Infestations by ectoparasites represent a significant threat to the health and welfare of dogs and cats. Ectoparasites can cause various dermatological signs, such as pruritus, allergies and even anaemia in heavy infestations (Beugnet et al, 2014). As an example, fleas can cause irritation, pruritus, hair loss and flea allergy dermatitis (Varloud and Hodgkins, 2015). Fleas can also act as vectors of pathogens such as Rickettsia spp. and Bartonella spp. and can serve as the intermediate host of the tapeworm Dipylidium caninum (Conboy, 2009; Bitam et al, 2010). Lice infestations can result in intense irritation, pruritus, and self-trauma. Scratching can lead to alopecia, crusts and secondary bacterial involvement (Pollmeier et al, 2002). Lice can also act as vectors for various disease-causing pathogens and, like fleas, the chewing louse (Trichodectes canis) can serve as an intermediate host of D. caninum. Ticks, in addition to causing irritation, feed on the blood of their host and can subsequently cause anaemia (Varloud and Hodgkins, 2015). Ticks can carry and transmit a number of pathogens while feeding on their host (Wengenmayer et al, 2014). A number of mite genera can also cause serious welfare concerns in companion animals, including Demodex, Sarcoptes, Otodectes, Notoedres and Cheyletiella (Becskei et al, 2016). Besides causing significant morbidity and mortality among companion animals, ectoparasites have a considerable economic impact because of the costs associated with treatment and control (Rafiqi et al, 2016).
It is important to treat and control ectoparasite infestations both for the health and wellbeing of animals and for public health reasons, given the zoonotic potential of some of these ectoparasites (Mencke, 2013). Ectoparasiticide treatments play a key role in minimising the impact of ectoparasite infestations, particularly by limiting the pathogens that can be transmitted by them (Geurden et al, 2017). A variety of ectoparasiticides from different chemical classes are commonly used in practice to prevent ectoparasite infestations. They come in the form of collars, spot-ons, sprays, injections and tablets (Maddison et al, 2008). Management of ectoparasite infestations can be achieved using chemical means (ectoparasiticides) and non-chemical means such as vacuuming the home environment, particularly during flea infestations (Rust and Dryden, 1997). In order to successfully control ectoparasite infestations, particularly fleas, it is necessary to implement an integrated pest control strategy designed to kill adult pests, disrupt the lifecycle and control environmental contamination (Beugnet et al, 2016). In addition to killing adult fleas using adulticides the use of combination products, or use alongside insect growth regulator (IGR) products such as lufenuron, improves environmental control of all lifecycle stages of flea populations. Although lufenuron is not technically an ectoparasiticide, it is widely used for flea control and is administered directly to the animal.
Ectoparasiticides are among the most commonly used drugs in companion animals. Over 40% of the global companion animal market is represented by parasite control, with half of this being for ectoparasite control (Witchey-Lakshmanan, 1999). In 2009, the market for antiparasitic drugs in carnivores was worth approximately US $3.5 billion, or 45% of the market for companion animal health (Beugnet and Franc, 2012). Unfortunately, some ectoparasiticides can cause morbidity and mortality from adverse reactions to the drug and the doses used in practice (Adelman et al, 2012). Additionally, confusion between products for canines and felines can lead to permethrin poisoning, with fatal consequences for felines (Malik et al, 2010). A Canadian survey regarding adverse effects of flea and tick control, as observed in cats and dogs, concluded that the most common adverse reactions to ectoparasiticides were of a dermatological, neurological or gastrointestinal nature (Turner et al, 2011).
A recent survey examined the association between isoxazoline ectoparasiticides and specific adverse events in dogs, based on the analysis of three separate data sets obtained from the USA Food and Drug Administration (FDA) and the European Medicines Agency's reported adverse events and Project Jake survey findings (Palmieri et al, 2020). Although this comparative analysis has some methodological limitations, the result suggested that isoxazoline products could be associated with neurological signs. However, it is important to recognise that most dogs and cats receiving isoxazoline treatments are not harmed. It is also worth noting that although the FDA provided a safety update for pet owners and veterinarians about the potential for neurological adverse events in dogs and cats treated with isoxazolines, but the FDA also considers isoxazoline products to be effective and safe. The expected benefits of any veterinary medicine should be greater than the risk of related harmful events (Dyer et al, 2010). However, it is essential for veterinarians and pet owners to remain vigilant and report any adverse drug events, because this information is necessary to support the evidence base for the risk-benefit profile of ectoparasiticide products.
The aim of this research was to review the frequency of adverse health events associated with the use of common ectoparasiticides in small animal practice. The frequency of adverse events to these drugs is summarised, together with an analysis of the most frequent adverse effects and most frequently affected systems. The study addressed the question ‘what are the adverse drug events associated with ectoparasiticides used in companion animals?’ Two hypotheses were investigated. First, the most common adverse drug events associated with spot-on ectoparasiticides are skin reactions and second, spot-on ectoparasiticides produce less adverse drug events than products with other methods of administration.
Literature search strategy and data sources
The primary search engine used for finding veterinary literature for this study was PubMed, covering the period from 1993 to 2017. ScienceDirect was also used for further papers if required, once the PubMed results were exhausted. The National Office of Animal Health (NOAH) compendium datasheet, website and handbook were used as references for information on the chosen drugs. Background reading using veterinary pharmacology textbooks was carried out to gain context, searching in the index for ‘ectoparasite’, ‘ectoparasiticide’, ‘adverse drug event’ and ‘drug-drug interaction’.
To obtain a wide overview of adverse drug events caused by ectoparasiticides for cats and dogs, each product chosen was categorised to a different chemical class. Because certain animals may develop adverse reactions to one or more ingredients in the product, only single active ingredient products were chosen for involvement in this study to ensure that any adverse event report is not complicated by possible involvement of multiple mechanisms of action. However, this does not preclude the possibility that adverse events were associated with inactive ingredients in administered products. The potential for inactive ingredients to be implicated in adverse events have been observed in other medications (Rivera et al, 2013; Reker et al, 2019). For an additional comparative overview, some products with different methods of administration were included in the analysis. Originally, when searching for information on each chosen drug, a filter for clinical trials only was applied when searching for keywords, but review articles were included when not enough results were obtained. Keywords for the searches included the scientific drug name or brand name, with the targeted parasite or companion animal. Examples of the searches made for Advantage™ spot-on are ‘imidacloprid and dog’, ‘imidacloprid and cat’ or ‘imidacloprid and flea’. The title was read to determine veterinary relevance and then the paper was read, if appropriate.
Where possible, results with only an abstract available to read were omitted. However, when there were no other accessible papers with the required information, abstracts were used. Care was taken not to confuse the drug being searched for with products containing a combination of drugs, such as imidacloprid plus moxidectin. The abstract was scanned for specific relevance to the drug in question and then the whole paper was read. If more information was required, the references in the paper were explored. The NOAH compendium was used in combination with research papers on each drug regarding pharmacokinetic properties and reports of adverse events.
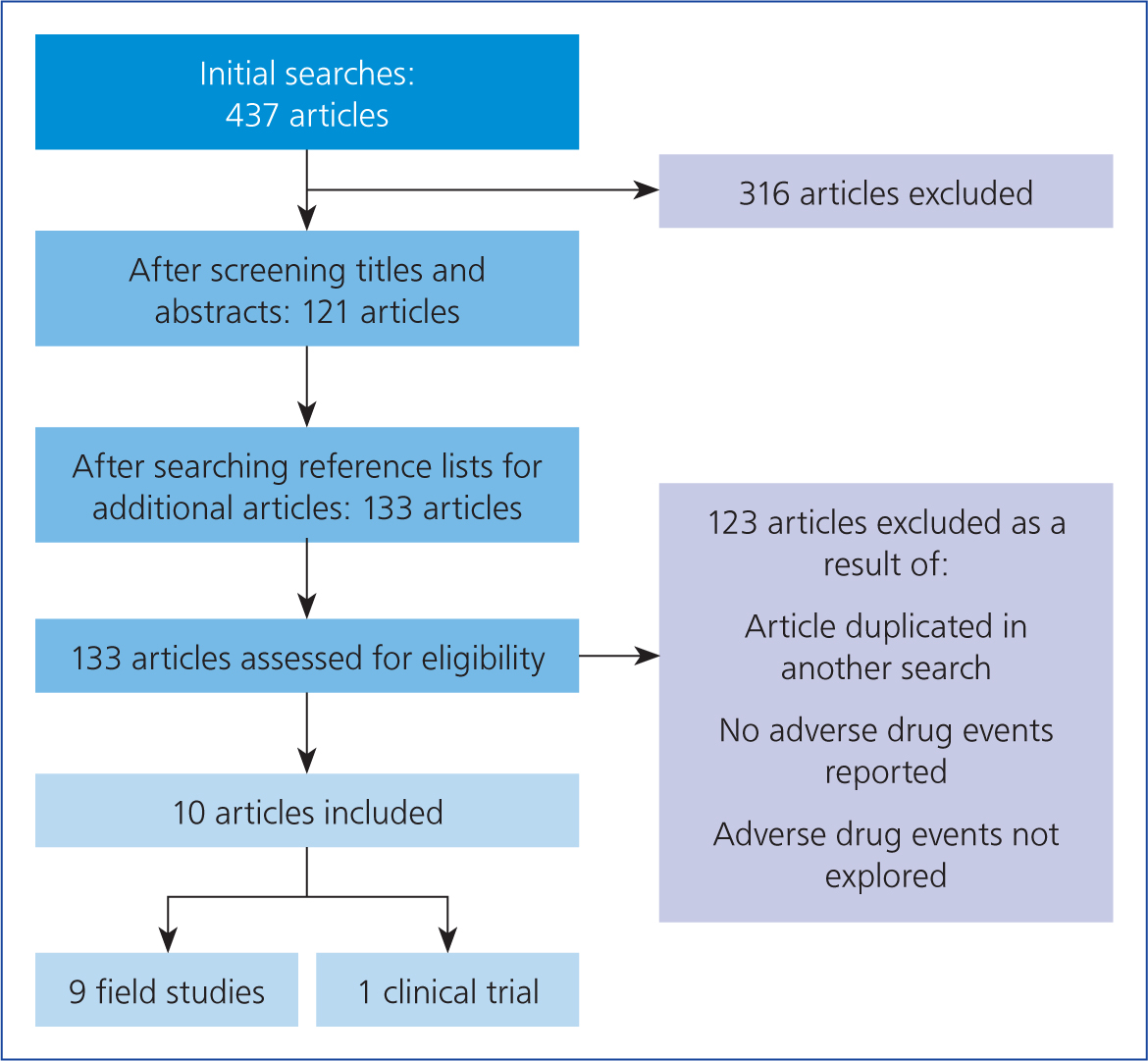
The search for articles reporting adverse drug events to the ectoparasiticides chosen for this review was undertaken between September and November 2017, and retrieved 437 potentially relevant citations. Screening of the title and abstract excluded 316 titles. Full papers were reviewed and duplicate citations were removed. Articles with no adverse drug events reported or mentioned were also removed, but were useful in terms of understanding the pharmacological actions of each drug. A total of ten citations met the inclusion criteria. Figure 1 shows the algorithm used for the inclusion or exclusion of articles.
Data analysis
One challenge in presenting the data was that the NOAH compendium of datasheets reported the frequencies of most adverse drug events using qualitative categories, instead of giving exact frequencies and percentages. Very common adverse drug events were classed at over 10%, common as less than 10% but more than 1%, and uncommon as less than 1% but more than 0.1%. Rare cases were classified as less than 0.1% but more than 0.01% and very rare was classed as less than 0.01%, including isolated reports. To overcome this, the adverse drug events reported by the NOAH were presented separately from those reported based on published studies in the veterinary literature. This was considered preferable to converting the numerical data from other studies to the same categories as the NOAH data, because this would compromise the precision of the results.
To ensure consistency in table presentation and ease of analysis, similar reactions were grouped together, for example, adverse drug events reported as transient itching or pruritus were all recorded as pruritus in the tables. Similarly, reactions reported as hypersalivation or drooling were all recorded as drooling and decreased appetite was recorded as inappetence.
The clinical signs of each reaction were categorised by body system of presentation. Some clinical signs could be allocated to more than one body system. Reports of skin reactions, such as pruritus, alopecia, crusts, scabs and lesions were all categorised as skin reactions. Polydipsia was categorised as a urinary system reaction. Vomiting, diarrhoea, inappetence, drooling and flatulence were all classified as gastrointestinal reactions. Signs classified as neurological included lethargy, apathy, tremors, neuromuscular problems, depression, nervous signs, disorientation, agitation and hyperaesthesia.
Discussion
This literature search was undertaken to obtain an initial idea about the prevalence of adverse drug events associated with the use of ectoparasiticides in cats and dogs. The following section summarises the observed frequency of adverse drug events associated with products representative of different chemical classes of ectoparasiticides.
Prevalence of adverse drug events in spot-on ectoparasiticides
As shown in Table 1, based on the NOAH compendium, skin reactions were the most prevalent adverse events associated with a topical isoxazoline treatment (Bravecto™ Spot-on is used as the class example) for both cats and dogs. The collective prevalence of gastrointestinal signs according to the NOAH compendium is largely unknown, potentially because there is a low reporting frequency. The data reported in a field trial (Meadows et al, 2017) showed gastrointestinal signs associated with oral isoxazolines (Table 1). This is probably related to the fact that fluralaner is distributed systemically (Klip et al, 2016). However, vomiting is likely a normal dog activity and is not necessarily caused by or related to the drug exposure and a causal relationship could not be not established. Also, gastrointestinal signs occurred at roughly comparable rates between dogs treated with fluralaner and dogs treated with fipronil-methoprene (positive control) (Meadows et al, 2017).
Table 1. Adverse drug events to topical isoxazoline example product
| Species | Total number | Adverse event | Frequency | % | Study type | Reference |
|---|---|---|---|---|---|---|
| Adverse drug events to Bravecto™ spot-on, based on published studies | ||||||
| Cats | 224 | Vomiting | 17 | 7.6% | Field | Meadows et al (2017) |
| Pruritus | 12 | 5.4% | ||||
| Diarrhoea | 11 | 4.9% | ||||
| Alopecia | 11 | 4.9% | ||||
| Inappetence | 8 | 3.6% | ||||
| Lethargy | 7 | 3.1% | ||||
| Scabs/lesions | 5 | 2.2% | ||||
| Adverse drug reactions to Bravecto™ spot-on, based on the NOAH compendium | ||||||
| Cats | Skin reaction | Common | ||||
| Apathy | Uncommon | |||||
| Tremors | Uncommon | |||||
| Anorexia | Uncommon | |||||
| Vomiting | Uncommon | |||||
| Drooling | Uncommon | |||||
| Dogs | Skin reaction | Common | ||||
As shown in Table 2, the most common type of adverse event for topical fipronil (Frontline™ spot-on is used as the class example) was dermatological reactions in dogs. Skin reactions are an expected occurrence for spot-on products, as they are applied topically. All of the listed adverse drug events on the NOAH compendium for this drug are very rare. However, the long experience with use of this product may lead to a lower reporting effect.
Table 2. Adverse drug events to topical fipronil class example
| Species | Total number | Adverse event | Frequency | % | Study type | Reference |
|---|---|---|---|---|---|---|
| Adverse drug events to Frontline™ spot-on, based on published studies | ||||||
| Dogs | 178 | Alopecia and crusts | 3 | 1.7% | Field | Rohdich et al (2014) |
| Pruritus | 1 | 0.6% | ||||
| Cats | 88 | Drooling | 1 | 1.1% | Field | Geurden et al (2017) |
| Pruritus | 1 | 1.1% | ||||
| Adverse drug reactions to Frontline™ spot-on, based on the NOAH compendium | ||||||
| Dogs and cats | Skin reaction | Very rare | ||||
| Pruritus | Very rare | |||||
| Alopecia | Very rare | |||||
| Drooling | Very rare | |||||
| Hyperaesthesia | Very rare | |||||
| Depression | Very rare | |||||
| Vomiting | Very rare | |||||
| Dogs | Respiratory signs | Very rare | ||||
Nervous disorders in dogs were the most frequent events to a neonicotinoid class product (Advantage™ spot-on used as the example) in the NOAH compendium (Table 3), suggesting that the body system most commonly affected by imidacloprid was the nervous system. There were no additional studies found in veterinary literature describing adverse drug events associated with Advantage™ spot-on for dogs or cats.
Table 3. Adverse drug reactions to topical neonicotinoid example product based on the National Office of Animal Health compendium
| Species | Adverse reaction | Frequency |
|---|---|---|
| Dogs and cats | Skin reaction | Very rare |
| Agitation | Very rare | |
| Disorientation | Very rare | |
| Drooling | Very rare | |
| Nervous signs | Very rare | |
| Dogs | Nervous disorders | Rare |
Neurological signs were most common type of adverse event associated with an avermectin example product (Stronghold™ spot-on) (Table 4). However, this indication is based on only one small-scale study, so the validity is inconclusive. Also, there were no studies involving cats with adverse events, but the NOAH compendium reports alopecia in cats as the most common adverse event (Table 4).
Table 4. Adverse drug events to topical avermectin class product
| Species | Total number | Adverse event | Frequency | % | Study type | Reference |
|---|---|---|---|---|---|---|
| Adverse drug events to Stronghold™ spot-on, based on published studies | ||||||
| Dogs | 236 | Hair discolouration | 1 | 0.4% | Field | Six et al (2000) |
| Dogs | 11 | Lethargy | 1 | 9.1% | Field | Gunnarsson et al (2005) |
| Adverse drug reactions to Stronghold™ spot-on, based on the NOAH compendium | ||||||
| Cats | AlopeciaFocal irritation | RareVery rare | ||||
| Dogs and cats | Neurological signs | Very rare | ||||
Gastrointestinal reactions were the most common with an example bioactivated sodium channel blocker (Activyl™ spot-on) treatment in dogs (Table 5). Rare to very rare adverse events in cats included neurological signs, vomiting and skin reactions.
Table 5. Adverse drug events to bioactivated sodium channel blocker product
| Species | Total number | Adverse event | Frequency | Percentage | Study type | Reference |
|---|---|---|---|---|---|---|
| Adverse drug events to Activyl™ spot-on, based on published studies | ||||||
| Dogs | 32 | Lethargy | 1 | 3.1% | Field | Dryden et al |
| Inappetence | 1 | 3.1% | (2013) | |||
| Vocalisation | 1 | 3.1% | ||||
| Vomiting | 2 | 6.3% | ||||
| Adverse drug reactions to Activyl™ spot-on, based on the NOAH compendium | ||||||
| Cats | Neurological signs | Rare | ||||
| Vomiting | Rare | |||||
| Skin reaction | Rare | |||||
| Alopecia | Very rare | |||||
| Lethargy | Very rare | |||||
| Hyperactivity | Very rare | |||||
| Vocalisation | Very rare | |||||
| Dogs | Skin reaction | Very rare | ||||
| Dogs and cats | Drooling | Very rare | ||||
Prevalence of adverse drug events of ectoparasiticides associated with other methods of administration
According to the NOAH compendium, orally administered isoxazolines in dogs can be associated with common adverse gastrointestinal events, correlating to a frequency of less than 10% but more than 1% each (Table 6). This may be associated with their direct impacts on the canine gastrointestinal tract. Interestingly, a clinical trial reported a lower incidence of adverse events for Bravecto tablets compared to the control dogs treated with spinosad and amitraz collars (Meadows et al, 2014).
Table 6. Adverse drug events to an oral isoxazoline in dogs
| Species | Total number | Adverse event | Frequency | Percentage | Study type | Reference |
|---|---|---|---|---|---|---|
| Adverse drug events to Bravecto™ tablets, based on published studies in veterinary literature | ||||||
| Dogs | 383 | Vomiting/diarrhoea | 2 | 0.5% | Field | Rohdich et al (2014) |
| Inappetence | 2 | 0.5% | ||||
| Dogs | 224 | Vomiting | 16 | 7.1% | Field | Meadows et al (2014) |
| Inappetence | 15 | 6.7% | ||||
| Lethargy | 12 | 5.4% | ||||
| Diarrhoea | 11 | 4.9% | ||||
| Polydipsia | 4 | 1.8% | ||||
| Flatulence | 3 | 1.3% | ||||
| Adverse drug reactions to Bravecto™ tablets, based on the NOAH compendium | ||||||
| Dogs | Diarrhoea | Common | ||||
| Vomiting | Common | |||||
| Inappetence | Common | |||||
| Drooling | Common | |||||
| Convulsions | Very rare | |||||
| Lethargy | Very rare | |||||
Pain on injection is the most frequent adverse event for the Program™ injection for cats, suggesting that neurological adverse drug events are the most common (Table 7). Pain is expected with this method of administration, but it is short-lived and does not cause a serious health problem. A range of dermatological, gastrointestinal and neurological signs were found with use of the Program™ injection, oral suspension and tablet.
Table 7. Adverse drug events to insect growth regulator formulations
| Species | Total number | Adverse event | Frequency | % | Study type | Reference |
|---|---|---|---|---|---|---|
| Adverse drug events to Program™ injection, based on published studies in veterinary literature | ||||||
| Cats | 294 | Pain during injection | N/A | 16% | Clinical trial | Food and Drug Administration (1998) |
| Lump/granuloma | N/A | 8.5% | ||||
| Vomiting | N/A | 2.5% | ||||
| Listlessness/lethargy | N/A | 1.9% | ||||
| Diarrhoea | N/A | 1.3% | ||||
| Anorexia | N/A | 1.1% | ||||
| Adverse drug events to Program™ oral suspension, based on published studies in veterinary literature | ||||||
| Cats | 99 | Nausea | 1 | 1.0% | Field | Maynard et al (2001) |
| Adverse drug reactions to the Program™ injection, oral suspension and tablet, based on the NOAH compendium | ||||||
| Cats/injection | Skin reaction | N/A | ||||
| Lethargy | N/A | |||||
| Cats/oral suspension | Vomiting | N/A | ||||
| Diarrhoea | N/A | |||||
| Malaise | N/A | |||||
| Pruritus | N/A | |||||
| Dogs/tablet | Vomiting | Rare | ||||
| Diarrhoea | Rare | |||||
| Pruritus | Rare | |||||
| Nervous signs | Rare | |||||
As shown in Table 8, topical administration of a synthetic pyrethroid in a collar presentation (Scalibor™ Protectorband collar for dogs) was associated with skin reactions. This can be expected as a result of the collar being in constant contact with skin. A mixture of gastrointestinal and neurological signs have been less commonly reported. Studies available in veterinary literature did not report any adverse drug events associated with the use of this product.
Table 8. Adverse drug reactions to topical synthetic pyrethroid collar, based on the National Office of Animal Health compendium
| Species | Adverse reaction | Frequency |
|---|---|---|
| Dogs | Skin reaction | Rare |
| Altered behaviour | Very rare | |
| Vomiting | Very rare | |
| Diarrhoea | Very rare | |
| Drooling | Very rare | |
| Neuromuscular problems | Very rare |
Implications of the study findings
This analysis could not determine which animal species is generally more susceptible to adverse drug events. This is because reports from the NOAH compendium often do not concur with published studies in the literature.
The search was limited to PubMed/MEDLINE and ScienceDirect, and the scanning of titles and abstracts of the search results was performed by one researcher. Also, the reports found of adverse drug events may not be representative of the whole population. It is also uncertain whether the adverse events were caused by the reported drug, as many factors such as underlying disease or non-drug related causes could also be the reason.
Finding articles about field trials and laboratory tests on each of the drugs mentioned in this article was not possible and the availability of safety studies was highly variable for the different drugs. This has precluded head-to-head comparison between ectoparasiticides in the same chemical class. Therefore, the findings should not be extrapolated to the wider companion animal populations and one must be cautious about making any conclusions from the results of this study when comparing ectoparasiticides. There was considerable variability in the duration, sample size and settings of the studies analysed in the present article. There was also great variability in the reporting of the types of reactions identified, as well as in the drugs responsible for adverse reactions, making it impossible to produce a summarised report of the most common ones. The NOAH categories for frequency of adverse drug events lack accuracy, as one cannot determine the precise frequency of their occurrence. This made it difficult to determine if one drug event was more common than the others. To overcome this, the NOAH reports were combined with reports from field trials and laboratory tests available in veterinary literature. It was not possible to distinguish the single most common adverse drug event, as reports vary significantly in detail. Therefore, the study could not definitively confirm that the most common adverse drug events to spot-on treatments were skin reactions.
Recommendations for future directions
The study findings suggest that adverse drug events can be a significant problem in cats and dogs. However, the nature of pharmacovigilance means that there will always be adverse events with any type of treatment. Therefore, it is important to be aware of what to expect with treatment and also to watch for safety signals that identify those unexpected or potentially severe events that may occur more rarely. The importance of adverse drug events reports is highlighted in pharmacovigilance monitoring to continually assess the safety of licenced products. Reports for new drugs in the isoxazoline chemical class may reveal previously unidentified treatment-associated events, or highlight the true extent of the known adverse drug events. Given the growing interest in understanding the adverse events associated with ectoparasiticide treatment, more comprehensive systematic reviews and meta-analysis of adverse events reported would be helpful in the provision of more reliable estimates of their prevalence across different settings and populations. Further investigations in this area with a large number of field trials involving different products from different chemical classes could help to improve understanding of the potential factors that contribute to the occurrence of adverse events.
Future studies should consider other ectoparasiticides and compare the adverse events of drugs within the same chemical class. This analysis could be extended to other animals, such as horses and farm animals. Documenting the information about each animal that has an adverse event to a drug could provide vital information about the susceptibility to a reaction. Perhaps a particular breed, age, gender or weight could have a predisposition to having an adverse response to a certain drug. It is important to report any adverse events associated with a product used in field conditions and to produce practical advice to clients and practitioners about these adverse events. This allows clinicians and owners to be more prepared for such an event. Failure to do so may result in late recognition of adverse drug events in individual animals. Unfortunately, information about the individual characteristics was not available in the studies analysed in this article, so these factors could not be examined. The development of standardised ways in which types of reaction and products contributing to these are reported in clinical observational studies would enable better comparison between studies performed across different countries, species and settings.
Conclusions
This preliminary study revealed trends of adverse drug events in a range of ectoparasiticides commonly used to treat and control ectoparasite infestations in dogs and cats.
Establishing the link between ectoparasiticides and suspected adverse drug events is fundamental to drug safety and to reducing their burden to cats and dogs. Dermatological reactions threaten the health and welfare of the animal and cause concern to the owner, particularly in the breeding and showing community, where the external appearance of an animal can be crucial to their success. For such clients, communication of any possible adverse reactions to a drug is essential and selection of the safest option should be suggested.
Many gastrointestinal and neurological reactions can occur as a result of the companion animal ingesting the product by licking their fur, which can happen when the product is applied at an inappropriate site. To avoid this, communication with the owner is key to explain proper application methods, alongside reading the product information. Similarly, administration of a correctly prescribed product and dose is vital for the safety of animals.
In this study, it was not possible to fully estimate the incidence of the adverse drug events associated with the use of ectoparasiticides. However, the low level of observations suggests that these reactions occur occasionally and many of them are not severe. Given the nature of pharmacovigilance, these events are likely to be reported and continued monitoring is essential. Therefore, the benefits of protection from ectoparasite infestations far outweigh the potential adverse events associated with such treatment.
KEY POINTS
- This study assessed several types of adverse drug events associated with ectoparasiticide use in dogs and cats.
- The skin, the gastrointestinal tract and the nervous system are the most commonly affected body systems.
- Veterinarians and pet owners should weigh the risks and benefits of treatment in the context of all available therapeutic options for ectoparasites.
- Determining how to recognise and report adverse events to drugs can help improve understanding of how to manage adverse events.
- The occurrence of adverse drug events highlights the need to create awareness of the potential for these events and educate owners.


