready ahead of time and available close to hand.
- Clinicians should understand the unique anatomical and physiological differences between rabbits and more commonly seen mammalian species.
- Ensure stress is minimised throughout and special care is taken to reduce the chance of hypothermia.
- Radiosurgery is an excellent aid to haemostasis in small mammals while simultaneously causing minimal collateral tissue damage.
- Monofilament, absorbable suture material is favoured in rabbits.


