As well as infecting rabbits, Encephalitozoon cuniculi may also colonise other mammals including mice, cats, dogs and sheep. It is important to recognise its zoonotic potential in immunocompromised humans. In rabbits, E. cuniculi most commonly presents as a neurological condition (Figure 1), but also affects the kidneys and eyes. Exposure to E. cuniculi is not uncommon in the UK, with one study finding that 52% of healthy rabbits were seropositive for E. cuniculi antibodies (Keeble and Shaw, 2006). Compared to the prevalence of E. cuniculi seropositivity, the prevalence of the ocular manifestation is fairly low (Williams, 2012).

Aetiopathogenesis
The classification of E. cuniculi is controversial. Previously classed as a protozoal parasite, and oft en still referred to as this, the current categorisation of E. cuniculi microsporidia is an obligate intracellular atypical fungi without mitochondria (Vergneau-Grosset and Sylvain, 2016). Three strains of E. cuniculi have been identified, with strain I infecting rabbits and humans, strain II infecting rodents, and strain III infecting dogs and humans (Didier et al, 1995).
E. cuniculi and other microsporidia infect host cells using a polar tube that pushes a sporoplasm against the cell membrane, causing invagination and uptake into a parasitophorous vacuole protected from the cell defence mechanisms (Bohne et al, 2011; Künzel and Fisher, 2018). Alternatively, infection can occur if the spore is phagocytosed by the host cell (Künzel and Fisher, 2018). Within the parasitophorous vacuoles, E. cuniculi meronts replicate and differentiate, first into sporonts and then into mature spores. Eventually, the parasitophorous vacuole ruptures because of overcrowding, releasing the spores (Künzel and Fisher, 2018) and continuing the cycle (Figure 2).
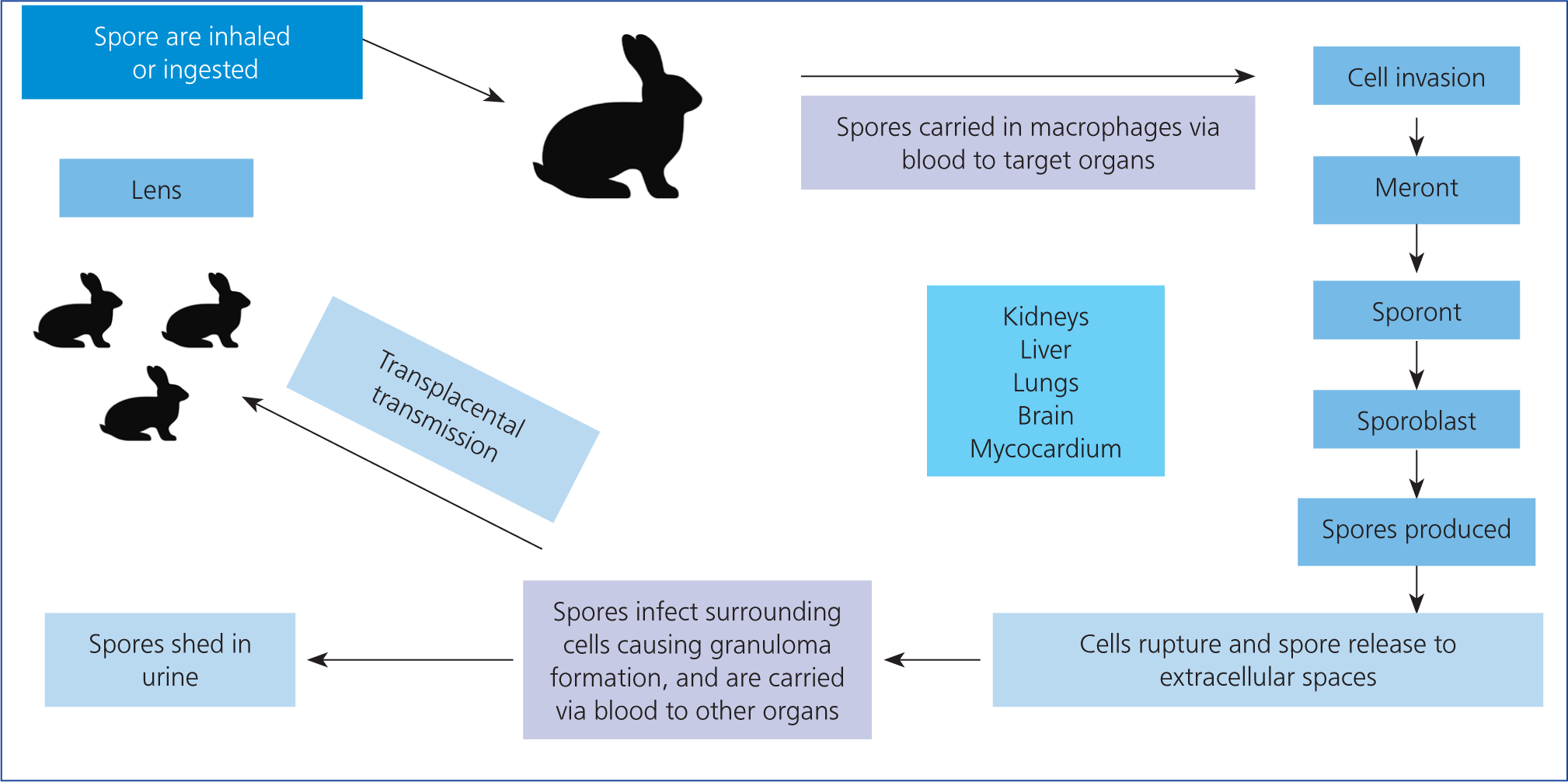
Figure 2 . Encephalitozoon cuniculi life cycle
E. cuniculi transmission can be vertical or horizontal. Horizontal transmission usually involves ingestion or inhalation of the microsporidian's environmentally resistant spores which are shed in infected urine (Künzel and Fisher, 2018). The parasite first affects the kidneys, liver and lungs, eventually targeting the brain (Künzel and Fisher, 2018). Vertical transmission, or transplacental from an infected mother, has been confirmed via polymer chain reaction (PCR) tests for E. cuniculi DNA in rabbit fetuses (Baneux and Pognan, 2003). It was originally unclear whether ocular infection occurred prenatally or postnatally, but further PCR testing on affected enucleated eyes confirmed that the parasite infects the ocular tissue in utero (Özkan et al, 2019). While rabbit embryos are developing, the early lens forms a few days before the protective lens capsule (the lens capsule is the basement membrane of the lens epithelial cells), so it is likely that the microsporidian infects the unprotected lens cells via its rich tunica vasculosa lentis blood supply during this time window (Boillot et al, 2015; Özkan et al, 2019). After birth and as the rabbit ages, the spores cause the lens capsule to rupture, leading to lens protein escape and resulting in phacoclastic uveitis and a pyogranulomatous inflammation (Felchle and Sigler, 2002). One report found rabbits with ocular granulomas caused by E. cuniculi were, on average, 18 months of age, with an age range of 3–60 months (Wolfer et al, 1993). In utero infection appears to only affect the eye, and not the other organs (Künzel and Joachim, 2010). However, one study by Jeklová et al (2019) suggested that in utero infection may not be the only aetiogenesis of ocular E. cuniculi infection. New Zealand White rabbits aged 4 months old were orally infected with E. cuniculi spores, then euthanised at 2, 4, 6 and 8 weeks post-infection, and their eyes collected for immunohistochemistry. While none of these rabbits developed any signs of uveitis, cataract or ocular granuloma, spores were detected most frequently in the cornea and sclera, and also in the lens in 11 out of 25 infected rabbits (Jeklová et al, 2019). This demonstrates that the microsporidian can access the lens postnatally, but the mechanism of this is currently unknown. In the adult rabbit, the lens is enclosed in the lens capsule and is avascular, receiving its nutritional support from the aqueous humour as the blood supply from the tunica vasculosa lentis regresses postnatally by day six (Boillot et al, 2015). It is therefore unclear how E. cuniculi entered the lens in these 4-month-old rabbits. Another question arising from the Jeklová (2019) study is whether these postnatally infected rabbits would eventually go on to develop phacoclastic uveitis similar to rabbits infected in utero, but as the study rabbits were euthanised this remains unanswered.
Clinical presentation
Ophthalmic E. cuniculi presents in a variety of ways and may be mistaken for pasteurellosis or uveal neoplasia. The ocular lesions are usually unilateral, but bilateral lesions can be seen (Harcourt-Brown and Holloway, 2003). It often presents as a white-yellow mass (granuloma), seen in the anterior chamber of the eye with neovascularisation, and a cataract (Figure 3) (Özkan and Alcigir, 2018). This mass begins at the lens, as it originates from a break in the lens capsule, with lens protein leakage and subsequent development of phacoclastic uveitis (Donnelly, 2003) (Figure 4). Williams (2012) reported that, unlike uveitis in other species, ocular E. cuniculi often does not present with a miotic pupil.
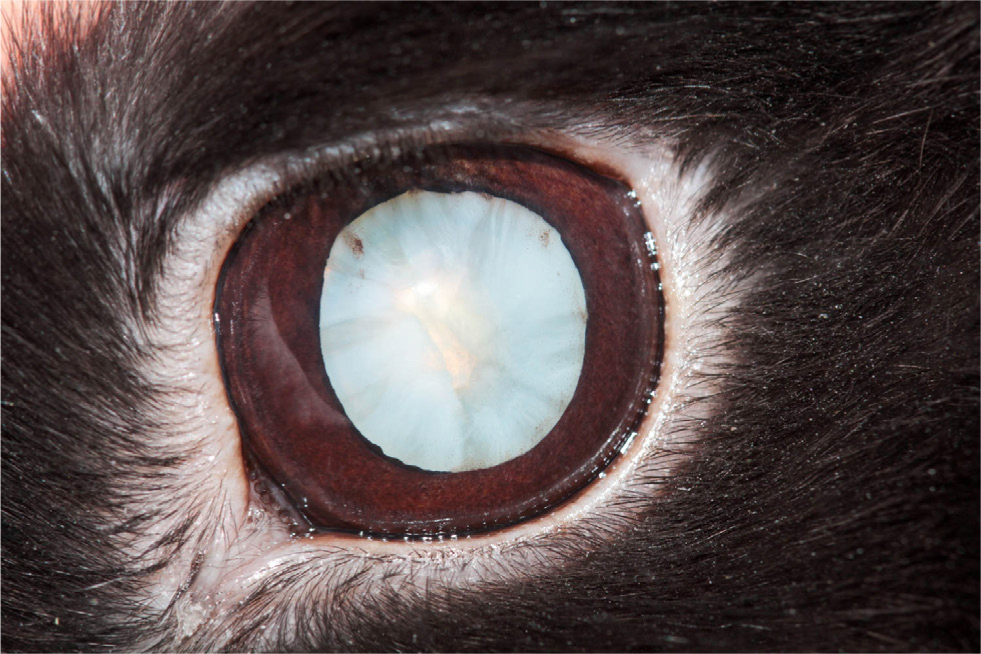
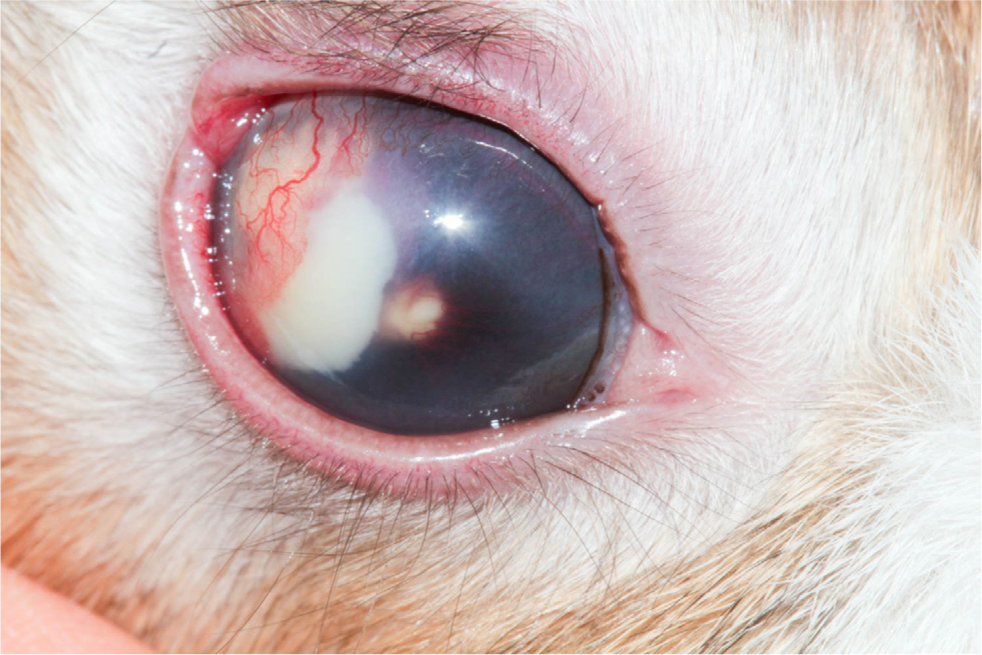
Aqueous flare will usually be seen with a slit lamp (Maini and Hartley, 2019). However, the posterior segment of the eye does not have pathological changes (Williams, 2012). On tonometry, the affected eyes will usually have a lowered intraocular pressure, but they may be normal (see later for normal reference intervals in rabbits) (Williams, 2012). If the uveitis is chronic, the affected eye can develop secondary glaucoma, synechiae or phthisis bulbi (Sandmeyer et al, 2011). Secondary glaucoma in dogs with lens-induced uveitis may be caused by blockage of the iridiocorneal drainage angle by macrophages full of phagocytosed lens (Wilcock and Peiffer, 1987). It is possible that a similar process could be extrapolated to rabbits.
Diagnosis
Diagnosing E. cuniculi can be challenging. Serology of IgG and IgM immunoglobulins is used in diagnosis, but may not always be straightforward. The severity of clinical signs does not correlate with serological titres because of the life cycle of the parasite (Figure 2) (Mancinelli, 2015). IgG immunoglobulin indicates exposure to E. cuniculi, but cannot be used alone to determine when infection occurred (Mancinelli, 2015). Following experimental infections of rabbits, there are three IgG responses that may occur: short, long, and episodic (Kunstýř et al, 1986). The short response is defined as an eventual decrease in IgG levels after days 45–63 post-infection, the long response is when IgG titre continuously rises even after 68 days post-infection, and episodic is when there are multiple, short periods of seroconversion (Kunstýř et al, 1986). It is still unclear why different rabbits show these three IgG responses, but it is thought to be influenced by individual immune system variations and exposure load (Latney et al, 2014). If IgM immunoglobulin levels are elevated, there is usually an acute or reactivated infection (Latney et al, 2014). Initial response and elevation of immunoglobulins post-infection can vary. One study looking at E. cuniculi humoral response detected IgM as early as 1-week post-infection, and IgG 1 week after when given high exposure loads (Jeklová et al, 2019). In general, IgM levels will increase between days 0–35 and decrease from weeks 5–18 (Latney et al, 2014). Unfortunately, the humoral responses do not appear to protect rabbits against E. cuniculi (Künzel and Joachim, 2010). For ocular E. cuniculi, a presumed diagnosis can be made based on the pathognomonic signs of cataract, uveitis, and a granuloma, and further supported with serology. However, it should be noted that a negative IgG titre does not rule out E. cuniculi as a potential cause of uveitis, since these cases can present chronically, and a wide variation in antibody response is reported in rabbits – with some individuals recording negative IgG titres 8 weeks post-infection (Keeble, 2013).
The diagnosis can be confirmed if phacoemulsification or enucleation is opted for with histology and immuno-histochemical staining. On histopathology of removed eyes, intralenticular heterophils, foamy macrophages and fibrous tissue with lymphocytes are seen (Van Der Woerdt, 2000). Immunohistochemical staining is performed using anti-E. cuniculi mouse anti-serum, in order to detect the spores in macrophages, lens epithelial cells and outside cells among inflammatory debris (Giordano et al, 2005).
PCR testing of the removed ocular tissue for E. cuniculi spore DNA is both highly sensitive and specific (Latney et al, 2014) and is a good option for confirming the diagnosis postoperatively. PCR testing on urine may be attempted, but is unreliable because of intermittent spore shedding (Varga, 2013).
Treatment options
Phacoemulsification
Phacoemulsification, involving lens breakdown through high frequency ultrasound, is the gold standard of treatment for ocular E. cuniculi associated with a cataract. An artificial intraocular lens may be placed in the lens capsule following phacoemulsification. Before performing this operation, it is very important to thoroughly examine the affected eye (Figure 5). If the rabbit's eye exhibits lens luxation, retinal detachment or chronic changes, then they may not be suitable surgical candidates for the procedure, on account of the eye being non-visual, or they may require a modified procedure (Sanchez et al, 2018). Retinal detachment can be assessed via ophthalmoscopy and ultrasonography. There is little point in proceeding to remove the cataract and place a lens if there is a poor prognosis for vision, because of underlying retinal pathology. Luxation or subluxation (instability) of the lens may occur as rabbits with uveitis have increased aqueous humour matrix metalloproteinases that degrade the lens zonules that support the lens (Colitz and O'Connell, 2015), which may complicate treatment.

Phacoemulsification is a specialist procedure performed by veterinary ophthalmologists. The rabbit's extraocular muscles can be paralysed with neuromuscular blocking agents to facilitate surgery. Atracurium (Felchle and Sigler, 2002) or cisatracurium (Ledbetter et al, 2018) have been described for rabbit phacoemulsification. The general procedure for cataract surgery is similar for canine, feline, rabbit and human patients (Gelatt, 2014). One or two small incisions are made into the eye adjacent to the limbus. The anterior chamber is stabilised with a special gel (viscoelastic) and a large circular hole is made in the anterior lens capsule. The lens material is removed via a phacoemulsification handpiece, and an artificial intraocular lens may be placed in the now empty lens capsular bag, or, alternatively, the eye can be left aphakic. The current recommended size and dioptre of an intraocular lens to reach emmetropia is 13mm diameter, with a dioptre power of +58D (Sanchez et al, 2017). An artificial intraocular lens will allow images projected onto the retina to be in focus, resulting in better visual acuity. In one operation, a rabbit received an artificial intraocular lens in one eye, and had the lens removed in the other eye, with no intraocular lens placed (aphakic). Postoperatively, the owner reported that the rabbit preferred to use the eye containing the intraocular lens to look at objects and the owner (Ledbetter et al, 2018). While the anecdotal evidence supports the use of an intraocular lens to improve rabbit vision, more needs to be done to assess and quantify the extent of visual improvement achievable with this technique. If hypopyon or granulomas are present, these can be aspirated at the time of phacoemulsification (Maini and Hartley, 2019).
Intraoperative complications
While any surgical procedure involves anaesthetic risks, there are several intraoperative complications of rabbit phacoemulsification, which should be fully considered before the procedure. Iris prolapse may occur through the incision, as the rabbit has a more shallow anterior chamber compared to other animals, such as the dog (Holmberg, 2007). Other intraoperative complications include lens luxation and expulsive choroidal haemorrhage (Sanchez et al, 2018). With any ocular surgery in rabbits, corneal ulceration is a risk. To prevent corneal ulcers, the downward facing eye can be protected with vacuum cushions and lubrication (Foote, 2020) or alternatively, the eye can be lubricated and cruciate skin sutures placed on the eyelids to close the eye (Sanchez et al, 2018).
Postoperative complications
Postoperative ocular hypertension is not uncommon following phacoemulsification, with evidence suggesting a prevalence of up to 75% of canine patients (Klein et al, 2011). As yet, the exact postoperative prevalence in rabbits has not been investigated, but it has been reported to occur (Ledbetter et al, 2018). In rabbits, the reference ranges for normal intraocular pressure are 15.4+/-2.2 mmHg with a Tono-Pen applanation tonometer and 9.5+/-2.2 mmHg with a TonoVet rebound tonometer (Maini and Hartley, 2019). The danger of postoperative ocular hypertension includes vision loss associated with injury to the optic nerve, retina and corneal endothelium (Miller et al, 1997). Fortunately, if postoperative ocular hypertension does occur, it is manageable. One study described successful treatment of a rabbit with brinzolamide every 8 hours, resulting in a normal intraocular pressure (Ledbetter et al, 2018). One option is to prophylactically prescribe a topical antihypertensive for 48 hours after phacoemulsification, in order to prevent postoperative hypertension from occurring (Sanchez et al, 2018). When assessing a rabbit's intraocular pressure postoperatively, the reading should ideally be taken at approximately the same time each day as diurnal variations can occur (Foote, 2020), which could lead to misinterpretation of results.
Another common postoperative complication is lens material regeneration. This phenomenon, called posterior capsular opacification, occurs when residual lens epithelial cells, attached to the lens capsule, proliferate in response to the trauma of cataract surgery (Wormstone et al, 2021). Rabbit lens epithelial cells may also differentiate into lens cells, that proceed to replicate, contributing to posterior capsular opacification, which does not occur in dogs (Williams, 2012). This may occur at varying degrees of severity, causing visual distortion as a result of the scattering of incoming light. While posterior capsular opacification also occurs in human and canine patients, rabbits can develop particularly rapid and severe posterior capsular opacification (Sanchez et al, 2017; Wormstone et al, 2021). It appears posterior capsular opacification is more likely to occur in juvenile rabbits (Ledbetter et al, 2018), which is significant as rabbits affected with E. cuniculi phacoclastic uveitis tend to be younger. Another possible contributing factor is that juvenile rabbits have an increased postoperative inflammatory response (Gwon, 2008). Other factors contributing to posterior capsular opacification include the degree of surgical trauma and the design of the intraocular lens (Gwon, 2008).
Prognosis
It is difficult to objectively assess vision after phacoemulsification, but owners' observations of their rabbits after surgery can indicate whether there has been a good improvement in vision (Sanchez et al, 2018). Owners of rabbits with bilateral cataracts reported decreased activity, curiosity, playfulness and social interaction with other rabbits. However, after cataract surgery, owners believed their rabbits improved, with vision returning to levels similar to before cataracts developed (Sanchez et al, 2018). This anecdotal evidence suggests phacoemulsification is a worthwhile procedure to improve the rabbit's quality of life.
Medical therapy
If cataract surgery is not an option or the rabbit is not a suitable surgical candidate, medical therapy may be attempted, but there is little published literature on its success or effect on long-term prognosis. Fenbendazole is licensed for treatment of E. cuniculi infection in rabbits and can be given orally daily for 28 days, in combination with topical ocular prednisolone acetate administered two to three times a day for 8 weeks, with or without topical atropine drops daily (Keeble, 2011; Williams, 2012). Some success has been reported with systemic dexamethasone and oxytetracycline treatment, with simultaneous ocular dexamethasone and tetracycline for 14 days (Ewringmann and Gobel, 1999). However, while this resolved the uveitis, the granuloma and cataracts remained (Ewringmann and Gobel, 1999). In one study, three rabbits awaiting serological results were treated with 0.1% dexamethasone sodium phosphate and 1% atropine sulphate eye drops topically, as well as with enrofloxacin and albendazole systemically (Giordano et al, 2005). However, the ocular lesions progressed in all three rabbits, leading to enucleation, as the owners declined phacoemulsification. While both of these treatment attempts used dexamethasone to reduce the inflammation in the eye, steroid use in E. cuniculi infections is controversial. As the humoral response is not protective, the major defence against infection is T-cell mediated destruction of infected cells (Latney et al, 2014). However, T-cell function and production is negatively altered by corticosteroid administration (Künzel and Joachim, 2010), so this may affect the rabbit's ability to eliminate infection. Additionally, the use of steroids is often avoided because of their strong immunosuppressant properties in rabbits, with even ocular steroids being systemically absorbed (Latney et al, 2014). If steroids are used, haematology must be carefully monitored. Alternatively, ocular non-steroidal anti-inflammatory drugs may be considered, provided the rabbit has adequate renal function (Mancinelli, 2015). Medical therapy is not always reliable, and standard treatment protocols have not been developed. However medical treatment may be useful as an initial treatment, before surgery, or if other options are not possible.
Alternative treatment options
Alternatively, the blind affected eye may be left if cataract surgery is not performed and medical therapy is unsuccessful, but only provided the eye is non-painful. This is essential to determine, as rabbits are prey species and naturally hide signs of pain, which could lead to welfare concerns for the affected animal. However, rabbits can cope with blindness if they live in a familiar environment with a companion (Fehr, 2013). An uncommon complication of chronic phacoclastic uveitis in rabbits is development of an intraocular sarcoma, thought to be a result of neoplastic transformation of lens epithelial cells secondary to injury (Dickinson et al, 2013). It is still to be determined whether neoplastic transformation also occurs in E. cuniculi-associated phacoclastic uveitis, but this is worth considering if an affected eye is to remain in situ.
Enucleation
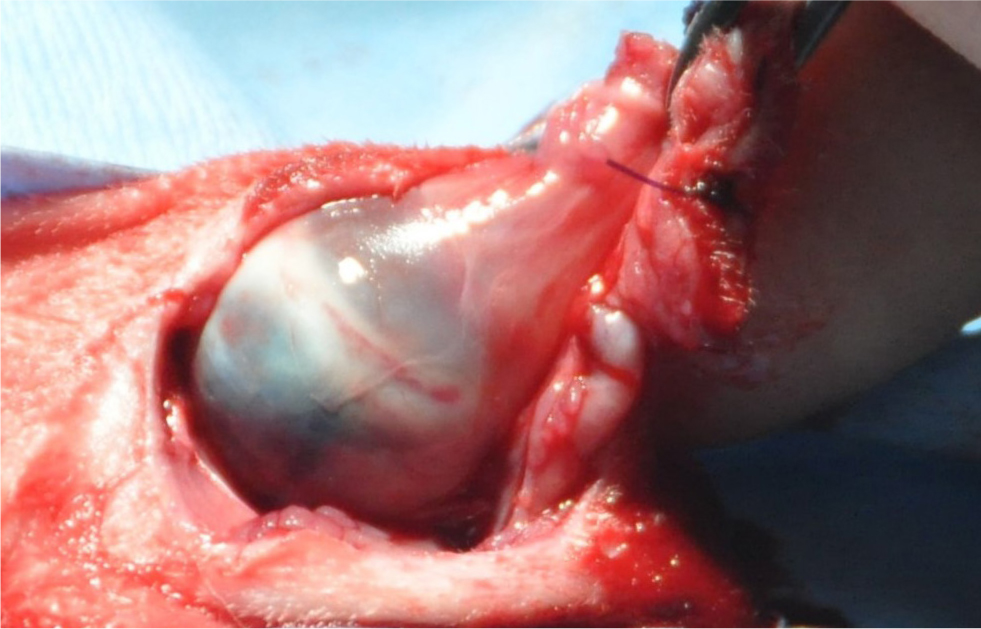
If the eye is painful, enucleation must be performed on welfare grounds (Figure 6). Signs of ocular pain in rabbits include blepharospasm, enophthalmos and epiphora (Gelatt and Plummer, 2017). Changes indicating the requirement for enucleation include chronic synechia, phthisis bulbi and glaucoma (Sandmeyer et al, 2011). While phthisis bulbi itself is not painful, if it causes entropion because of the eye being retracted, or it is associated with chronic inflammation, enucleation is required (Mitchell, 2008) as these conditions are associated with pain. Acute glaucoma is associated with severe pain and chronic glaucoma is associated with continuous dull pain, that is relieved after enucleation (Maggio, 2015). So again, if present, the eye should be removed on welfare grounds (Figure 7). The main complication in rabbit enucleation is an increased haemorrhage risk because of the orbital venous plexus behind the globe (Williams, 2012), which cannot be ligated if haemorrhaging, rather relying on pressure to control bleeding. Haemorrhage can be prevented by performing a transconjunctival enucleation rather than the transpalpebral method used in dogs and cats, as this technique involves dissecting closer to the globe, to better avoid damaging the orbital venous plexus (Diehl and McKinnon, 2016). Enucleated eyes can be submitted for PCR or immunohistochemistry to confirm E. cuniculi. Postoperative complications may include infection, dehiscence, haemorrhage or, less commonly, orbital emphysema if the nasolacrimal duct remains patent (Diehl and McKinnon, 2016).
Conclusions
Ocular E. cuniculi, while not as common as the neurological presentation, still has a significant impact on rabbit health and welfare. Despite rabbits now being popular pets with dedicated owners, there are still numerous gaps in our knowledge of the disease process, treatment, and management of the ocular manifestation of this common condition in pet rabbits. Current treatment options for this condition include medical management, enucleation and phacoemulsification, with the option of benign neglect in select cases that are veterinary assessed as non-painful. Phacoemulsification is considered the optimal treatment for rabbits affected by ocular E. cuniculi as it removes the source of inflammation and gives the best visual prognosis. Unfortunately, studies focused on cataract surgery in rabbits primarily have a human medical research aim, rather than being in the interest of pet rabbits. Therefore, visual acuity and its impact on quality of life in rabbits after placement of an artificial lens following phacoemulsification remains an important area for further research.
KEY POINTS:
- Encephalitozoon cuniculi is a microsporidian parasite affecting rabbits as well as other mammals, including immunocompromised humans.
- In rabbits, E. cuniculi most commonly presents as a neurological condition, but it also affects the kidneys and eyes.
- Ocular E. cuniculi typically presents with phacoclastic uveitis, a yellow-white granuloma and a cataract.
- Current treatment options for ocular E.cuniculi include medical management, enucleation and phacoemulsification, with the option of benign neglect in select cases that are assessed by a veterinarian and deemed non-painful.
- Phacoemulsification, involving lens breakdown through high frequency ultrasound, is the gold standard treatment for ocular E. cuniculi associated with a cataract.


